Abstract
Ischaemia‐reperfusion syndrome (IRS) is a condition that may require early fasciotomy. In the past, fasciotomies ultimately required prolonged hospitalisation. Vacuum‐assisted closure (VAC) therapy system is an innovative method which promotes wound healing by reducing wound oedema, increasing microcirculation, and stimulation of granulation tissue. The aim of this retrospective study was to compare the VAC treatment with the conservative treatment of the fasciotomy wound until definitive surgical closure. The researchers retrospectively identified 15 patients, 3 females and 12 males, with a mean age of 69 years, who underwent a fasciotomy between January 2003 and December 2009 at the University Hospital of Geneva. All of the fasciotomies performed on the patients were on account of IRS. Seven patients were subjected to wound treatment using the VAC‐system device and eight patients underwent treatment through the usual conservative method. The data were analysed by comparing the operative wound size, length of time for wound closure and duration of hospital stay in both groups. The number of days after fasciotomy until surgical wound closure in the VAC‐system group (n = 7) ranged from 8 to 13 days with a mean of 11 days. The wound size at the day of closure was decreased in length by a mean of 58% (range 29–67%) and in diameter by a mean of 56% (range 33–75%). The duration of hospital stay for this group ranged from 12 to 18 days with a mean of 14 days. No signs of infections were observed and no re‐operation was required after first closure. In the conservative group (n = 8), the time to wound closure ranged between 12 and 20 days with a mean of 15 days. The wound size was decreased in length by a mean of 40% (range 32–53%) and in diameter by a mean 46% (range 30–70%). The mean duration of hospital stay was 18·5 days. Three of the patients in the conservative treatment group manifested wound infection during the course of the treatment. VAC device could be a new standard for treatment of fasciotomy wound. VAC therapy is a recent innovation and becoming more and more a necessary complementary therapy to hasten wound healing. In our preliminary study, the VAC‐system device showed significantly reduction of the wound size, decreased tissue oedema, duration of hospital days and improvement of granulation tissue.
Keywords: Compartment syndrome, Fasciotomy closure, Fasciotomy treatment, VAC, Vascular surgery
INTRODUCTION
Ischaemia‐reperfusion syndrome (IRS) is a condition in which increased pressure within a closed compartment compromises the circulation and function of the tissues within that space (1). Elevated tissue pressure in the compartments of the leg or forearm, upper arm, thigh, foot, buttocks, hand and abdomen may cause loss of function or necrosis of the enclosed nerve and muscle. IRS of the upper extremity and lower limb has multiple aetiologies, including fracture, intensive use of muscles, venous obstruction, burns, crush or electrical injury, iatrogenic, idiopathic reasons and after surgical intervention 2, 3. IRS is a source of significant morbidity and mortality (4). In the Department of Vascular Surgery, IRS is very often observed in cases of post‐surgical revascularisation (5), that is indicated by artery occlusion, induced traumatic, thrombo‐embolic or acute aggravation of vascular disease. The incidence of IRS occurs up to 20% in acute ischaemic limbs that have been revascularised (6). In these cases an emergency fasciotomy is required to reduce the compartment pressure 7, 8, 9. Primary conservative closure of fasciotomy wounds is difficult and can cause wound oedema (10). In this case, there are many opportunities to treat this wound until final closure. In this study, the researchers compared the results of using the vacuum‐assisted closure (VAC) device vis‐à‐vis the conservative treatment of fasciotomy wound among patients with IRS.
Materials and methods
With the aid of the cardiovascular database at the University Hospital of Geneva, the researchers retrospectively identified 15 patients who underwent fasciotomies as a result of IRS between January 2003 and December 2009. These patients constituted the groups that were compared with respect to the results of the treatment using the VAC‐system device and conservative treatment of the fasciotomy wound until final surgical closure, respectively.
The researchers performed the VAC‐system therapy on seven patients and the common wound treatment with wound dressings was done on the other eight cases or the conservative treatment group. The characteristics of patients specific to operation indication, ischaemia time, operation type, the interval between first operation and development of IRS, fasciotomy type and the wound size are presented in 1, 2. Thirteen patients were under full‐dose unfractionated heparin therapy for therapeutic anticoagulation, activated partial thromboplastin time (aPTT) ratio (at least daily) and adjustment of heparin doses according to a local protocol, and two patients were under low‐dose unfractionated heparin (5000 IU 8–12 hours or 7500 IU 12 hours) that was administered subcutaneously as a routine prophylaxis for venous thromboembolism.
Table 1.
Characteristic of the patients (group VAC therapy)
| Patient no. | Age (years) | Gender | Operation indication | Ischaemia time pre‐OP (hours) | Operation type | Interval OP → IRS * (hours) | Fasciotomy type [wound size (cm)] |
|---|---|---|---|---|---|---|---|
| 1 | 54 | M | Traumatic popliteal artery rupture | 3 | Popliteal artery repair + replacement | 3 | Bilateral (30 × 10) |
| 2 | 75 | W | Acute lower limb artery occlusion | 8 | Arterial desobstruction + femoro‐popliteal bypass | 5 | Bilateral (28 × 10) |
| 3 | 50 | M | Chronically subocclusion of subcalvian artery | >8 | A subclavia bypass | 20 | Unilateral (17 × 7) |
| 4 | 62 | M | Acute lower limb artery occlusion | 10 | Arterial desobstruction + femoro‐popliteal bypass | 12 | Bilateral (28 × 9) |
| 5 | 39 | W | Acute lower limb artery occlusion | 6 | Arterial desobstruction | 8 | Bilateral (29 × 9) |
| 6 | 79 | M | Acute lower limb artery occlusion | 7 | Arterial desobstruction + endarteriectomie A. femorale | 15 | Unilateral (31 × 9) |
| 7 | 71 | M | Acute lower limb artery occlusion | 5 | Arterial desobstruction | 10 | Bilateral (30 × 12) |
Pre‐OP, preoperative.
*Time between first surgical intervention and IRS.
Table 2.
Characteristic of the patients (group conservative therapy)
| Case no. | Age (years) | Gender | Operation indication | Ischaemia time (hours) | Operation type | Interval OP → IRS * (hours) | Fasciotomy [wound size (cm)] |
|---|---|---|---|---|---|---|---|
| 1 | 81 | M | Acute artery occlusion | 6 | Arterial desobstruction | 2 | Bilateral (30 × 11) |
| 2 | 73 | M | Acute artery occlusion | 8 | Arterial revascularisation | 8 | Bilateral (32 × 10) |
| 3 | 69 | M | Acute artery occlusion | 12 | Arterial revascularisation | 20 | Unilateral (29 × 10) |
| 4 | 55 | M | Acute artery occlusion | 8 | Arterial desobstruction | 15 | Bilateral (30 × 9·5) |
| 5 | 75 | W | Acute artery occlusion | 7 | Arterial desobstruction | 8 | Bilateral (28 × 9) |
| 6 | 69 | M | Acute artery occlusion | 15 | Arterial desobstruction | 6 | Unilateral (30 × 10) |
| 7 | 45 | M | Traumatic artery rupture | 8 | Arterial revascularisation | 9 | Bilateral (25 × 8) |
| 8 | 70 | M | Acute artery occlusion | 10 | Arterial revascularisation | 6 | Bilateral (25 × 9) |
*Time between first surgical intervention and IRS.
VAC‐system group
Among the seven patients who comprised the VAC‐group, two were females and five were males (Table 1).
Six of these patients were operated for thromboembolism, acute artery occlusion or major indications of a developing vascular disease. Five of them showed acute artery occlusion of the lower limb and one patient showed occlusion of the upper limb. The age of the patients ranged between 39 and 79 years with a mean of 61·4 years. Preoperatively, the ischaemia time ranged between 3 and 10 hours with a mean of 6·7 hours. Four of the patients required lower limb arterial desobstruction, and two required arterial bypasses by a graft. The mean time after the surgical intervention until the manifestation of IRS was 10·4 hours. All the fasciotomies were performed for IRS by the same department staff. Six patients underwent lower limb fasciotomy and one patient had upper extremity fasciotomy. Five patients required bilateral fasciotomy while the two other patients required unilateral fasciotomy. The wound size length ranged between 17 and 31 cm with a mean of 27 cm and the width ranged between 7 and 12 cm with a mean of 9·4 cm (Figure 1).
Figure 1.
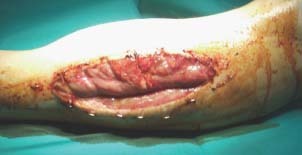
A 44‐year‐old patient after car accident needed a femoro‐popliteal bypass. After compartment syndrome developed, an emergency lateral and median fasciotomy was necessary.
VAC system, also called negative pressure wound system (11) [Kinetic Concepts Inc. (KCI), San Antonio, TX], consists of an open‐cell foam dressing covered with an adhesive drape. The dressing is connected to a vacuum pump that creates and maintains a subatmospheric pressure intermittent or continuous, which depends on the wound size, wound surface and wound area (Figure 2). The details of the VAC implantation were explained in this section. It comprises an open pore foam conduit (cut to fit a wound), which is covered by a semi‐permeable adhesive drape, attached to a microprocessor‐controlled therapy unit. The unit applies negative pressure to the foam–wound interface through a specialised tube, which also transfers accumulated fluid to a canister (12).
Figure 2.
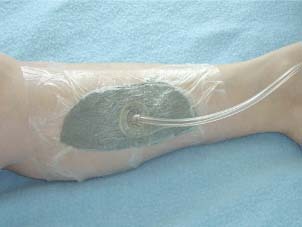
Fasciotomy wound with VAC‐system.
VAC‐system therapy protocol
In all seven cases, the first VAC implantation was postoperatively done between the first and second days. After the fasciotomy and before starting with the VAC therapy the researchers measured the maximum length and width or diameter of the fasciotomy wound. Bacteriological cultures were also taken during the changing of wound dressing. The surgical debridement and cleaning of the wound for removal of necrotic tissue was performed. The procedure using the VAC device involved fitting a cut piece of VAC GranuFoam® (polyurethane Foam, KCI, San Antonio, TX) into the fasciotomy wound and fixing this with a special adhesive drape. A small opening (2 cm × 2 cm) was cut in the adhesive drape for the VAC pad. The VAC pad was then implanted into the fasciotomy wound with the fixed foam (13) (Figure 3). The VAC‐system was adjusted between 75 and 125 mm Hg in a continuous mode. The open wound now became a closed, controlled system.
Figure 3.
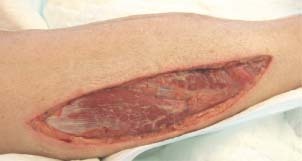
Fasciotomy wound after 1 week treatment with VAC‐system before direct closure.
The VAC foam was changed every 2–4 days by a wound care nurse. This was done under clean and clear but not necessarily sterile conditions. When the wound size and wound oedema decreased and the wound was macroscopically and microscopically infection‐free as indicated by a negative culture, then primary closure surgery or a delayed primary closure was undertaken.
All patients were treated with intravenous antibiotics for at least 72 hours before surgical wound closure.
Conservative therapy group
The conservative treatment group consisted of eight patients, seven of whom were males (Table 2). The age of the patients ranged between 45 and 81 years with a mean of 67 years. Seven of the patients were operated for thromboembolism, acute occlusion or major indication of a developing vascular disease of the lower limb. Three of these patient required artery desobstruction and the other four patients received emergency arterial revascularisation (bypass). One patient was operated after a traumatic artery ruptured in the lower limb. The preoperative ischaemia time ranged between 6 and 15 hours with a mean of 9·1 hours. The time that IRS developed after the surgical intervention ranged between 2 and 15 hours with a mean of 9·2 hours. All the fasciotomies were performed for IRS by the same department staff. Six patients required bilateral fasciotomy while two patients required unilateralfasciotomy The length of the fasciotomy wound ranged between 25 and 32 cm with a mean of 28 cm and width of the fasciotomy wound ranged between 8 and 11 cm with a mean 9·5 cm.
Conservative therapy protocol (gauze dressing)
Before starting the conservative wound treatment after surgery, the researchers measured the maximum length and width (diameter) of the fasciotomy wound. Bacteriological cultures were taken during the changing of the wound dressing. Surgical debridement and cleaning of the wound for removal of necrotic tissue was also performed. In all the eight patients, the researchers used sterile compresses that were fixed with semi‐elastic bandage after cleaning the fasciotomy with NaCl 0·9% Tüll‐Betadine® (Mundipharma, Hamilton, Bermuda).
Changing of wound dressing was done two to three times per day for reasons of dressing mobilisation and exudation of wound liquid. After the wound size and wound oedema decreased and the wound was macroscopically and microscopically infection‐free as indicated by a negative culture then primary closure surgery was done. All patients were treated with intravenous antibiotics for least at 72 hours before surgical wound closure.
DISCUSSION
Conservative primary closure of fasciotomy wounds is constrained by wound oedema and the large wound size (10). Moreover, using the conservative methods (gauze dressing) of treatment may prove to be very unsatisfactory. The common methods of the fasciotomy closure described in the literature include the use of skin‐stretching devices (7), split thickness skin grafts (8), vessel loop techniques (14) and dermatotraction techniques 15, 16, 17.
Recent advances in technology combined with better understanding of the complex cellular and biochemical mechanisms of the wound healing have resulted in the development of a plethora of advanced wound‐healing modalities such as hyperbaric oxygen, tropical growth factors, bioengineered skin and tissue equivalents and negative pressure wound therapy (NPWT) 18, 19, 20, 21, 22, 23. The NPWT (VAC; KCI) was developed at the Wake Forest University at Winston‐Salem, North Carolina in the early 1990s (11). VAC therapy is a non invasive wound closure system that uses controlled, localised subatmospheric pressure to help promote healing in chronic and acute wounds. VAC promotes wound healing in pressure ulcers; diabetic foot; and other types of acute and traumatic wounds. Because of its ability to also manage wound exudates, VAC is very useful in managing large and heavy drainage wounds. Both characteristics can serve as a catalyst to secondary wound healing and as a bridge between debridement and definitive closure 24, 25, 26, 27, 28. Many studies reported that the VAC therapy effectively prepares the wound bed for grafting or delayed primary wound closure (25). VAC helps to decrease the frequency of dressing change and the time between debridement (fasciotomies) and definitive closure and lowers the costs of the hospital stay 25, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38.
RESULTS
For the seven patients who underwent post‐fasciotomy, it was found that the wound size length ranged between 17 and 30 cm with a mean of 27·5 cm. The width of the wound ranged between 7 and 12 cm with a mean of 9·4 cm. It took an average of 2 days before the VAC therapy commenced after the fasciotomy (Table 3). The variations were due to multiple factors that were related to active bleeding and the general medical condition of the patient. Pressure settings were between 75 and 125 mm Hg that was applied on a continuous mode depending on the amount of bleeding. With the VAC, a higher suction would stimulate microcirculation on the wound tissue, but it also increases the diffuse wound bleeding. When the bleeding tendency decreased after the VAC implantation usually within 24 hours, the valve of the suction was gradually opened to increase the pressure. Twenty‐four hours after the VAC implantation the mean volume of exudates that accumulated in the canister was 630 ml in all cases. The volume of the exudates decreased daily that gradually reduced the wound oedema. The VAC dressing changing time was between 2 and 4 days (Table 4). The mean duration of the VAC therapy was 11·5 days. No complications were recorded for any of the patient. All seven patients who underwent the VAC therapy had their wound closed at an average of 11 days after fasciotomy. During the last 24 hours before wound closure, it was observed that the mean volume of exudates that accumulated in the canisters was 39 ml in all cases. At the day of wound closure, the length of the wound had a mean of 11·57 cm (range 10–14 cm) and the diameter of the wound had a mean was 4·1 cm (range 3–6 cm). The length of the wound decreased by a mean of 58% (range 29–67%) and the width by a mean of 56% (range 33–75%). Five patients underwent surgical closure with adjacent skin and the other two patients required a small skin graft. The mean duration of hospital stay in the VAC‐system group was 15 days (range 12–18 days). It was also significantly observed that no infections occurred and no other intervention was required during the first 4 weeks after the VAC therapy.
Table 3.
Outcomes of vacuum‐assisted closure (VAC) for the treatment of fasciotomy wound (group VAC therapy)
| Patient no. | Interval between fasciotomy and VAC (days) | Accumulated fluid in the canister [VAC starting (total ml/24 hours)] | Accumulated fluid in the canister [VAC finished (total ml/24 hours)] | VAC changing time (days) | VAC therapy * (total days) | Wound closure time † (days) | Wound size at the day of the closure [maximum length × maximum width (cm)] | Type of wound closure | Hospital stay ‡ [total (days)] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 970 | 50 | 2 | 10 | 10 | 14 × 3 | Adjacent skin | 13 |
| 2 | 2 | 750 | 35 | 3 | 13 | 13 | 12 × 4 | Adjacent skin | 15 |
| 3 | 2 | 250 | 30 | 3 | 10 | 10 | 12 × 4 | Adjacent skin | 12 |
| 4 | 2 | 550 | 50 | 4 | 9 | 12 | 11 × 3 | Adjacent skin | 15 |
| 5 | 1 | 630 | 20 | 3 | 12 | 12 | 12 × 6 | Skin graft | 18 |
| 6 | 1 | 330 | 30 | 2 | 7 | 8 | 10 × 6 | Skin graft | 12 |
| 7 | 2 | 850 | 60 | 3 | 10 | 12 | 10 × 3 | Adjacent skin | 13 |
*Total time of VAC therapy.
†Time between fasciotomy and surgical wound closure.
‡Time between fasciotomy and discharge from hospital.
Table 4.
Outcomes of the treatment of fasciotomy wound conservative therapy (group conservative therapy)
| Patient no. | Wound dressing (changing/day) | Wound infection | Wound closure time * (days) | Wound size at the day of the closure (cm) | Type of wound closure † | Hospital stay ‡ [total (days)] |
|---|---|---|---|---|---|---|
| 1 | 2 | None | 16 | 18 × 4 | Adjacent skin | 18 |
| 2 | 2 | None | 15 | 15 × 3 | Adjacent skin | 20 |
| 3 | 1 | None | 13 | 15 × 7 | Skin graft | 17 |
| 4 | 2 | None | 12 | 19 × 5 | Adjacent skin | 15 |
| 5 | 2 | MRSA | 16 | 19 × 5 | Adjacent skin | 20 |
| 6 | 2 | Enterococcus | 20 | 18 × 8 | Skin graft | 25 |
| 7 | 2 | Escherichia coli | 16 | 16 × 4 | Adjacent skin | 18 |
| 8 | 2 | None | 12 | 16 × 5 | Adjacent skin | 15 |
*Time between fasciotomy and the day of the surgical wound closure.
†Surgical closure with or without skin graft.
‡‡Time between fasciotomy and discharge from hospital.
In the conservative treatment group (Table 4) eight patients underwent a fasciotomy. The calculation of the volume of exudates was impossible because of the irregular and multiple changing of the wound dressing. At the beginning of the treatment the fasciotomy wound size showed a maximum mean length of 28·6 cm (range 25–32 cm) and a maximum mean width of 9·56 cm (range 8–11 cm) (Table 2). During the course of the conservative therapy, the fasciotomy wound of three patients was infected with methicillin‐resistant staphylococcus (MRSA), Enterococcus faecalis and Escherichia coli. These patients were subjected to antibiotic therapy for a minimum of 2 weeks until the bacteriological cultures showed negative microbial growth. Thereafter, the fasciotomy wound was closed. The mean time interval between fasciotomy and wound closure was 15 days (range 15–25 days). At the day of the surgical wound closure the maximum mean length of the wound was 17 cm (range 15–19 cm) and the maximum mean diameter was 5·3 cm (range 3–8 cm). The wound size decreased in length by a mean of 40% (range 32–53%) and the width decreased by a mean of 46% (30–70%). In six cases, a delayed primary closure was done with adjacent skin, and the fasciotomy wound was closed with small skin graft in the remaining two cases. The mean duration of hospital stay in this group was 18·5 days (range 15–25 days). Just like in the former group no infection occurred and no other interventions were required after the first 4 weeks after surgical closure.
CONCLUSION
The aim of this study was to retrospectively compare the VAC treatment with the conservative treatment of the fasciotomy wound until surgical closure.
In our preliminary study the VAC‐system device showed significant reduction of the wound size, tissue oedema, duration of hospital days and evidence of improvement of granulation tissue. As a whole, the use of VAC system prior to closure of fasciotomy wound following an IRS after revascularisation is an innovative method which promotes excellent wound healing, permit earlier closure with adjacent skin or skin graft and protect the wound from infection. VAC device could be a new standard for treatment of fasciotomy wound. VAC therapy is a recent innovation and becoming more and more a necessary complementary therapy to hasten wound healing. Our results are preliminary and needs further studies of long‐term trials.
REFERENCES
- 1. Tuckey J. Bilateral compartment syndrome complicating prolonged lithotomy position. Br J Anaesth 1996;77:546–9. [DOI] [PubMed] [Google Scholar]
- 2. Fronek J, Mubarak SJ, Hargens AR, Lee YF, Gershuni DH, Garfin SR, Akeson WH. Management of chronic exertional anterior compartment syndrome of the lower limb. Clin Orthop Relat Res 1987;220:217–27. [PubMed] [Google Scholar]
- 3. Sheridan GW, Matsen FA. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am 1976;58:112–5. [PubMed] [Google Scholar]
- 4. Bradley EL III. The anterior tibial compartment syndrome. Surg Gynecol Obstet 1973;136:289–97. [PubMed] [Google Scholar]
- 5. Ernst CB, Kaufer H. Fibulectomy‐fasciotomy: an important adjunct in the management of lower limb arterial trauma. J Trauma 1971;11:365–80. [PubMed] [Google Scholar]
- 6. Brown M, Sayers R. Compartment syndromes. In: Fitrige R, Thomspm M, editors. Mechanism of vascular disease: a textbook for vascular surgeon. Cambridge: Cambridge University Press, 2007:275–90. [Google Scholar]
- 7. Hirshowitz B, Lindenbaum E, Har‐Shai Y. A skin‐stretching device for the harnessing of the viscoelastic properties of skin. Plast Reconstr Surg 1993;92:260–70. [DOI] [PubMed] [Google Scholar]
- 8. Narayanan K, Futrell JW, Bentz M, Hurwitz D. Comparative clinical study of the sure‐closure device with conventional wound closure techniques. Ann Plast Surg 1995;35:485–91. [DOI] [PubMed] [Google Scholar]
- 9. Patman RD, Thompson JE. Fasciotomy in peripheral vascular surgery. Report of 164 patients. Arch Surg 1970;101:663–72. [DOI] [PubMed] [Google Scholar]
- 10. Medina C, Spears J, Mitra A. The use of an innovative device for wound closure after upper limb fasciotomy. Hand (N Y). 2008;3:146–51. [E‐pub 1 December 2007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gregor S, Maegele M, Sauerland S, Krahn JF, Peinemann F, Lange S. Negative pressure wound therapy: a vacuum of evidence? Arch Surg 2008;143:189–96. [DOI] [PubMed] [Google Scholar]
- 12. Hunter JE. Evidence base medicine: Vacuum‐assisted closure in wound care management. Int wound J. 2007;4:256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleck T, Simon P, Burda G, Wolner E, Wollenek G. Vacuum assisted closure therapy for the treatment of sternal wound infections in neonates and small infants. Interact Cardiovasc Thorac Surg 2006;5:285–8. [E‐pub 1 March 2006] [DOI] [PubMed] [Google Scholar]
- 14. Asgari MM, Spinelli HM. The vessel loop shoelace technique for closure of fasciotomy wounds. Ann Plast Surg 2000;44:225–9. [DOI] [PubMed] [Google Scholar]
- 15. Blomqvist G. ETE (External Tissue Expansion): a new method for external tissue extension. Ann Chir Plast Esthet 1996;41:577–81. [PubMed] [Google Scholar]
- 16. Blomqvist G, Steenfos H. A new partly external device for extension of skin before excision of skin defects. Scand J Plast Reconstr Surg Hand Surg 1993;27:179–82. [DOI] [PubMed] [Google Scholar]
- 17. Yang CC, Chang DS, Webb LX. Vacuum‐assisted closure for fasciotomy wounds following compartment syndrome of the leg. J Surg Orthop Adv 2006;15:19–23. [PubMed] [Google Scholar]
- 18. Healing chronic wound: technologic for today and tomorrow. Wound Care 2000;32:13. [PubMed] [Google Scholar]
- 19. Gough A, Clapperton M, Rolando N, Foster AV, Philpott‐Howard J, Edmonds ME. Randomised placebo‐controlled trial of granulocyte‐colony stimulating factor in diabetic foot infection. Lancet 1997;350:855–9. [DOI] [PubMed] [Google Scholar]
- 20. Steed DL. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity diabetic ulcers: Diabetic Ulcer Study Group. J Vasc Surg 1995;21:71–8; discussion 79–81. [DOI] [PubMed] [Google Scholar]
- 21. Donaghue VM, Chrzan JS, Rosenblum BI, Giurini JM, Habershaw GM, Veves A. Evaluation of a collagen‐alginate wound dressing in the management of diabetic foot ulcers. Adv Wound Care 1998;11:114–9. [PubMed] [Google Scholar]
- 22. Steed DL, Donohoe D, Webster MW. Lindsley effect of extensive debridement and treatment on the healing of diabetic foot ulcers: Diabetic Ulcer Study Group. J Am Coll Surg 1996;183:61–4. [PubMed] [Google Scholar]
- 23. Hopf HW, Humphrey LM, Puzziferri N, West JM, Attinger CE, Hunt TK. Adjuncts to preparing wounds for closure: hyperbaric oxygen, growth factors, skin substitutes, negative pressure wound therapy (vacuum‐assisted closure). Foot Ankle Clin 2001;6:661–82; review. [DOI] [PubMed] [Google Scholar]
- 24. Banwell P, Withey S, Holten I. The use of negative pressure to promote healing. Br J Plast Surg 1998; 51(1):79. [DOI] [PubMed] [Google Scholar]
- 25. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76; discussion 577. [PubMed] [Google Scholar]
- 26. Müllner T, Mrkonjic L, Kwasny O, Vécsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. Br J Plast Surg 1997;50:194–9. [DOI] [PubMed] [Google Scholar]
- 27. Agarwal JP, Ogilvie M, Wu LC, Lohman RF, Gottlieb LJ, Franczyk M, Song DH. Vacuum‐assisted closure for sternal wounds: a first‐line therapeutic management approach. Plast Reconstr Surg 2005; 116(4):1035–1041‐3. [DOI] [PubMed] [Google Scholar]
- 28. McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum‐assisted closure versus saline‐moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Manage 2000;46:28–32, 34. [PubMed] [Google Scholar]
- 29. Van Schie CH, Whalley A, Armstrong DG, Vileikyte L, Boulton AJ. The effect of silicone injections in the diabetic foot on peak plantar pressure and plantar tissue thickness: a 2‐year follow‐up. Arch Phys Med Rehabil 2002;83: 919–23. [DOI] [PubMed] [Google Scholar]
- 30. Giovannini UM, Settembrini F, Colonna MR, Teot L, Giofrè C, Amadeo G, Strano A, Stagno D’Alcontres F. Topical negative therapy and vacuum assisted closure: New strategies and devices in surgical reconstruction. Minerva Chir 2005;60:191–4. [PubMed] [Google Scholar]
- 31. Arca MJ, Somers KK, Derks TE, Goldin AB, Aiken JJ, Sato TT, Shilyansky J, Winthrop A, Oldham KT. Use of vacuum‐assisted closure system in the management of complex wounds in the neonate. Pediatr Surg Int 2005;21:532–5. [E‐pub 17 June 2005] [DOI] [PubMed] [Google Scholar]
- 32. Caniano DA, Ruth B, Teich S. Wound management with vacuum‐assisted closure: experience in 51 pediatric patients. J Pediatr Surg 2005;40:128–32. [DOI] [PubMed] [Google Scholar]
- 33. O’Connor J, Kells A, Henry S, Scalea T. Vacuum‐assisted closure for the treatment of complex chest wounds. Ann Thorac Surg 2005;79:1196–2000. [DOI] [PubMed] [Google Scholar]
- 34. Bickels J, Kollender Y, Wittig JC, Cohen N, Meller I, Malawer MM. Vacuum‐assisted wound closure after resection of musculoskeletal tumors. Clin Orthop Relat Res 2005;441:346–50. [DOI] [PubMed] [Google Scholar]
- 35. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C‐reactive protein, interleukin‐6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 2005;112:976–83. [E‐pub 8 August 2005] [DOI] [PubMed] [Google Scholar]
- 36. Cowan KN, Teague L, Sue SC, Mahoney JL. Vacuum‐assisted wound closure of deep sternal infections in high‐risk patients after cardiac surgery. Ann Thorac Surg 2005;80:2205–12. [DOI] [PubMed] [Google Scholar]
- 37. Cherr GS, Zimmerman PM, Wang J, Dosluoglu HH. Patients with depression are at increased risk for secondary cardiovascular events after lower extremity revascularization. J Gen Intern Med 2008;23:629–34. [E‐pub 26 February 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paul JC. Vacuum assisted closure therapy: a must in plastic surgery. Plast Surg Nurs 2005;25:61–5. [DOI] [PubMed] [Google Scholar]


