Abstract
Dermatofibrosarcoma protuberans (DFSP) is a locally invasive neoplasia with a pattern of infiltrative growth that leads to extended resections. To avoid unnecessary resections and spare tissues, its treatment requires an adequate assessment of the margins. We present a case where artificial dermis (Matriderm®) was used followed by skin graft for reconstruction. We present a 50‐year‐old woman with a DFSP in the occipital region. She was referred to us after a first surgery with positive margins. A wide local excision with a 2‐cm margin was performed and periosteal tissue was also removed, which led to exposure of the skull. Matriderm was placed on the bone surface and dressings were changed every other day. Meanwhile, margins were evaluated by the complete circumferential and peripheral deep margin assessment (CCPDMA) and were positive for DFSP in the superior margin. After 4 weeks the area was completely covered by granulation tissue and a new resection followed by reconstruction with a skin graft was performed. With regard to the difficulties in the margin assessment in DFSP, we present artificial dermis (Matriderm) as an option for reconstructive surgery in these patients, especially when a skin graft cannot be performed as a first option.
Keywords: Artificial dermis, CCPDMA, Dermatofibrosarcoma protuberans
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare mesenchymal tumour that arises from the skin. It corresponds to 1% of soft tissue sarcomas and to 0·1% of malign neoplasias. Although classified as a low‐grade sarcoma that rarely metastasises, it is very invasive and reports high rates of local recurrence 1.
Although surgery has been reported as the main therapeutic option in DFSP, there are some controversies in managing these cases. The most frequent techniques are Mohs surgery, wide local excision with 2‐ or 3‐cm margin and surgery followed by 3D complete circumferential and peripheral deep margin assessment (CCPDMA) 2, 3.
By using the CCPDMA technique, the tumour is removed with a smaller margin than the traditional 3 cm one. The material is processed in paraffin and margins are completely evaluated. Immunohistochemistry (IHQ) may be useful for margin assessment in some cases. This technique is associated with a more accurate evaluation of the margins and shows very low rates of recurrence in literature 1, 2.
Nevertheless, this technique has some limitations. It may take some time until the material is completely studied and, in some cases, patients might need another surgery in the case of positive margins. Some patients can be submitted to primary closure after the resection, and another surgery is completely feasible in these cases whenever necessary. Nonetheless, some patients could have larger resections and primary closure will not be possible.
Skin graft may be a very interesting option in large resections; but whenever bones, nerves and tendons are exposed, skin grafts should be avoided. Skin flaps are not good options for reconstruction because they can lead to a very difficult second surgery if necessary.
Dermal substitutes have been widely used in plastic, reconstructive and burn surgery for many years. Matriderm (Dr. Suwelack Skin & Health Care AG, Billerbeck, Germany)—an acellular lyophilised collagen and elastin‐based biomatrix—was introduced several years back, which is a thin (1 or 2 mm) dermal template. It has been successfully used in one‐step procedures in combination with split‐thickness skin grafts 4.
Although the use of Matriderm in burns and trauma surgery has been widely demonstrated, there are only few reports available on its use in surgical oncology 5, 6 and none has yet reported it for DFSP.
Case report
We present a 50‐year‐old woman who was diagnosed as having a DFSP in the occipital region. She had her first surgery in another hospital during which the lesion had been removed and a skin flap was performed. She was referred to us because the pathological studies showed positive margins for DFSP.
A wide local excision was performed and the periosteal tissue had to be removed, which led to exposure of the skull (Figure 1A). While waiting for the final pathology report before performing the reconstructive surgery, Matriderm 2 mm was placed at the site (Figure 1B,C). A compressive dressing was done and it was removed 6 days after surgery.
Figure 1.
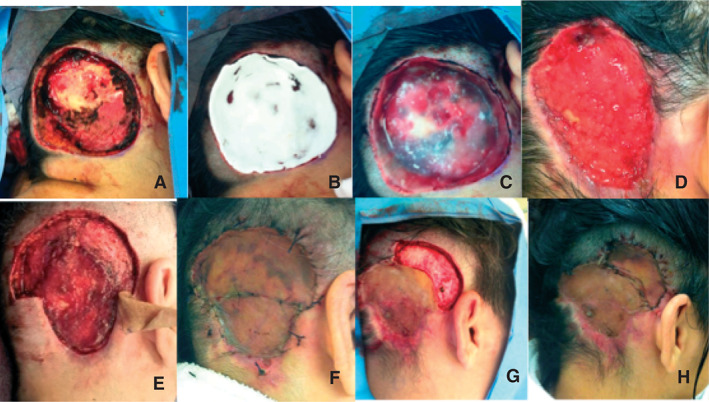
After the removal of the lesion, skull was exposed (A). Matriderm® 2 mm was placed (B and C). After 4 weeks the wound was completely granulated and the edges were retracted (D). Two other excisions were necessary (E–G) until clear margins could be reached and the final reconstructive surgery was performed (H).
Following this, the patient underwent change of dressings every other day and in 4 weeks the site was completely covered by granulation tissue (Figure 1D). Negative pressure wound therapy (NPWT) was not used mainly because of the topography of the lesion. Meanwhile, the pathological studies showed that superior margins were positive for DFSP again. Therefore, another wide local excision was performed in this area, but with preservation of the periosteal tissue, and skin grafts were also performed (Figure 1F).
The CCPDMA showed another area with DFSP in the upper lateral portion of the lesion. Hence, the patient was submitted to another wide local excision, and another skin graft was placed. All the procedures were well tolerated by the patient; the last CCPDMA showed clear margins, and she is now disease‐free, 6 months after the last surgery.
Discussion
DFSP represents a complex challenge for the oncologic and reconstructive surgeon because of its high rate of recurrence. The first challenge is the adequate size of the margins, which has been largely discussed in the literature 3, 7. The current method of performing adequate margin assessment can be done either by Mohs surgery or by paraffin following the CCPDMA protocols 2, 3, 8, 9. In our service, we practice a wide local excision with a 2‐cm margin followed by CCPDMA and IHQ whenever necessary 1.
This case is a good example to show the difficulty involved in accessing free margins in DFSP. While a 1‐cm margin was enough in the lower part of the lesion, more than a 4‐cm margin was necessary in the upper lateral portion of the lesion.
The second challenge is with regard to reconstruction. Whenever primary closure is feasible, this method is followed. Even when the CCPDMA shows positive margins, the region can be easily accessed for another local excision. Nevertheless, in larger wounds, skin graft is the first option.
In the reported case, skin graft was not possible owing to bone exposure. Matriderm has already been used for covering deperiosted bones 10, as in other situations where skin graft was not possible 11, 12. Nevertheless, there are no reports of its use in the reconstruction of DFSP excisions in the literature.
This method was of benefit to the patient because by using Matriderm her skull could be covered during the surgery, which otherwise would have only been possible with the use of a skin flap. Also, because of the deperiosted bone, a 2‐mm layer of Matriderm was used instead of the 1‐mm one.
The healing process occurred while the margins were fully assessed in the pathological department and it was reported that the superior margins were positive. We considered it a second benefit of Matriderm as it allowed another resection and reconstruction with a skin graft in a large area of resection according to oncological principles.
There are evidences in literature that the use of NPWT associated with other artificial dermis, such as Pelnac® and Integra®, could hasten the healing process 13, 14, 15. In this case, we did not consider the use of NPWT owing to the localisation of the lesion, as it would be very hard to place the vacuum device in the occipital region.
Matriderm can be used with or without NPWT 16, which was also favourable in this case. Nonetheless, there is a clear benefit of NPWT associated with artificial dermis, and we intend to use both of them in future cases, not only for DFSP but also for larger reconstructions in general.
This is the first case reported in the literature where Matriderm was used in DFSP. We believe that this could become an interesting option in selected patients who will need large resections, especially when a skin graft cannot be performed as a first option.
References
- 1. Molina AS. Dermatofibrosarcoma protuberans: análise dos marcadores de proliferação celular, invasividade e apoptose. Estudo da fusão de col‐1α1/pdgf‐β por fish e correlação com a recidiva. Paper presented at Fundação Antônio Prudente for Master in Sciences graduation, March 2012. URL http://accamargo.phlnet.com.br/MESTRADO/2012/AndreMolina/AndreMolina.
- 2. Häfner HM, Moehrle M, Eder S, Trilling B, Röcken M, Breuninger H. 3D Histological evaluation of surgery in dermatofibrosarcoma protuberans A\and malignant fibrous histiocytoma: differences in growth patterns and outcome. Eur J Surg Oncol 2008;34:680–6. [DOI] [PubMed] [Google Scholar]
- 3. Farma JM, Ammori JB, Zager JS, Marzban SS, Bui MM, Bichakjian CK, Johnson TM, Lowe L, Sabel MS, Wong SL, Douglas Letson G, Messina JL, Cimmino VM, Sondak VK. Dermatofibrosarcoma protuberans: how wide should we resect? Ann Surg Oncol 2010;17:2112–8. [DOI] [PubMed] [Google Scholar]
- 4. Böttcher‐Haberzeth S, Biedermann T, Schiestl C, Hartmann‐Fritsch F, Schneider J, Reichmann E, Meuli M. Matriderm® 1 mm versus Integra® single layer 1.3 mm for one‐step closure of full thickness skin defects: a comparative experimental study in rats. Pediatr Surg Int 2012;28:171–7. [DOI] [PubMed] [Google Scholar]
- 5. Pauchot J, Elkhyat A, Rolin G, Mac S, Grumblat A, Fotso A, Humbert P, Tropet Y. Dermal equivalents in oncology: benefit of one‐stage procedure. Dermatol Surg 2013;39(1 pt 1):43–50. [DOI] [PubMed] [Google Scholar]
- 6. Sohn WI, Han SH, Jung SN. One‐stage skin grafting of the exposed skull with artificial dermis after cancer removal: long‐term experiences. Head Neck Oncol 2012;4:73. [Google Scholar]
- 7. Buck DW 2nd, Kim JY, Alam M, Rawlani V, Johnson S, Connor CM, Dumanian GA, Wayne JD. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol 2012;67:861–6. [DOI] [PubMed] [Google Scholar]
- 8. Matin RN, Acland KM, Williams HC. Is Mohs micrographic surgery more effective than wide local excision for treatment of dermatofibrosarcoma protuberans in reducing risk of local recurrence? A Critically Appraised Topic. Br J Dermatol 2012;167:6–9. [DOI] [PubMed] [Google Scholar]
- 9. Roh MR, Bae B, Chung KY. Mohs' micrographic surgery for dermatofibrosarcoma protuberans. Clin Exp Dermatol 2010;35:849–52. [DOI] [PubMed] [Google Scholar]
- 10. Heckmann A, Radtke C, Rennekampff HO, Jokuszies A, Weyand B, Vogt PM. One‐stage defect closure of deperiosted bone and exposed tendons with MATRIDERM® and skin transplantation: possibilities and limitations. Unfallchirurg 2012;115:1092–8. [DOI] [PubMed] [Google Scholar]
- 11. Cervelli V, Brinci L, Spallone D, Tati E, Palla L, Lucarini L, De Angelis B. The use of MatriDerm® and skin grafting in post‐traumatic wounds. Int Wound J 2011;8:400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryssel H, Radu CA, Germann G, Otte M, Gazyakan E. Single‐stage Matriderm® and skin grafting as an alternative reconstruction in high‐voltage injuries. Int Wound J 2010;7:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eo S, Kim Y, Cho S. Vacuum‐assisted closure improves the incorporation of artificial dermis in soft tissue defects: Terudermis(®) and Pelnac(®). Int Wound J 2011;8:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta S. Optimal use of negative pressure wound therapy for skin grafts. Int Wound J 2012;9(Suppl 1):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sinna R, Qassemyar Q, Boloorchi A, Benhaim T, Carton S, Perignon D, Robbe M. Role of the association artificial dermis and negative pressure therapy: about two cases. Ann Chir Plast Esthet 2009;54:582–7. [DOI] [PubMed] [Google Scholar]
- 16. Atlan M, Naouri M, Lorette G, Estève E, Zakine G. Original treatment of constitutional painful callosities by surgical excision, collagen/elastin matrix (MatriDerm(®)) and split thickness skin graft secured by negative wound therapy. Ann Chir Plast Esthet 2011;56:163–9. [DOI] [PubMed] [Google Scholar]


