Abstract
Chronic painful wounds, a major health problem, have a detrimental impact on the quality of life due to associated pain. Some clinical reports have suggested that local administration of morphine could be beneficial. The aim of this study was to evaluate the analgesic effect of topically applied morphine on chronic painful leg ulcers. Twenty‐one patients were randomly assigned to receive either morphine or placebo in a randomised, placebo‐controlled, crossover pilot study. Each patient was treated four times in total. Pain was measured by the visual analogue score (VAS) before application of gel, directly after and after 2, 6, 12 and 24 hours. Although an overall, clinically relevant, reduction of pain was observed upon treatment with morphine, the difference was not statistically significant. Morphine reduced pain scores more than placebo on treatment occasions 1 and 2. The difference was statistically significant only 2 hours after dressing on the first treatment occasion. Thus, our study did not demonstrate a consistent and globally significant difference in nociception in patients treated with morphine. However, the relatively small number of patients included in our study and other methodological limitations makes it difficult for us to draw general conclusions regarding efficacy of topically applied morphine as an effective treatment for some painful ulcers. Further studies are warranted to evaluate the value of topically applied morphine in the treatment of patients with chronic painful leg ulcers.
Keywords: Leg ulcers, Morphine, Pain, Topical, VAS
INTRODUCTION
Chronic leg ulcers represent a major health problem in clinical practice, causing pain and considerable social discomfort, which can have a detrimental impact on the quality of life of affected patients in addition to generating considerable healthcare costs 1, 2. In Sweden, the reported prevalence of leg ulcers was 0·12% 3, 4. International studies have calculated a prevalence of 1% in the adult population and up to 5% in the population over 65 years of age (5). Venous insufficiency is the predominant cause of leg ulceration. Almost half of the patients report pain and two thirds of these patients have described their pain as ‘severe’, ‘horrible’ or ‘excruciating’. One third of these patients suffered from inadequate pain relief and considered pain to be the most important problem associated with their leg ulcers, more than lack of healing or any other aspect of the ulceration (6). A prevalence study found that approximately 25% of patients with chronic ulcers, who were treated with analgesics, did not achieve pain relief (7).
It has been established that peripheral nerve terminals in inflamed tissue express opioid receptors (8). The receptors are synthesised in the cell bodies of small afferent fibres of the dorsal root and are expressed on the ends of both central and peripheral nerve cells (9). These receptors can be detected on the cell surface after the onset of inflammation and/or after peripheral injury (10). Expression of receptors is upregulated and axonal transport enhanced, often within minutes to hours, after initiation of an inflammatory reaction, contributing to an increase in density of the receptors 11, 12. Activation of peripheral opioid receptors results in interactions with specific proteins which attenuates excitability of the peripheral nerve terminal, suggesting that these receptors achieve a clinically significant nociceptive response. The hypothesis is, therefore, that extremely small doses of opioids may reduce the need for high systemic doses resulting in fewer opioid‐related side effects.
Recent studies have suggested that local administration of morphine could be beneficial for some groups of patients. However, most of these studies have examined the intra‐articular application of morphine during knee surgery and arthritis 13, 14, 15, 16, 17, 18, 19. Several case reports have been published describing the effectiveness of topical opioids on painful ulcers such as pressure ulcers, cancer‐related ulcers and burns 9, 20, 21, 22, 23, 24, 25, 26, 27, 28. There were, until recently, very few controlled studies that presented objective evidence regarding the efficacy of topically administered morphine. Divergent results have been obtained from some randomised clinical trials describing the use of topically applied morphine and diamorphine for various painful skin conditions, such as pressure ulcers 29, 30, 31, 32, 33, chronic leg ulcers 34, 35 and burns 36, 37, 38. Morphine gel has been produced extemporaneously and used by many doctors to treat individual patients in Sweden. The magnitude and clinical significance of this treatment strategy is still poorly documented. The aim of this study was to evaluate the effect of topically applied morphine on chronic painful leg ulcers in a double‐blind, placebo‐controlled and crossover clinical trial.
MATERIALS AND METHODS
Subjects
Twenty‐one patients with painful leg ulcers were enrolled in the study. All subjects gave their written informed consent to participate in the study. Almost all participants used a combination of different drugs due to their medical conditions. Analgesics such as paracetamol, NSAID and/or opioid (mostly oxycodone) were commonly used drugs. All previous therapies remained unchanged. The patients were asked to note if they used any analgesia on demand beside their ordinary drugs.
Study design
We conducted a unicentre, randomised, double‐blinded, placebo‐controlled, crossover pilot study. Patients were recruited either at the Department of Dermatology at the University Hospital or at different primary care centres within the County Council of Östergötland and Jönköping.
Inclusion criteria: Patients were eligible if they had painful leg ulcers. Pain was defined as visual analogue score (VAS) ≥4.
Exclusion criteria: Patients not capable of completing VAS or understanding the patient information sheet were excluded. Patients were excluded if the ulcer was infected or they were allergic to morphine gel. Patients were assigned randomly to either morphine or placebo according to a computer‐generated randomisation scheme. Each patient was treated four times, of which two times were with placebo. A washout period of at least 3 days and at most 10 days was allowed between each treatment occasion. Topical morphine/placebo gel was applied after cleaning and washing the ulcers.
The gel was obtained from APL, Apoteket Production and Laboratories (Stockholm, Sweden). It was made extemporaneously as a sterile hydrogel containing hydroxypropyl methylcellulose, a semisynthetic, inert viscoelastic polymer and morphine hydrochloride. The placebo gel was manufactured similarly with exactly the same components except morphine. A 10–20 ml syringe filled with morphine or placebo gel was used to apply on the ulcers. The amount of gel that should be applied on the ulcers was calculated based on the size of the ulcers. Approximately 0·5 mg of morphine was applied on 1 cm2of ulcer. It was not possible to apply this dose on smaller ulcers as the ulcers were superficial. Therefore, a gel with different strength of morphine, 1–3 mg/ml, was ordered.
Morphine gel was manufactured according to Good Manufacturing Practice (GMP) standard.
Assessment of pain
VAS 0–10 cm (0 = no pain, 10 = unbearable pain) was used for assessment of pain. Pain was measured before, directly after and 2, 6, 12, 24 hours after application of morphine/placebo gel. Patients received a form for documentation after each treatment occasion. The form was taken home by each participant and was returned after completion. They were asked to carefully note their pain and also the use of any on‐demand analgesics on this form. Any adverse effects of topically applied morphine/placebo gel were also documented by both patients and medical staff.
Ethical considerations
The study was approved by, both, the Regional Ethical Review Board in Linköping and the Swedish Medical Products Agency.
Statistics
Statistical analysis was performed under the guidance of an independent statistician. The mean pain score was compared between morphine and placebo groups on different treatment occasions and measurement time points. We also compared mean pain score in each patient. We used a general linear model, analysis of variance (ANOVA), to investigate the difference between placebo and morphine by using mean VAS for each groups and each patient. P < 0·05 was considered statistically significant.
RESULTS
Subjects
Twenty‐one patients were recruited to the study out of which four did not complete the study. Two patients were excluded because their legs with painful ulcers were amputated due to prior complications related to their disease; one patient (treated with placebo gel) experienced burning pain after the first application and asked medical staff to clean the ulcer from applied gel, one patient was excluded because she wanted to try another dressing. Data from 17 patients was analysed statistically. Patient's demographic data are shown in Table 1. Majority of patients recruited to the study were women (88%). Venous leg ulcers were the most common type of ulcers noted (76%). The type of ulcers was not characterised in two patients. The mean age was 75 ± 11 years. The dosages varied (mean 6·6 mg ± 5·06) in patients. There was a large variation in ulcer size, mean 28·6 ± 39·4 cm2.
Table 1.
Demographic data of participants included in the final analysis. Patient number 3, 7, 8 and 14 were excluded
| Patients | Sex | Age | Ulcer characteristics | Use of rescue medication | ||
|---|---|---|---|---|---|---|
| Size (cm2) | Morphine (mg) | Aetiology | ||||
| 1 | Female | 82 | 6 | 1·8 | Venous | + |
| 2 | Female | 71 | 4 | 1·6 | Venous | − |
| 4 | Female | 68 | 32 | 15 | Venous | − |
| 5 | Female | 80 | 0·4 | 0·2 | Venous | − |
| 6 | Female | 78 | 6·64 | 4·5 | Venous | − |
| 9 | Female | 51 | 13·6 | 8 | Venous | − |
| 10 | Female | 93 | 10·5 | 20 | Venous | − |
| 11 | Female | 82 | 24 | 10 | Arterial | − |
| 12 | Female | 75 | 16·5 | 15 | Not characterised | − |
| 13 | Female | 82 | 38 | 12 | Not characterised | − |
| 15 | Female | 85 | 0·72 | 1·5 | Venous | − |
| 16 | Female | 56 | 45·5 | 5 | Venous | + |
| 17 | Female | 68 | 150 | 14 | Venous | − |
| 18 | Female | 68 | 94·5 | 5 | Arterial and venous | − |
| 19 | Male | 64 | 8·75 | 2 | Venous | − |
| 20 | Female | 86 | 20·5 | 2 | Venous | − |
| 21 | Male | 90 | 0·25 | 1·2 | Venous | − |
Overall efficacy evaluation
The difference between mean pain score was not statistically significant between the morphine and placebo group when we analysed the study population as a whole (P = 0·172) even though the mean of the overall pain score was higher in the placebo group (4·3 ± 2·8) compared to the morphine group (3·8 ± 2·7). Figure 1 shows a comparison of mean pain score evaluated using variance analysis. The pain score was higher directly after application of gel in almost all patients in both groups compared to pain score before application of gel. The pain scores were significantly reduced in both treatment groups compared to pain scores before application of gel. The mean pain score for each measurement time point was higher in placebo group compared to the morphine group, but the reduction in pain score was almost the same for measurement time points other than 12 hours after dressing. There were no statistically significant differences in pain reduction between the groups at any measurement time point.
Figure 1.
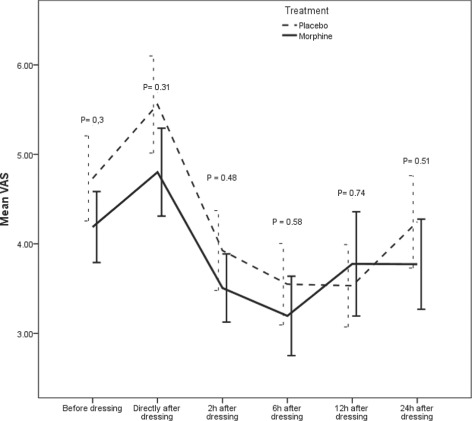
Mean pain scores at different measurement time points for all patients. There was no statistically significant difference between placebo and morphine at any measurement time point. Data is presented as mean (of 33 observations at each measurement time point and treatment group) ± SE.
Efficacy measurement at different treatment occasion
The pain scores at the different treatment occasions and measurement time points were analysed statistically. The results are shown in Figure 2. Randomisation leads to unequal number of patients in each treatment group at different treatment occasions. On the first and fourth treatment occasion the number of patients in the placebo group was almost double the number of patients in the morphine group. Consequently, the number of patients in the morphine group was higher than placebo on treatment occasions 2 and 3. Morphine reduced pain scores more than placebo on treatment occasions 1 and 2 but the difference between placebo and morphine was statistically significant only 2 hours after dressing on the first treatment occasion.
Figure 2.
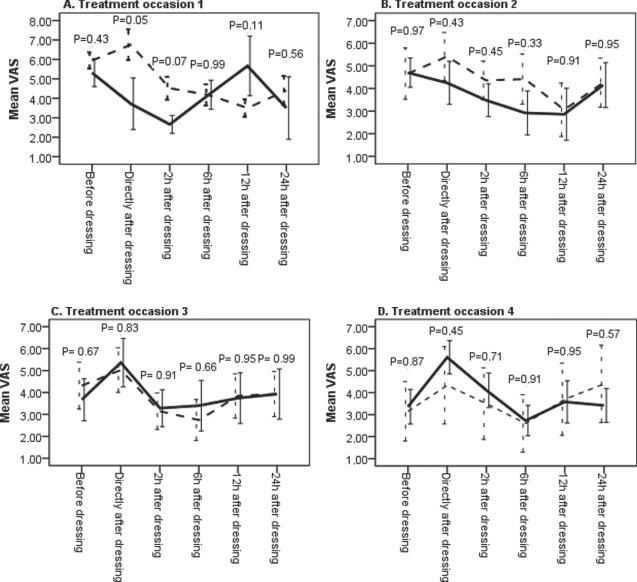
Comparison between morphine and placebo at different treatment occasions. Pain scores were measured before gel application, directly after and 2, 6, 12 and 24 hours after application. The dashed lines represent the mean value of measurements after application of placebo gel and the solid lines represent the mean value of measurements after application of morphine gel. The value represents a mean of two observations at each measurement time point and treatment group for all patients except patient no. 21 who had only one value for each measurement time point and treatment group. The number of patients that received morphine respective placebo were, in treatment occasion 1 (6, 11), in treatment occasion 2 (10, 7), in treatment occasion 3 (7, 9) and in treatment occasion 4 (10, 6).
Efficacy measurement in each patient
Statistical analysis was also done for each patient (Figure 3). There was wide interindividual variation in response to topically applied morphine/placebo gel in this study population. The pain score varied initially at different treatment occasions. In some patients, the value of VAS was initially lower than four at second, third and/or fourth treatment occasion which resulted in a mean value of VAS lower than four in some patients.
Figure 3.

Data represent mean visual analogue score (VAS) at different measurement time point for each patient. The value represents a mean of two observations from each measurement time point and treatment group for all patients except patient no. 21 who had only have one value for each measurement time point and treatment group.
The mean VAS was increased in some patients whereas it remained unchanged in some. The mean pain score was significantly reduced in patient no. 19. Mean VAS, in this patient, was significantly lower 2 and 6 hours after dressing (P = 0·03; 0·017, respectively) upon treatment with morphine gel. This patient continued to receive morphine gel after completing the study.
Adverse events
Adverse events were reported by patients and medical staff during the study period (Table 2). The table lists the number of patients who developed new symptoms during the study period. The frequency of most symptoms was similar in both treatment groups. Burning pain from ulcers after application of gel was the most commonly reported adverse reaction but the incidence was almost the same in both treatment groups. Drowsiness was observed more frequently when placebo gel was used compared to morphine. Medical staff reported redness around the ulcers in three cases after application of gel. No systemic adverse effects were reported by any patients.
Table 2.
Adverse reactions reported by participants in each group. The data presented here is based on reported adverse events from 17 patients who were included in this analysis
| Adverse reaction | Placebo | Morphine | ||
|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | |
| Itching | 2 | 6% | 0 | 0% |
| Drowsiness | 2 | 6% | 1 | 3% |
| Redness | 1 | 3% | 2 | 6% |
| Smarting pain/burning pain | 7 | 21% | 6 | 18% |
| Total | 12 | 36% | 9 | 27% |
DISCUSSION
Chronic wounds are common medical problems that result in dramatic alterations in the quality of life due to associated pain, disability and social isolation. They disproportionately affect the elderly and are therefore increasing in incidence and prevalence. During the last decades, characterisation of topical opioid receptors on nerve terminals in inflamed tissues provided the theoretical base for possible use of topically applied opioids such as morphine and diamorphine for treatment of painful ulcers. Chronic venous leg ulcers are characterised by ongoing and unregulated inflammation which is a pre‐requisite for presentation of opioid receptors on peripheral nerve terminals (39).
The objective of the present study was to investigate the efficacy of topically applied morphine on painful leg ulcers. Our results suggest that overall efficacy of topically applied morphine for the treatment of painful leg ulcers might be limited. There was no statistically significant difference between placebo and morphine groups as a whole (Figure 1). Even though the mean pain score was lower in the morphine group versus placebo, the difference was too small to have clinical significance as judged by reduction in pain score (Figure 1).
In the present study, the pain score was higher directly after dressing which could partly be explained by the treatment procedure. Paradoxically, both groups experienced a clinically relevant reduction of pain when compared to VAS directly after application of gel. A reduction of pain score by roughly two VAS units was judged as clinically relevant. This suggests a potential placebo effect of the gel vehicle.
The pain was measured after application of the morphine/placebo gel at four consecutive dressing changes. The study period was at least 2 weeks and at most 4 weeks. The condition of ulcers and pain varied during this period which had an important influence on the results. There was a large variation in the intensity of pain between and within the patients before applying the gel. For example, patient no. 5, had a pain score less than four (VAS 2·7 and 0 before applying the gel) when she started treatment with morphine on both treatment occasions and patient no. 13 had no pain when she started the treatment with placebo gel.
We also analysed the results from the first treatment occasion, at which the pain scores were above or equal to four for all participants, to further understand if there were any differences between these two treatment groups. The results showed a significant difference between the treatment groups, both, directly after and 2 hours subsequent to application of gel. We are aware that limited conclusions can be drawn from this analysis, not only because of the limited number of patients but also because of the unequal number of participants in each groups and because the study was not designed for such comparison.
Our study does have a number of limitations that could have an impact on our conclusions. Lack of significance between the morphine and placebo groups could be due to our methodology not being rigorous or sensitive enough to pick up a small yet clinically significant difference.
We opted for a crossover design as we wanted to evaluate morphine versus placebo under similar conditions. More specifically we had hoped that each individual patient would have similar pain scores before entering the morphine or placebo arm of the study. Unfortunately, most patients varied in their baseline pain scores at different treatment occasions. Indeed when patients had an initial high pain score (Figure 2(A)), morphine gel induced a significant reduction of pain. It could, therefore, be speculated that a parallel group design with a single‐point comparison would have been more suitable.
It has been suggested that peripheral nerves in inflamed tissue express opioid receptors that are upregulated, often within minutes to hours 8, 9, 10, 11, 12. It is also possible that the lack of efficiency of topically applied morphine could be due to insufficient inflammatory involvement in the individual leg ulcers.
When the results were analysed for each patient, topically applied morphine seemed to be significantly better than placebo only for one patient. We could not find any explanation for as to why this patient responded differently to the treatment. In another patient, extra analgesic drugs were used during the treatment with placebo but not during the morphine treatment. It could be speculated as indicative of a positive morphine effect in specific individuals.
As described earlier, there is some evidence for the existence and activity of opioid receptors on peripheral sensory nerves which makes it theoretically possible to provide local analgesia for painful ulcers. So far there are some case reports and a few controlled clinical trials studying the effectiveness of topically applied morphine and other opioids on painful ulcers. Contradictory results have been obtained. To our knowledge, there are only few controlled studies that have evaluated the effect of topically applied morphine on chronic painful ulcers. Recent studies have suggested that topical morphine does not have a clinically relevant analgesic effect in patients with painful ulcers which is in line with our results 34, 35. In these studies, the analgesic efficacy of morphine was compared with placebo. In one study, 10 mg of morphine hydrochloride/water for injection were mixed in Intrasite and then applied daily for 5 days (34) and in another one a hydrogel containing 0·5% of morphine was evaluated. Neither of these studies showed a statistically nor clinically relevant analgesic effect of topically applied morphine. In another study, the analgesic effect of topically applied morphine in Intrasite gel(10 mg morphine /8 ml Intrasite gel) was compared to placebo in a crossover study on five patients with painful sacral pressure sores (30). Patients were treated for 2 days with either 10 mg morphine sulphate or placebo (water for injection) applied topically to the ulcers. After a 2‐day washout period, patients were crossed over for a further 2 days of the alternative treatment. All patients reported clinically and statistically significant lower pain scores with morphine compared to placebo. In a follow‐up letter to editor, the author presented results from 17 patients with the same magnitude of pain reduction with topically applied morphine (31). Even topically applied diamorphine indicated significant pain relief in patient with pressure sacral sores. Our results do not support these case reports and studies. However, it is difficult to compare results from these previous studies with present study because of differences in study design and differences in ulcer aetiology, pathogenesis and localisation 40, 41.
There are very few reports that have studied the efficacy of topical morphine in a controlled manner which forced us to reach an arbitrary decision as to the dose used in our study. The average quantity of morphine gel applied was higher in most studies than the average morphine quantity used in this study. It is, therefore, perfectly feasible that topical morphine is effective, albeit at a higher dose. A pharmacokinetic and dose titration study would be necessary to explore absorption and distribution of topically applied morphine. In addition, the difference in aetiology of these ulcers and the location of ulcers may play an important role in response to topically applied morphine. The gel that is used in Sweden is an extemporaneously produced hydrogel. Theoretically, this gel has physical properties similar to the commercially available Intrasite gel. We studied the release of morphine from our extemporaneously produced gel by diffusion cell (Franz Cell) using human skin as a diffusion membrane, in order to ensure that the morphine was available on the surface of the wound. A maximal release of morphine was observed 2 hours after the application of morphine (data not shown).
An important part of wound care is to avoid interference with the delicate process of wound healing. Topically applied morphine can have a negative effect on wound healing. It has been suggested that sensory neuropeptides play an important role in wound repair (42). The effect of topically applied morphine on wound healing was examined on a standardised model of cutaneous wound healing in the rat (43). The results from this study demonstrated a significant overall concentration‐depended delay in the time course of wound contraction in morphine treated animals compared to placebo (only gel‐treated). However, this effect has not been described in the human clinical trials at present.
CONCLUSION
The results of this study suggest that topically applied morphine is not an effective treatment strategy for pain relief in patient with chronic painful leg ulcers which is in congruence with recently published studies. This study, however, does not exclude the possibility that topically applied morphine, could be useful in some individuals.
Further studies are warranted to evaluate if topically applied morphine could be an effective treatment strategy in some patients with chronic painful leg ulcers.
ACKNOWLEDGEMENT
This work was financially supported by Forskningsrådet I Sydöstra Sverige, FORSS.
REFERENCES
- 1. Ragnarson Tennvall G, Hjelmgren J. Annual costs of treatment for venous leg ulcers in Sweden and the United Kingdom. Wound Repair Regen 2005;13:13–8. [DOI] [PubMed] [Google Scholar]
- 2. Faresjo T, Frodin T, Vahlquist C, Klevbrand M, Elfstrom J, Leszniewska D, Larsson A Costs of the treatment of leg ulcers: initiating a quality assurance process. Int J Health Care Qual Assur Inc Leadersh Health Serv 1997;10:125–30. [DOI] [PubMed] [Google Scholar]
- 3. Ebbeskog B, Lindholm C, Ohman S. Leg and foot ulcer patients. Epidemiology and nursing care in an urban population in south Stockholm, Sweden. Scand J Prim Health Care 1996;14:238–43. [DOI] [PubMed] [Google Scholar]
- 4. Ebbeskog B, Skonda E, Lindholm C, Grauers M, Ohman S. A follow‐up study of leg ulcer patients in south Stockholm. J Wound Care 1999;8:170–4. [DOI] [PubMed] [Google Scholar]
- 5. Gottrup F, Karlsmark T. Leg ulcers: uncommon presentations. Clin Dermatol 2005;23:601–11. [DOI] [PubMed] [Google Scholar]
- 6. Persoon A, Heinen MM, van der Vleuten CJ, de Rooij MJ, van de Kerkhof PC, van Achterberg T. Leg ulcers: a review of their impact on daily life. J Clin Nurs 2004;13:341–54. [DOI] [PubMed] [Google Scholar]
- 7. Nemeth KA, Harrison MB, Graham ID, Burke S. Pain in pure and mixed aetiology venous leg ulcers: a three‐phase point prevalence study. J Wound Care 2003;12:336–40. [DOI] [PubMed] [Google Scholar]
- 8. Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med 1995;332:1685–90. [DOI] [PubMed] [Google Scholar]
- 9. Krajnik M, Zylicz Z, Finlay I, Luczak J, van Sorge AA. Potential uses of topical opioids in palliative care–report of 6 cases. Pain 1999;80:121–5. [DOI] [PubMed] [Google Scholar]
- 10. Truong W, Cheng C, Xu QG, Li XQ, Zochodne DW. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann Neurol 2003;53:366–75. [DOI] [PubMed] [Google Scholar]
- 11. Laduron PM. Axonal transport of opiate receptors in capsaicin‐sensitive neurones. Brain Res 1984;294:157–60. [DOI] [PubMed] [Google Scholar]
- 12. Hassan AH, Ableitner A, Stein C, Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience 1993;55:185–95. [DOI] [PubMed] [Google Scholar]
- 13. Stein A, Yassouridis A, Szopko C, Helmke K, Stein C. Intraarticular morphine versus dexamethasone in chronic arthritis. Pain 1999;83: 525–32. [DOI] [PubMed] [Google Scholar]
- 14. Kalso E, Tramer MR, Carroll D, McQuay HJ, Moore RA. Pain relief from intra‐articular morphine after knee surgery: a qualitative systematic review. Pain 1997;71:127–34. [DOI] [PubMed] [Google Scholar]
- 15. Likar R, Kapral S, Steinkellner H, Stein C, Schafer M. Dose‐dependency of intra‐articular morphine analgesia. Br J Anaesth 1999;83:241–4. [DOI] [PubMed] [Google Scholar]
- 16. Likar R, Schafer M, Paulak F, Sittl R, Pipam W, Schalk H, Geissler D, Bernatzky G. Intraarticular morphine analgesia in chronic pain patients with osteoarthritis. Anesth Analg 1997;84:1313–7. [DOI] [PubMed] [Google Scholar]
- 17. Rosseland LA. No evidence for analgesic effect of intra‐articular morphine after knee arthroscopy: a qualitative systematic review. Reg Anesth Pain Med 2005;30:83–98. [DOI] [PubMed] [Google Scholar]
- 18. Rosseland LA, Stubhaug A, Grevbo F, Reikeras O, Breivik H. Effective pain relief from intra‐articular saline with or without morphine 2 mg in patients with moderate‐to‐severe pain after knee arthroscopy: a randomized, double‐blind controlled clinical study. Acta Anaesthesiol Scand 2003;47:732–8. [DOI] [PubMed] [Google Scholar]
- 19. Rosseland LA, Stubhaug A, Skoglund A, Breivik H. Intra‐articular morphine for pain relief after knee arthroscopy. Acta Anaesthesiol Scand 1999;43:252–7. [DOI] [PubMed] [Google Scholar]
- 20. Twillman RK, Long TD, Cathers TA, Mueller DW. Treatment of painful skin ulcers with topical opioids. J Pain Symptom Manage 1999;17:288–92. [DOI] [PubMed] [Google Scholar]
- 21. Ballas SK. Treatment of painful sickle cell leg ulcers with topical opioids. Blood 2002;99:1096. [DOI] [PubMed] [Google Scholar]
- 22. Flock P, Gibbs L, Sykes N. Diamorphine‐metronidazole gel effective for treatment of painful infected leg ulcers. J Pain Symptom Manage 2000;20:396–7. [DOI] [PubMed] [Google Scholar]
- 23. Watterson G, Howard R, Goldman A. Peripheral opioids in inflammatory pain. Arch Dis Child 2004;89:679–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Back IN, Finlay I. Analgesic effect of topical opioids on painful skin ulcers. J Pain Symptom Manage 1995;10:493. [DOI] [PubMed] [Google Scholar]
- 25. Farley P. Should topical opioid analgesics be regarded as effective and safe when applied to chronic cutaneous lesions? J Pharm Pharmacol 2011;63:747–56. [DOI] [PubMed] [Google Scholar]
- 26. Barker S. Analgesic effect of locally applied morphine to pyoderma gangrenosum. Clin Exp Dermatol 2009;34:91–2. [DOI] [PubMed] [Google Scholar]
- 27. Turris M. The use of topical opioids for systemic pain management. J Pain Symptom Manage 2008;36:e13–4. [DOI] [PubMed] [Google Scholar]
- 28. van Ingen IL, Jansen MM, Barrera P. Topical opioids for painful ulcers in systemic sclerosis. Ann Rheum Dis 2008;67:427. [DOI] [PubMed] [Google Scholar]
- 29. Flock P. Pilot study to determine the effectiveness of diamorphine gel to control pressure ulcer pain. J Pain Symptom Manage 2003;25:547–54. [DOI] [PubMed] [Google Scholar]
- 30. Zeppetella G, Paul J, Ribeiro MD. Analgesic efficacy of morphine applied topically to painful ulcers. J Pain Symptom Manage 2003;25:555–8. [DOI] [PubMed] [Google Scholar]
- 31. Zeppetella G, Ribeiro MD. Morphine in intrasite gel applied topically to painful ulcers. J Pain Symptom Manage 2005;29:118–9. [DOI] [PubMed] [Google Scholar]
- 32. Faktorovich EG, Basbaum AI. Effect of topical 0·5% morphine on postoperative pain after photorefractive keratectomy. J Refract Surg 2010;26:934–41. [DOI] [PubMed] [Google Scholar]
- 33. Vayne‐Bossert P, Escher M, de Vautibault CG, Dulguerov P, Allal A, Desmeules J, Herrmann FR, Pautex S. Effect of topical morphine (mouthwash) on oral pain due to chemotherapy‐ and/or radiotherapy‐induced mucositis: a randomized double‐blinded study. J Palliative Med 2010;13:125–8. [DOI] [PubMed] [Google Scholar]
- 34. Vernassiere C, Cornet C, Trechot P, Alla F, Truchetet F, Cuny JF, Commun N, Granel Brocard F, Barbaud A, Schmutz JL. Study to determine the efficacy of topical morphine on painful chronic skin ulcers. J Wound Care 2005;14:289–93. [DOI] [PubMed] [Google Scholar]
- 35. Jansen MM, van der Horst JC, van der Valk PG, Kuks PF, Zylicz Z, van Sorge AA. Pain‐relieving properties of topically applied morphine on arterial leg ulcers: a pilot study. J Wound Care 2009;18:306–11. [DOI] [PubMed] [Google Scholar]
- 36. Welling A. A randomised controlled trial to test the analgesic efficacy of topical morphine on minor superficial and partial thickness burns in accident and emergency departments. Emerg Med J 2007;24:408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Long TD, Cathers TA, Twillman R, O’Donnell T, Garrigues N, Jones T. Morphine‐infused silver sulfadiazine (MISS) cream for burn analgesia: a pilot study. J Burn Care Rehabil 2001;22:118–23. [DOI] [PubMed] [Google Scholar]
- 38. Draxler J, Schuch M, Paul A, Sycha T, Valenta C, Likar R, Gustorff B. Topical application of morphine and buprenorphine gel has no effect in the sunburn model. Schmerz 2008;22:571–4. [DOI] [PubMed] [Google Scholar]
- 39. Lundqvist K, Sorensen OE, Schmidtchen A. Increased levels of human neutrophil alpha‐defensins in chronic venous leg ulcers. J Dermatol Sci 2008;51:131–4. [DOI] [PubMed] [Google Scholar]
- 40. Woo KY, Sibbald RG. Chronic wound pain: a conceptual model. Adv Skin Wound Care 2008;21:175–88. [DOI] [PubMed] [Google Scholar]
- 41. Popescu A, Salcido RS. Wound pain: a challenge for the patient and the wound care specialist. Adv Skin Wound Care 2004;17:14–20. [DOI] [PubMed] [Google Scholar]
- 42. Delgado AV, McManus AT, Chambers JP. Exogenous administration of Substance P enhances wound healing in a novel skin‐injury model. Exp Biol Med (Maywood) 2005;230:271–80. [DOI] [PubMed] [Google Scholar]
- 43. Rook JM, McCarson KE. Delay of cutaneous wound closure by morphine via local blockade of peripheral tachykinin release. Biochem Pharmacol 2007;74:752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


