Abstract
Selecting an appropriate treatment for a given case of skin wound is crucial for inducing optimal healing. We used an animal model developed from normal rabbit ears in order to assess the efficacy of treatments for skin wounds with or without a wet dressing, anti microbial reagent or topical wound‐stimulatory reagents. The degree of healing in each group was evaluated and compared using four histological parameters: (i) degree of reepithelialisation, (ii) amount of granulation tissue formation, and (iii) the number of capillary lumens and (iv) fibroblasts in the granulation tissue. Treatment using wet dressings resulted in an increase in capillary number compared with the open dry wound. Although the retention of povidone‐iodine (PI) in wound tissue after application significantly inhibited reepithelialisation (P < 0·05), rinsing PI off with saline was comparable in effect to using only a wet dressing. The three topical reagents, namely, basic fibroblast growth factor, prostaglandin E1 and dibutyryl cyclic adenosine monophosphate, significantly improved reepithelialisation (P < 0·05). In conclusion, wounds should be kept hydrated by applying topical reagents. If there are any signs of bacterial infection, PI can be applied and rinsed later with saline in order to minimise its cytotoxic effects.
Keywords: Animal model; Povidone‐iodine; Skin wounds; Topical reagent; Wet dressing
Introduction
Wound healing occurs in three phases: inflammation, granulation tissue and matrix formation and remodelling (1). Selecting an appropriate treatment for a given case of skin wound is crucial for inducing optimal healing. It is commonly observed, for instance, that skin wounds heal faster in a hydrated environment than in a dry one 2, 3.
Povidone‐iodine (PI), a compound of iodine and polyvinylpyrrolidone, is a common anti microbial agent (4) and has been used as a surgical scrub or a skin cleanser in various forms. Although PI has a strong anti microbial effect, the use of PI as a topical agent was limited because of its cytotoxicity (5). Tissue damage due to anti microbial agents should be minimised unless there is no recourse because of severe bacterial infection. Sugar and related products from various natural sources have been used to promote wound healing to good effect (6). A paste consisting of 70% sugar and 3% PI(SP) is commercially available in Japan under the brand name, U‐PASTA® (Kowa Company Ltd, Nagoya, Japan) and is reportedly clinically effective in promoting rapid healing of wounds and reducing bacterial contamination 7, 8.
Application of basic fibroblast growth factor (bFGF) accelerated wound healing in both a diabetic mouse model (9) and clinical cases (10). Prostaglandin E1 (PGE1) (11) and dibutyryl cyclic adenosine monophosphate (DBcAMP) (12) are also effective topical reagents for skin wounds. These three reagents are commercially available and widely used in Japan in spray or ointment form to treat skin wounds.
In this study, we used an animal model prepared from normal rabbit ears in order to assess the efficacy of treatments for skin wounds with or without a wet dressing, anti‐microbial reagent or topical reagents, and analysed the histopathology of these wounds quantitatively.
Materials and methods
Reagents
PI (10%) was purchased from Meiji Co., Ltd (Tokyo, Japan). The SP paste, consisting of 70% sugar and 3% PI (US patent 4844898, U‐PASTA®), was manufactured by Kowa Company Ltd. It contains a water‐soluble base including polyethylene glycol 400, glycerin and water, in addition to sugar and PI. For the bFGF spray, Fibrast®spray (Trafermin), purchased from Kaken Pharmaceutical Co., Ltd (Tokyo, Japan), was used. For the PGE1 ointment, Prostandin® ointment (Alprostadil alfadex), purchased from Ono Pharmaceutical Co., Ltd (Osaka, Japan), was used. For the DBcAMP ointment, Actosin® ointment (Bucladesine sodium), purchased from Maruho Co., Ltd (Osaka, Japan), was used.
Animals and wounding
The wound design and sampling for histological assessment were conducted according to our previously reported methods (13). Normal female rabbits (Jla:JW, 2·5 kg, 8 week old) were purchased from Saitama Experimental Animals Supply Co., Ltd (Sugito, Japan). All rabbits were housed individually and maintained on a standard laboratory diet and received water ad libitum. They were anaesthetised with sodium pentobarbital solution (Dainippon Sumitomo Pharma Co., Ltd, Osaka, Japan 25 mg/kg, injected into the femoral muscle) and with 3 ml of intravenous ketamine hydrochloride (Daiichi Sankyo Co., Ltd, Tokyo, Japan). Four round, full‐thickness wounds were prepared on the inner surface of each ear using a punch biopsy instrument (6‐mm diameter; Nipro Medical Industries Co., Ltd, Tatebayashi, Japan) (Figure 1). The wounds, treated with or without the reagents, were covered either with a sterilised transparent dressing (Cathereep; Nichiban Co., Tokyo, Japan) or with a cotton gauze, or were kept open according to the experimental protocol. Later, each ear was covered with a gauze and bandage; 0·1 ml of PI was applied to the wounds and allowed to remain for 3 minutes. The wounds were rinsed with 2 ml of saline forced through a syringe (2·5 ml). Then the saline was removed from the wound by gently pressing cotton gauze onto the wound surface. The PI was removed using cotton gauze in the same way in which the saline was removed after an incubation period of 3 minutes. The reagent was applied to the wounds and then removed by rinsing with saline every other day over 7 days, for a total of four times. Four wounds on the right ear were treated using the same procedure, whereas the wounds on the left ear were treated using a different procedure. The same experiment was performed twice, so that there were a total of 16 wounds for each treatment. The rabbits were anaesthetised with sodium pentobarbital solution (Dainippon Sumitomo Pharma Co., Ltd) and sacrificed by cervical dislocation on day 7. The wounds were excised and fixed in 10% buffered formalin solution. All studies were approved by the Animal Care Committee of the Tokyo Medical University animal facility.
Figure 1.
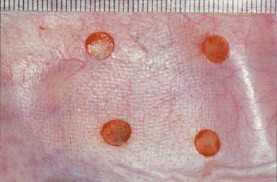
Wounding of the rabbit ear skin. Four round, full‐thickness wounds, 6 mm in diameter, were prepared on the inner surface of the rabbit ear.
Experimental design
Four assessment categories were formed in order to determine the effect of various treatment procedures on wound healing in an animal model consisting of normal rabbit ears.
Effect of dressing and rinsing on wound healing
Four procedures for inducing wound healing were compared. Group 1: wounds kept open for 7 days; group 2: wounds covered with sterilised transparent dressing; group 3: wounds covered with sterilised transparent dressing and rinsed with saline; and group 4: wounds covered with sterilised dry cotton gauze and rinsed with saline.
Effect of the application of PI
The five procedures for the treatment of wounds covered with sterilised transparent dressing were compared. Group 1: wounds occluded for 7 days without application of PI; group 2: PI previously applied to wounds allowed to remain; group 3: PI applied to wounds and later rinsed off with saline; group 4: PI applied to wounds and later removed with cotton gauze; and group 5: SP applied to wounds.
Effect of the application of various vehicles on wounds
The wounds were filled with the following vehicles and then covered with a sterilised transparent dressing. Group 1: wounds occluded for 7 days without application of any vehicle; group 2: wounds filled using white petrolatum; group 3: wounds filled with plastibase; group 4: wounds filled with a cream base; group 5: wounds filled with polyethylene glycol; and group 6: wounds filled with sugar.
Assessment of topical reagents for skin wounds
The effects of the three topical reagents for skin wounds, namely, bFGF spray, PGE1 ointment and DBcAMP ointment, were compared with the negative control. Group 1 (control group): wounds occluded for 7 days with sterilised transparent dressing; group 2: wounds treated with bFGF spray; group 3: wounds treated with PGE1 ointment; and group 4, wounds treated with DBcAMP.
Histological evaluation
After overnight fixation, the tissue was trimmed and cut through at the widest margin, then embedded in paraffin and sliced into 5‐µm sections. The sections were made perpendicular to the proximal–distal axis of the ear and the wound surface. Three sections were placed on a slide and stained with haematoxylin and eosin. Specimens that showed signs of bacterial infection or were excised improperly were excluded from the analyses. The sections with the widest original wound margin were used for assessment. The samples were assessed according to the following parameters: degree of reepithelialisation, area of granulation tissue, number of capillaries and number of fibroblasts in the dermis. Each of the parameters was assigned a numerical score, as described below.
Reepithelialisation
The degree of reepithelialisation was measured by computerised morphometric analysis (IPAP WIN; Sumika Technosevice Co., Ltd, Takarazuka, Japan) and was given a percentage value, 0% being equivalent to no closure and 100% equivalent to complete wound closure.
Area of granulation tissue
The amount of granulation tissue was quantified by measuring the area of granulation tissue (mm2) in the section perpendicular to the surface of the wound. Granulation tissue was traced by computerised morphometric analysis (IPAP WIN).
Capillary number
The number of capillary lumens in the granulation tissue was counted in the complete wound cross section at 100× magnification.
Fibroblast number
The number of fibroblasts in the granulation tissue was counted in the complete wound cross section at 100× magnification.
Statistical analysis
In order to compare the effects of the treatments using (1) wet dressing or vehicles, and (2) PI with or without washing or as a SP paste, the data obtained were analysed by analysis of variance (ANOVA) and the Tukey–Kramer test using statistical software Stat View 5·0 (Abacus Concepts Inc., Piscataway, NJ). To assess the effects of the topical reagents on the skin ulcers, namely, bFGF spray, PGE1 ointment and DBcAMP ointment, the data obtained were analysed by unpaired Student's t‐test and compared with the negative controls using Stat View 5·0 (Abacus Concepts Inc.). The results were expressed as mean ± standard error of the mean.
Results
Effect of dressing and rinsing on wound healing
The four procedures for inducing wound healing were compared. In group 1, wounds were kept open for 7 days; in group 2, wounds were covered with a sterilised transparent dressing; in group 3, wounds were covered with a sterilised transparent dressing and rinsed with saline; and in group 4, wounds were covered with sterilised dry cotton gauze and rinsed with saline. Each group comprised of 16 wounds. Rinsing of the wounds with sterilised saline was performed every other day, over 7 days for a total of four times. The rabbits were sacrificed on day 7. There was no significant difference in reepithelialisation (Figure 2A) among the four groups (P = 0·06 by ANOVA). In comparison with the other procedures, covering with a dry gauze delayed reepithelialisation, although not to a statistically significant degree. Dry gauzes always adhered to the wound surface when the treatments were performed every other day. Closed wounds (group 2) showed significant increases in capillary number (P < 0·05 by Tukey–Kramer test) compared with open wounds (group 1) (Figure 2B). There was no statistically significant difference among the four groups in terms of the area of granulation tissue and fibroblast number (data not shown; P = 0·70, 0·69 by ANOVA, respectively). Representative histopathological images of open wounds (group 1) and closed wounds (group 2) on day 7 are shown in Figure 2C. Reepithelialisation was complete in both open and closed wounds, whereas synthesis of capillaries in granulation tissue was noticeable in the closed wounds.
Figure 2.
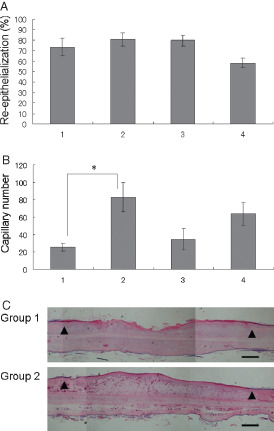
Effects of dressing and rinsing on wound healing. (A) Reepithelialisation; (B) capillary number. Lane 1: wounds open for 7 days, n = 9; lane 2: covered wounds with sterilised transparent dressing, n = 14; lane 3: covered wounds with sterilised transparent dressing and rinsed with saline, n = 14; lane 4: covered wounds with sterilised dry cotton gauze and rinsed with saline, n = 10. *P < 0·05. The data obtained were analysed by analysis of variance and the Tukey–Kramer test. Each bar represents mean ± standard error of the mean. (C) Representative histopathology of open and closed wounds on day 7. Group 1: an open wound; group 2: a covered wound with sterilised transparent dressing. Arrow heads indicate the original wound edges (haematoxylin and eosin staining, scale bar: 500 µm).
Influence of the application of PI
The five procedures for the treatment of wounds covered with sterilised transparent dressing were compared. In group 1, wounds were closed for 7 days without application of PI; in group 2, PI applied to the wounds was allowed to remain; in group 3, PI was applied to the wounds and later rinsed off with saline; in group 4, PI was applied to the wounds and later removed with a cotton gauze; and in group 5, SP was applied to the wounds. Each group comprised of 16 wounds. Application of PI or SP was performed every other day over 7 days for a total of four times. The rabbits were sacrificed on day 7. The retention of PI in the wounds in group 2 significantly inhibited reepithelialisation (P < 0·05) compared with the wounds in group 3, in which the PI was removed after application using saline. There was no significant difference between rinsed wounds (group 3) and non‐treated wounds (group 1) (Figure 3A). The number of capillary lumens in group 1 was significantly higher (P < 0·05) compared with the other groups (groups 2, 3 and 5), in which the wounds were treated using PI (Figure 3B). There was no significant difference in the area of granulation tissue and fibroblast number (P = 0·39 and 0·19 by ANOVA, respectively; data not shown). The representative histopathology of wounds in which PI was either retained or removed by rinsing (groups 2 and 3, respectively) on day 7 is presented in Figure 3C. Retention of PI delayed reepithelialisation, whereas removal of PI did not and led to complete reepithelialisation.
Figure 3.
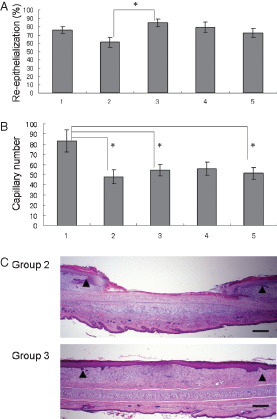
Effect of the application of povidone‐iodine (PI). (A) Reepithelialisation; (B) capillary number. Lane 1: without application of PI, n = 13; lane 2: retention of PI, n = 12; lane 3: rinsing the PI off with saline after application, n = 15; lane 4: wiping the PI off with gauze after application, n = 11; lane 5: application of SP (a paste comprising 70% sugar and 3% PI), n = 15. *P < 0·05. The data obtained were analysed by analysis of variance and the Tukey–Kramer test. Each bar represents mean ± standard error of the mean. (C) Representative histopathology of wounds applied with PI on day 7. Group 2: a wound retaining PI; group 3: a wound following removal of PI using saline. Arrow heads indicate the original wound edges (haematoxylin and eosin staining, scale bar: 500 µm).
Effect of the application of various vehicles on wounds
The wounds were filled with the following vehicles and then covered with a sterilised transparent dressing. In group 1, wounds were closed for 7 days without application of any vehicle; wounds in group 2 were filled using white petrolatum; in group 3, plastibase; in group 4, a cream base; in group 5, polyethylene glycol and in group 6, sugar. Application of each vehicle was performed every other day for a total of four times. The rabbits were sacrificed on day 7. There was no statistical difference in reepithelialisation (Figure 4) among the six groups (P = 0·25 by ANOVA) although reepithelialisation was most extensive after the application of white petrolatum. There was also no significant difference in the area of granulation tissue, capillary number or fibroblast number among the six groups (P = 0·12, 0·13 and 0·38 by ANOVA, respectively) although the area of granulation tissue and the number of capillaries were greatest following the application of polyethylene glycol, and the number of fibroblasts was greatest when the wounds were covered only with a sterilised, transparent dressing (data not shown).
Figure 4.
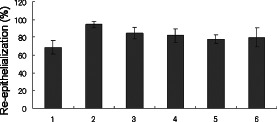
Effect of the application of various vehicles on wounds. Lane 1: wounds occluded for 7 days without application of vehicles; lane 2: vaseline application; lane 3: plastibase application; lane 4: cream application; lane 5: polyethylene glycol application; lane 6: sugar application. The data obtained were analysed by analysis of variance (P = 0·25). Each bar represents mean ± standard error of the mean.
Assessment of topical reagents for skin wounds
The effects of the three topical reagents namely, bFGF spray, PGE1 ointment and DBcAMP ointment on skin wounds were compared with the negative control. Each reagent was applied to a wound, which was then occluded for 7 days with a sterilised transparent dressing. In group 1 (control group), the wounds were occluded for 7 days with a sterilised transparent dressing; in group 2, the wounds were treated with bFGF spray; in group 3, the wounds were treated with PGE1 ointment and in group 4, the wounds were treated with DBcAMP. Each group comprised of 16 wounds. Application of the reagents was performed every other day for a total of four times, and the rabbits were sacrificed on day 7. Application of bFGF, PGE1 and DBcAMP significantly increased reepithelialisation (P < 0·05, 0·01 and 0·05, respectively, by Student's unpaired t‐test) compared with the control group (Figure 5A). Application of bFGF significantly increased the area of granulation tissue (P < 0·05 by Student's unpaired t‐test) compared with the control group (Figure 5B), whereas application of PGE1 and DBcAMP did not. There was no significant difference in terms of the number of capillaries or fibroblasts between each reagent group and the control (data not shown). The representative histopathology of wounds treated with bFGF and PGE1 (groups 2 and 3, respectively) on day 7 is presented in Figure 5C. Reepithelialisation was complete in both wounds, and granulation tissue had increased remarkably after the application of bFGF.
Figure 5.
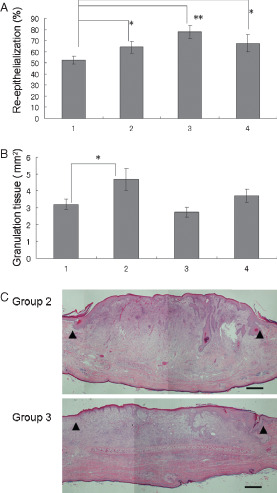
Assessment of topical reagents for skin wounds. (A) Reepithelialization; (B) area of granulation tissue. Lane 1: control (dressing only), n = 11; lane 2: application of basic fibroblast growth factor (bFGF) spray, n = 14; lane 3: application of prostaglandin E1 (PGE1) ointment, n = 12; lane 4: application of dibutyryl cyclic adenosine monophosphate ointment, n = 11. *P < 0·05, **P < 0·01. The data obtained were analysed by unpaired Student's t‐test. Each bar represents mean ± standard error of the mean. (C) Representative histopathology of wounds applied with topical reagents. Group 2: a wound treated with bFGF spray; group 3: a wound treated with PGE1 ointment. Arrow heads indicate the original wound edges (haematoxylin and eosin staining, scale bar: 500 µm).
Discussion
In this study, we showed that treatment using wet dressings and various vehicles resulted in progressive reepithelialisation and increased granulation tissue formation, compared with the open, dry wound. Although the retention of PI in wound tissue after application significantly inhibited reepithelialisation, removal of PI using saline after application was comparable in effect to using only the wet dressing. The three topical reagents produced significant improvement in reepithelialisation, and bFGF increased the amount of granulation tissue. Because we employed an excisional wound model using the ears of normal rabbits, the results obtained in this study reflect normal wound healing. Skin ulcers, on the other hand, can be caused by various aetiological factors such as bacterial infection, ischaemia and malnutrition. Nonetheless, the results of this study afford some insights into the healing process of complicated skin ulcers as well.
Since the 1950s, it has commonly been observed that occlusive dressing accelerates wound closure although dry treatment of wounds has one advantage: it reduces the incidence of certain Gram‐negative infections (14). The moist or wet healing environment resulted in less necrosis and faster and better quality of healing in the formation of newly regenerated epidermis (15), and showed a more rapid progression towards healthy levels of vascularisation than dry wounds (16). Unfortunately, in developing countries, most patients with chronic ulcers are not treated with moist wound dressings because of their high cost and physicians' traditional management of wounds (17). Another crucial problem in the treatment of wounds is the cytotoxicity of PI. Various studies have provided evidence showing that in most instances, PI failed to effectively promote optimal wound healing. Most studies showed impaired wound healing (4). Our opinion that these negative findings regarding the application of PI to wounds need to be balanced by the recognition of the usefulness of PI led us to undertake this study.
Although PI is a commonly used, effective anti microbial agent (4), its toxicity to fibroblasts is well known, as stated above, causing progressive retardation at concentrations of 0·01% and 0·025%, and complete inhibition at 0·1% and 1%, in solution (18). Reepithelialisation of wounds treated with PI was delayed on the dorsum of ears in male hairless mouse (19). It was also shown that application of PI decreased the blood flow in the capillary circulation of the granulation tissue by using a laser Doppler flow meter in rabbit ear chambers (20). However, in this study we showed that these adverse effects can be minimised by rinsing with saline. Furthermore, the ameliorative effects of SP paste on wound healing have been shown in an animal model in our previous in vivo study. Application of SP significantly accelerated reepithelialisation and decreased the colony‐forming units of methicillin‐resistant Staphylococcus aureus (MRSA) in MRSA‐infected wounds, compared with the non‐treated group, in a study of the wound healing process using a bacterial infection model with diabetic db/db mice (6). In Europe and North America, as well as Japan, Cadexomer‐iodine, a commercially available ointment that releases iodine slowly from beads of dextrin and epichlorohydrin, is commonly used in treatments. This preparation is an effective debridement and antiseptic agent for treating chronic, exudative wounds 21, 22, 23, because it removes necrotic tissue, bacterial components and biofilm synthesised by bacteria 24, 25, and stimulates epidermal regeneration (26).
In a comparison of the five vehicle groups and the groups using only sterilised transparent dressings in this study, white petrolatum was clearly conducive to reepithelialisation, polyethylene glycol to increasing the area of granulation tissue and capillary formation and the sterilised transparent dressing to increasing fibroblast count. However, there was no statistical difference in these benefits, which suggests that the chemical reagents used for wound healing can be mixed with any of these vehicles provided that they form a chemically stable combination. In clinical practice, bFGF is sprayed on wounds and can be covered with any vehicle according to the manufacturer's protocol (Fibrast®spray; Kaken Pharmaceutical Co., Ltd). The commercially available vehicles of choice for PGE1 ointment (Prostandin® ointment; Ono Pharmaceutical Co., Ltd) and DBcAMP ointment (Actosin® ointment; Maruho Co., Ltd) are white petrolatum and polyethylene glycol, respectively.
bFGF stimulated the migration of cultured keratinocytes (27) and accelerated wound healing in a diabetic mouse model (9) and in pressure ulcers (10). PGE1 increased the intracellular cyclic AMP and reduced platelet aggregation 28, 29 and was also found to increase tissue blood flow in an animal model (30). Furthermore, intravenous administration of PGE1 was effective in treating venous ulcers (31), diabetic skin ulcers (32) and skin ulcers stemming from collagen diseases (33). PGE1 ointment was effective in treating patients with incurable peripheral ischaemic ulcers (34) and burn patients presenting rapid epithelialisation (11). DBcAMP stimulated the growth of keratinocytes and fibroblasts by inducing secretion of several cytokines 35, 36, and its topical application resulted in significant acceleration of wound healing in an animal model (12). Clinical study of this ointment has also shown its benefits in the treatment of pressure ulcers (37). Although these reagents are commercially available in Japan only, they are effective and useful for accelerating wound healing as we have shown in this study. We expect that they will be used globally for various types of skin wounds such as pressure ulcers, venous ulcers, diabetic skin ulcers and burns.
Acknowledgements
This work was supported by research grants from the Private University Strategic Research‐Based Support Project (Molecular Information‐based Intractable Disease Research Project Epigenetics: Research project aimed at general cancer cure using epigenetic targets, no. S0891020) from the MEXT (Ministry of Education, Culture, Sports, Science and Technology of Japan) (RT). This work was also supported by Grant‐in‐Aid for Scientific Research(C) (no. 70221421) (RT).
References
- 1. Clark RA. Cutaneous tissue repair: basic biologic considerations. J Am Acad Dermatol 1985;13:701–25. [DOI] [PubMed] [Google Scholar]
- 2. Svensjö T, Pomahac B, Yao F, Slama J, Eriksson E. Accelerated healing of full‐thickness skin wounds in a wet environment. Plast Reconstr Surg 2000;106:602–12. [PubMed] [Google Scholar]
- 3. Vranckx JJ, Slama J, Preuss S, Perez N, Svensjö T, Visovatti S, Breuing K, Bartlett R, Pribaz J, Weiss D, Eriksson E. Wet wound healing. Plast Reconstr Surg 2002;110:1680–7. [DOI] [PubMed] [Google Scholar]
- 4. Kramer SA. Effect of povidone‐iodine on wound healing: a review. J Vasc Nurs 1999;17:17–23. [DOI] [PubMed] [Google Scholar]
- 5. Burks RI. Povidone‐iodine solution in wound treatment. Phys Ther 1998;78:212–8. [DOI] [PubMed] [Google Scholar]
- 6. Shi CM, Nakao H, Yamazaki M, Tsuboi R, Ogawa H. Mixture of sugar and povidone‐iodine stimulates healing of MRSA‐infected skin ulcers on db/db mice. Arch Dermatol Res 2007;299:449–56. [DOI] [PubMed] [Google Scholar]
- 7. Miyachi Y, Imamura S. Use of sugar and povidone‐iodine in the treatment of refractory cutaneous ulcers. J Dermatol Treat 1990;1:191–3. [Google Scholar]
- 8. Shiraishi T, Oka R, Nakagawa Y. Pharmaceutical and bacteriological study on povidone‐iodine sugar ointment. Dermatology 1997;195 Suppl 2:100–3. [DOI] [PubMed] [Google Scholar]
- 9. Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth factor stimulates wound healing in healing‐impaired db/db mice. J Exp Med 1990;172:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohura T, Nakajo T, Moriguchi T, Oka H, Tachi M, Ohura N Jr. Nogami R, Murayama S. Clinical efficacy of basic fibroblast growth factor on pressure ulcers: case‐control pairing study using a new evaluation method. Wound Repair Regen 2011;19:542–51. [DOI] [PubMed] [Google Scholar]
- 11. Gunji H, Ono I, Tateshita T, Kaneko F. Clinical effectiveness of an ointment containing prostaglandin E1 for the treatment of burn wounds. Burns 1996;22:399–405. [DOI] [PubMed] [Google Scholar]
- 12. Asai J, Takenaka H, Katoh N, Kishimoto S. Dibutyryl cAMP influences endothelial progenitor cell recruitment during wound neovascularization. J Invest Dermatol 2006;126:1159–67. [DOI] [PubMed] [Google Scholar]
- 13. Tsuboi R, Shi CM, Sato C, Cox GN, Ogawa H. Co‐administration of insulin‐like growth factor (IGF)‐I and IGF‐binding protein‐1 stimulates wound healing in animal models. J Invest Dermatol 1995;104:199–203. [DOI] [PubMed] [Google Scholar]
- 14. Hinman CD, Maibach H. Effect of air exposure and occlusion on experimental human skin wounds. Nature 1963;200:378–9. [DOI] [PubMed] [Google Scholar]
- 15. Vogt PM, Andree C, Breuing K, Liu PY, Slama J, Helo G, Eriksson E. Dry, moist, and wet skin wound repair. Ann Plast Surg 1995;34:493–500. [DOI] [PubMed] [Google Scholar]
- 16. Dyson M, Young SR, Hart J, Lynch JA, Lang S. Comparison of the effects of moist and dry conditions on the process of angiogenesis during dermal repair. J Invest Dermatol 1992;99:729–33. [DOI] [PubMed] [Google Scholar]
- 17. Fu X, Sheng Z, Cherry GW, Li Q. Epidemiological study of chronic dermal ulcers in China. Wound Repair Regen 6:21–7. [DOI] [PubMed] [Google Scholar]
- 18. Balin AK, Pratt L. Dilute povidone‐iodine solutions inhibit human skin fibroblast growth. Dermatol Surg 2002;28:210–4. [DOI] [PubMed] [Google Scholar]
- 19. Kjolseth D, Frank JM, Barker JH, Anderson GL, Rosenthal AI, Acland RD, Schuschke D, Campbell FR, Tobin GR, Weiner LJ. Comparison of the effects of commonly used wound agents on epithelialization and neovascularization. J Am Coll Surg 1994;179:305–12. [PubMed] [Google Scholar]
- 20. Brennan SS, Leaper DJ. The effect of antiseptics on the healing wound: a study using the rabbit ear chamber. Br J Surg 1985;72:780–2. [DOI] [PubMed] [Google Scholar]
- 21. Bianchi J. Cadexomer‐iodine in the treatment of venous leg ulcers: what is the evidence? J Wound Care 2001;10:225–9. [DOI] [PubMed] [Google Scholar]
- 22. Danielsen L, Cherry GW, Harding K, Rollman O. Cadexomer iodine in ulcers colonised by Pseudomonas aeruginosa . J Wound Care 1997;6:169–72. [DOI] [PubMed] [Google Scholar]
- 23. Zhou LH, Nahm WK, Badiavas E, Yufit T, Falanga V. Slow release iodine preparation and wound healing: in vitro effects consistent with lack of in vivo toxicity in human chronic wounds. Br J Dermatol 2002;146:365–74. [DOI] [PubMed] [Google Scholar]
- 24. Akiyama H, Huh WK, Yamasaki O, Oono T, Iwatsuki K. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in mouse skin: does S. aureus generally produce a biofilm on damaged skin?. Br J Dermatol 2002;147:879–85. [DOI] [PubMed] [Google Scholar]
- 25. Akiyama H, Oono T, Saito M, Iwatsuki K. Assessment of cadexomer iodine against Staphylococcus aureus biofilm in vivo and in vitro using confocal laser scanning microscopy. J Dermatol 2004;31:529–34. [DOI] [PubMed] [Google Scholar]
- 26. Lamme EN, Gustafsson TO, Middelkoop E. Cadexomer‐iodine ointment shows stimulation of epidermal regeneration in experimental full‐thickness wounds. Arch Dermatol Res 1998;290:18–24. [DOI] [PubMed] [Google Scholar]
- 27. Tsuboi R, Sato C, Shi CM, Ogawa H. Stimulation of keratinocyte migration by growth factors. J Dermatol 1992;19:652–3. [DOI] [PubMed] [Google Scholar]
- 28. Iyú D, Jüttner M, Glenn JR, White AE, Johnson AJ, Fox SC, Heptinstall S. PGE1 and PGE2 modify platelet function through different prostanoid receptors. Prostaglandins Other Lipid Mediat 2011;94:9–16. [DOI] [PubMed] [Google Scholar]
- 29. Taub ML, Wang Y, Yang IS, Fiorella P, Lee SM. Regulation of the Na,K‐ATPase activity of Madin‐Darby canine kidney cells in defined medium by prostaglandin E1 and 8‐bromocyclic AMP. J Cell Physiol 1992;151:337–46. [DOI] [PubMed] [Google Scholar]
- 30. Hamamoto Y, Ogata T, Morino T, Hino M, Yamamoto H. Prostaglandin E1 analog increases spinal cord blood flow at the point of compression during and after experimental spinal cord injury. Spinal Cord 2010;48:149–53. [DOI] [PubMed] [Google Scholar]
- 31. Milio G, Minà C, Cospite V, Almasio PL, Novo S. Efficacy of the treatment with prostaglandin E‐1 in venous ulcers of the lower limbs. J Vasc Surg 2005;42:304–8. [DOI] [PubMed] [Google Scholar]
- 32. Miyata T, Yamada N, Miyachi Y. Efficacy by ulcer type and safety of lipo‐PGE1 for Japanese patients with diabetic foot ulcers. J Atheroscler Thromb 2010;17:805–16. [DOI] [PubMed] [Google Scholar]
- 33. Murota H, Kotobuki Y, Umegaki N, Tani M, Katayama I. New aspect of anti‐inflammatory action of lipo‐prostaglandin E1 in the man agement of collagen diseases‐related skin ulcer. Rheumatol Int 2008;28:1127–35. [DOI] [PubMed] [Google Scholar]
- 34. Sakakibara Y, Jikuya T, Mitsui T. Application of lipid microspheres containing prostaglandin E1 ointment to peripheral ischemic ulcers. Dermatology 1997;195:253–7. [DOI] [PubMed] [Google Scholar]
- 35. Takahashi H, Honma M, Miyauchi Y, Nakamura S, Ishida‐Yamamoto A, Iizuka H. Cyclic AMP differentially regulates cell proliferation of normal human keratinocytes through ERK activation depending on the expression pattern of B‐Raf. Arch Dermatol Res 2004;296:74–82. [DOI] [PubMed] [Google Scholar]
- 36. Zhou LJ, Ono I. Stimulatory effects of dibutyryl cyclic adenosine monophosphate on cytokine production by keratinocytes and fibroblasts. Br J Dermatol 2000;143:506–12. [DOI] [PubMed] [Google Scholar]
- 37. Toba K, Sudoh N, Nagano K, Eto M, Mizuno Y, Nakagawa H, Kawabata Y, Yamami N, Hara M, Fukushima Y, Ouchi Y. Randomized prospective trial of gentian violet with dibutyryl cAMP and povidone‐iodine with sugar as treatment for pressure sores infected with methicillin‐resistant Staphylococcus aureus in elderly patients. Nihon Ronen Igakkai Zasshi 1997;34:577–82 (in Japanese). [DOI] [PubMed] [Google Scholar]


