Abstract
It is well documented that diabetic foot ulceration contributes to increased morbidity and mortality associated with renal replacement therapy. Much less is known about the risk of foot ulceration and lower limb amputation in the non‐diabetic dialysis population. The aim of this study was to determine if the prevalence of risks factors for lower limb amputation in a stable haemodialysis population was greater in the diabetic cohort compared with the non‐diabetic cohort. The study design is a prospective observational cohort study. Sixty patients attending a satellite haemodialysis unit in Cardiff were invited to have a comprehensive foot assessment as part of a Podiatry service review. The medical notes and hospital information system were used to identify the diabetic cohort. Patients were classified according to diabetic status (diabetic versus non‐diabetic). The Renal Foot Screening Tool was developed to prospectively identify risk factors associated with foot ulceration. The assessment included peripheral neuropathy (PN), peripheral arterial disease (PAD) and foot pathology (FP). Fifty‐seven patients gave informed verbal consent prior to inclusion. Risk factors for foot ulceration were recorded at baseline in the diabetic (n = 24) and non‐diabetic (n = 33) groups and mortality data was revisited after a 3‐year period. FP was identified in 79% of patients. Eighteen per cent of the non‐diabetic patients had PN. PAD was identified in 45% of diabetic and 30% of non‐diabetic patients. Forty‐nine per cent of the total cohort had ≥2 of the 3 independent risk factors for foot ulceration (16/24 diabetic versus 12/33 non‐diabetic). The presence of PAD and PN was predictive of mortality independent of age. The limitations of this study are its small sample size and patients were from a single satellite dialysis unit. There was a high prevalence of risk factors for foot ulceration in this population, which were not confined to the diabetic cohort. These findings suggest that non‐diabetic patients on haemodialysis therapy are also at risk of developing foot ulceration. Further work on strategies to monitor and prevent FP in this high‐risk cohort is needed to minimize morbidity and mortality associated with foot ulceration.
Keywords: Diabetes mellitus, Foot ulceration, Haemodialysis, Lower limb amputation
Introduction
Survival of patients on haemodialysis is significantly reduced when compared with the general population. The UK Renal Registry report (2007) suggested that foot ulceration was the most important predictor of death within the first 90 days of dialysis initiation and second only to malignancy in the first year of dialysis (1). Although the excess in morbidity and mortality is multifactorial, foot ulceration and the associated increased risk of lower limb amputation has been postulated to represent one contributing factor to the reduced survival in the general dialysis population 2, 3, 4.
It is well documented that foot ulceration is an important cause of morbidity and mortality in patients with diabetes mellitus. All stages of diabetic nephropathy have been associated with increased risk of foot ulceration. A previous study showed that 20% of patients with diabetes develop foot ulceration within the first year of commencing haemodialysis representing a threefold increase in comparison with the general diabetic population 5, 6 and dialysis therapy has been independently associated with diabetic foot ulceration (7). Neuropathy and ischaemia have been identified as the two major risk factors in the causation and subsequent poor healing of diabetic foot ulceration (8). The lack of protective sensation in sensory neuropathy leads to repetitive trauma on the skin and callus from areas of high pressure lead to a wound which results in ulceration. Consequentially, arterial damage in the lower limb, commonly observed in the diabetic population as a result of vascular calcification, has a profound negative effect on the wound healing potential 9, 10.
Much less is known about the risk of ulceration in the non‐diabetic haemodialysis population, although a recent study suggests that this population may also be at high risk of foot ulceration (11).
This audit was designed to establish the prevalence of risk factors for lower limb ulceration presenting in patients on haemodialysis and to compare these risk factors in individuals with and without diabetes.
Methods
Patient identification
A cohort of patients undergoing haemodialysis therapy at a satellite unit in Cardiff, South Wales were offered a comprehensive foot assessment by a trained podiatrist as part of an increase in service provision for foot care. The data was collected as part of an audit between June and August 2007 and required no formal ethical approval. However, all patients gave verbal consent prior to inclusion.
Fifty‐seven of the 60 patients attending the haemodialysis unit were assessed, 3 patients were excluded because of a dialysis modality switch or transplant during data collection period. Demographic and medical history were ascertained by patient interview, review of the medical records and the laboratory results reporting system. Patients were classified according to diabetic status into the following two groups (diabetic versus non‐diabetic).
Screening protocol
A single podiatrist used non‐invasive techniques to assess both feet for physiological risk factors in accordance with evidence‐based practice (12). The Renal Foot Screening Tool was a pro forma developed to prospectively identify risk factors associated with foot ulceration. The tool focussed on identifying the three important risk factors for foot ulceration: peripheral neuropathy (PN), peripheral arterial disease (PAD) and foot pathology (FP).
Peripheral neuropathy
Multiple forms of standardised assessment were used for each patient. Light touch sensation was tested using the Semmes‐Weinstein 10g monofilament at 10 sites on each foot. The patient was instructed to close their eyes; the 10g monofilament was applied at a 90° right angle to the selected site until it buckled and held for 1 second before moving on to the next test site (13).
Nine plantar sites; distal great toe, third and fifth toes; first, third and fifth plantar metatarsal heads; medial arch, lateral foot and heel and one dorsal site located between the first and second digital webbing line were used in the assessment. A positive score for neuropathy was defined as the inability to identify two or more separate test sites.
Vibration perception threshold was assessed by a neurothesiometer. The device was held with the tractor balanced vertically on the pulp of the hallux and the voltage increased on the base unit until the patient could perceive the vibration. A mean of three readings were recorded for each foot with a positive score for neuropathy defined as a threshold value of ≥25 V (14). This value of 25 V and over has been determined as the threshold for classifying the patient ‘at risk’ of developing a foot ulcer (15).
Patients with detectable neuropathy by either assessment method were classed as having PN to ensure that all patients with neuropathy were identified.
Peripheral arterial disease
The handheld Doppler ultrasound was used to locate the dorsalis pedis and posterior tibial arteries. The probe was lightly applied to the skin to avoid occlusion of the artery and arterial insufficiency was recorded when both the dorsalis pedis and posterior tibial signals were monophasic in a single lower limb (16).
The Edinburgh Claudication Questionnaire was used to diagnose symptomatic vascular insufficiency (17). Patients scored positive for symptomatic vascular insufficiency if they described either intermittent claudication or rest pain. This data was compared against the hard outcome measures derived from the Doppler assessment.
Foot pathology
It is recognised that coexistent structural FP is an additional risk factor for foot ulceration in a neuropathic or arterial insufficient limb (8). FP was ascertained by the podiatrist and was defined as one of the following: claw toes, hallux valgus, prominent metatarsal heads, corns, callosities and nail pathologies.
The results were recorded using SPSS Windows v 12.0.0 (SPSS Inc, Chicago, IL) in preparation for data analysis. Survival analysis was made with Kaplan–Meier plots and log‐rank (Mantel–Cox) testing for a linear trend using SPSS 15 (PC).
For the purposes of survival analysis, correction for subject's ages was achieved by stratification by the median. All subjects were censored at 32 months follow‐up irrespective of dialysis modality switch or transplantation.
Results
Patient demographics
Twenty‐four of the 57 haemodialysis patients screened had a history of diabetes mellitus (Table 1). The clinical characteristics (age and gender) were similar in both cohorts except that the patients with diabetes had a higher body mass index (BMI) and a shorter length of time on haemodialysis.
Table 1.
Baseline demographics of haemodialysis patient cohort
| Age ≥ 65 (years) | Male: female ratio | Mean body mass index | Smoker | |
|---|---|---|---|---|
| Diabetic patients, N = 24 | 6 (25%) | 14:10 | 29·65 | 2 (8%) |
| Non‐diabetic patients, N = 33 | 9 (27%) | 23:10 | 26·45 | 5 (15%) |
| All patients, N = 57 | 15 (26%) | 37:20 | 28·1 | 7 (12%) |
Smoking status
There were 35 patients with a current or past history of smoking. Of the 21 patients with objective evidence of peripheral vascular disease, 15 had a smoking history (8/15 diabetic). Presence of PN was also associated with a smoking history in 12 of the 18 patients from the total cohort.
Peripheral neuropathy
In this haemodialysis cohort, 18 patients (32%) were classified as having PN (Table 2). There were 12 (50%) from the diabetic patient group and 6 (18%) from the non‐diabetic group.
Table 2.
Peripheral neuropathy was identified if patients were positive for either neurothesiometer ≥25 V or diminished 10 g monofilament
| Neurothesiometer ≥ 25 V | 10 g monofilament absent | Tested positive for neuropathy | |
|---|---|---|---|
| Diabetic patients, N = 24 | 11 (46%) | 9 (37%) | 12 (50%) |
| Non‐diabetic patients, N = 33 | 3 (9%) | 4 (12%) | 6 (18%) |
| All patients, N = 57 | 14 (25%) | 13 (23%) | 18 (32%) |
As can be seen from Table 2, neither method of detecting PN alone was sufficient alone to identify all patients with neuropathy. Only 10 of the 18 had both reduced vibration perception (≥25 V) and diminished 10 g monofilament sensation suggesting that both measures are needed to identify neuropathy in this patient cohort.
Peripheral arterial disease
Arterial insufficiency was documented in 21 (37%) patients from the total cohort (11 of the diabetic cohort and 10 of the non‐diabetic cohort; Table 3). There was no significant difference between the diabetic and non‐diabetic cohorts in the prevalence of PAD (chi‐squared P = 0·23). Six patients (25%) with diabetes had objective evidence of both PN and arterial disease compared with four patients (12·1%) in the non‐diabetic cohort.
Table 3.
Patients with monophasic signals in both the dorsalis pedis and posterior tibial arteries were classified with arterial insufficiency. Those patients that reported symptoms of arterial disease are also shown but were not classified as having arterial disease unless supported by doppler evidence
| Arterial insufficiency by doppler analysis | Symptoms of peripheral arterial disease | |
|---|---|---|
| Diabetic patients, N = 24 | 11 (45%) | 10 (41%) |
| Non‐diabetic patients, N = 33 | 10 (30%) | 18 (75%) |
| All patients, N = 57 | 21 (37%) | 23 (40%) |
Symptoms of arterial insufficiency (claudication or rest pain) was reported by 23 (40%) of the total cohort and of these 10 were diabetic. A greater percentage of the symptomatic diabetic population compared with their non‐diabetic counterparts had objective evidence of PAD as defined by the Doppler (45% versus 30%). Ten patients (4/10 = diabetic) had symptoms identified by the Edinburgh Claudication Questionnaire but had biphasic pedal pulses.
Asymptomatic PAD was identified in eight patients from the total cohort (5/8 diabetic), three (37%) of which had evidence of coexistent neuropathy.
Other risk factors
FP was identified in 45 (79%) patients (Table 4). The same percentage of diabetic and non‐diabetic patients had FP on examination. Only 13 patients from the total cohort presented with no evidence of FP on inspection.
Table 4.
Some of the other risk factors for lower limb ulceration were recorded
| Foot pathology | Current lower limb ulcer | Previous lower limb ulcer | |
|---|---|---|---|
| Diabetic patients, N = 24 | 19 (79%) | 1 (4%) | 7 (29%) |
| Non‐diabetic patients, N = 33 | 26 (79%) | 3 (9%) | 5 (15%) |
| All patients, N = 57 | 45 (79%) | 4 (7%) | 12 (21%) |
Four (7%) of the total cohort had evidence of a foot ulcer at the time of assessment (3/4 non‐diabetic versus 1/4 diabetic). Seven patients (29%) with diabetes had a history of previous foot ulceration compared with five patients (15%) from the non‐diabetic cohort.
Cumulative risk factors
Given the high prevalence of risk factors in this haemodialysis population, the number of risk factors (PN, PAD and FP) for foot ulceration was calculated for each individual patient (Figure 1).
Figure 1.
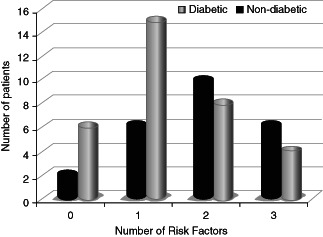
Graphical representation of cumulative risk factors for foot ulceration in diabetic and non‐diabetic haemodialysis patients.
Forty‐nine per cent of the total cohort (n = 57) had two or more risk factors for foot ulceration (16/24 diabetic versus 12/33 non‐diabetic). Only seven (12%) patients (2/7 diabetic) had normal feet with no evidence of FP or objective evidence of neurovascular disease.
Mortality
Three years after the baseline assessment, the cohort was revisited to assess mortality. Of the original 57 patients 22 (38%) had deceased: 10 out of 24 from the diabetic cohort and 12 out of 33 from the non‐diabetic cohort. There was no statistical difference in the number of deaths observed between the cohorts. The Kaplan–Meier survival analysis can be seen in Figure 2. The presence of both PAD and neuropathy was predictive even when corrected for patient age (chi‐square 3·898; P = 0·048).
Figure 2.
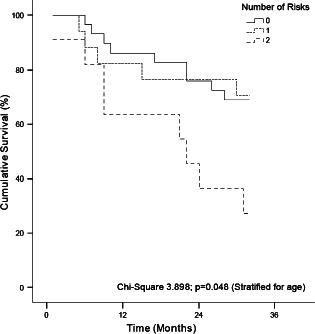
Kaplan–Meier plot of survival by presence of the number of risk factors for foot ulceration.
Discussion
The key finding of this article suggests a high prevalence of risk factors for foot ulceration in a general haemodialysis population. Our findings have important clinical implications as they alert the prevalence of risk factors for foot ulceration in the non‐diabetic haemodialysis population. Three patients from the non‐diabetic cohort had a foot ulcer at the time of assessment compared with one patient from the diabetic cohort. Foot ulceration in the general dialysis population is often considered a minor clinical problem but the 2007 UK Renal Registry report signalled the importance of this observation by identifying foot ulceration as a predictor of mortality in the haemodialysis population (1).
This study is applicable to the general dialysis population in that the demographics of the population are similar to those reported by the 2007 UK Renal Registry despite the prevalence of diabetes in this haemodialysis population being approximately double that of the UK haemodialysis population. Both cohorts were similar in most respects. The diabetic patients had a higher BMI than their counterparts and the duration of haemodialysis therapy in the diabetic cohort did not exceed 5 years, whereas 15·2% of the non‐diabetic cohort had been on dialysis longer than 5 years.
The study had several limitations. The patients were from a single satellite dialysis unit and this restricted the number of patients we could assess during the audit. Our study sample, despite being representative in most respects of the general UK haemodialysis population, was insufficient to draw any statistically significant conclusions on the causal relationship between this dialysis modality and the development of foot ulceration. In addition, the data was gathered as part of a service review audit and no ethical approval was given to test patients for diabetes. We adopted the American Diabetes Association criteria for diagnosis on the revision of the medical notes and results reporting system.
There are many studies detailing the prevalence of risk factors and foot ulceration in the diabetic renal failure population 6, 7, 11. By contrast, the prevalence of risk factors for foot ulceration in the non‐diabetic dialysis population is not well defined. It is clear that foot ulceration may herald a clinical course ultimately ending in amputation. It is well documented that the diabetic population with renal disease have a ten times higher risk of lower limb amputation than the general diabetic population. The rate of amputation in the diabetic dialysis population is in the order of 4% per year (18). Yet, reported rates for lower limb amputation in the non‐diabetic dialysis population were approximately 80% less than the diabetic population with annualised rates of between 2 and 3 per 100 patients (3). This is despite very similar prevalence rates of risk factors between the diabetic and non‐diabetic haemodialysis patients in our study, which suggest that there are other factors that influence this process.
The pathogenesis of foot ulceration and thus amputation in the diabetic haemodialysis group of patients is multifactorial with both ischaemic and neuropathic damage contributing to minor lesions developing after relatively innocuous trauma. These lesions develop into overt ulceration and progress as a result of PN with or without lower limb ischaemia 2, 19. There is evidence that the initiation of dialysis in the diabetic population is associated with a threefold risk of new ulceration in the first year of dialysis treatment (6). Information for non‐diabetic patients developing new ulceration is to our knowledge not available. The mechanism by which dialysis initiation could influence ulcer formation may relate to decreased transcutaneous oxygen tension and cutaneous microcirculation (20).
This has been showed in diabetic patients and could lead to critical limb ischaemia, which would compound the risk factors already present in these patients 21, 22.
Although we expected to find risk factors in the diabetic population, we were surprised to find the high prevalence of multiple risk factors in the non‐diabetic cohort. PN had a high prevalence in the diabetic population but was also evident in 18% of the non‐diabetic cohort. These results are consistent with other publications, which suggested that PN might develop with the progression of chronic kidney disease (CKD) secondary to uraemia 5, 23.
PAD is an important risk factor for foot ulceration in both diabetic and non‐diabetic patients and was present in 45% and 30% of our patients, respectively. It is well known that both diabetic and non‐diabetic renal failure patients have a high risk of PAD (24) and data suggests that patients with impaired renal function have a twofold risk of peripheral vascular disease (25). The reason for this increased risk is not only due to the traditional risk factors associated with arterial disease but also specific renal failure risk factors (26). These include, but are not limited to, hyperphosphataemia, hyperparathyroidism and chronic inflammation.
By contrast to the above hypothetical risk factors, smoking history is one of the traditional risk factors for PAD and was shown in similar high percentages of those with documented PAD in both cohorts of this study (26). There was also a strong link between smoking history and the development of PN in this data with two‐thirds of those with PN reporting a history of smoking.
Foot deformity in the presence of neuropathy has been shown to contribute to ulcer formation (27). The baseline assessment showed a high prevalence of FP in this general haemodialysis population. A considerable proportion of our total cohort (72% diabetic versus 44% non‐diabetic) had two or more risk factors for foot ulceration. The presence of two or more risk factors is known to significantly increase the clinical risk of lower limb threatening complications developing in the diabetic population (28).
The data presented in this study suggests that there is a potential health risk for non‐diabetic patients on haemodialysis therapy developing foot ulceration. Resources for the detection of these risk factors are currently confined to the diabetic population. Current CKD guidelines and recommendations fail to recognise the association between haemodialysis and the development of foot ulceration. We suggest that further work on strategies to monitor and prevent FP in this high‐risk population is needed to minimise morbidity and mortality associated with foot ulceration.
References
- 1. Ansell D, Feest T, Tomson C, Williams AJ, et al. UK Renal Registry Report 2007. Bristol: UK Renal Registry, 2007. [Google Scholar]
- 2. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513–21. [DOI] [PubMed] [Google Scholar]
- 3. Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end‐stage renal disease population. Kidney Int 1999;56:1524–33. [DOI] [PubMed] [Google Scholar]
- 4. Koch M, Trapp R, Kulas W, Grabensee B. Critical limb ischaemia as a main cause of death in patients with end‐stage renal disease: a single‐centre study. Nephrol Dial Transplant 2004;19:2547–52. [DOI] [PubMed] [Google Scholar]
- 5. Fernando DJ, Hutchison A, Veves A, Gokal R, Boulton AJM. Risk factors for non‐ischaemic foot ulceration in diabetic nephropathy. Diabet Med 1991;8:223–5. [DOI] [PubMed] [Google Scholar]
- 6. Game FL, Chipchase SY, Hubbard R, Burden RP, Jeffcoate WJ. Temporal association between the incidence of foot ulceration and the start of dialysis in diabetes mellitus. Nephrol Dial Transplant 2006; 21:3207–10. [DOI] [PubMed] [Google Scholar]
- 7. Ndip A, Rutter MK, Vileikyte L, Vardhan A, Asari A, Jameel M, Tahir HA, Lavery LA, Boulton AJM. Dialysis Treatment is an independant risk factor for foot ulceration in patients with diabetes and stage 4 or 5 chronic kidney disease. Diabetes Care 2010;33: 1811–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lavery LA, Armstrong DG, Vela SA, Quebedeaux TL, Herschlt JG. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med 1998;158:157–62. [DOI] [PubMed] [Google Scholar]
- 9. Pham HT, Economides PA, Veves A. The role of endothelial function on the foot. Microcirculation and wound healing in patients with diabetes. Clin Podiatr Med Surg 1998;15:85–93. [PubMed] [Google Scholar]
- 10. David Smith C, Gavin Bilmen J, Iqbal S, Robey S, Pereira M. Medial artery calcification as an indicator of diabetic peripheral vascular disease. Foot Ankle Int 2008;29:185–90. [DOI] [PubMed] [Google Scholar]
- 11. Freeman A, May K, Frescos N, Wraight PR. Frequency of risk factors for foot ulceration in individuals with chronic kidney disease. Intern Med J 2007;38(5):314–20. [DOI] [PubMed] [Google Scholar]
- 12. Apelgvist J, Bakker K, Van Houtum WH, Nabuurs‐Franssen MH, Schaper NC. International Consensus and Practical Guidelines on the management and prevention of the diabetic foot. International Working Group on the Diabetic Foot. Diab Metab Res Rev 2000;(Suppl 1): 84S–92S. [DOI] [PubMed] [Google Scholar]
- 13. Smieja M, Hunt DL, Edelman D, Etchells E, Cornuz J, Simel D. Clinical examination for the detection of protective sensation in the feet of diabetic patients. International Cooperative Group for Clinical Examination Research. J Gen Intern Med 1999;14:418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bloom S, Till S, Sonksen P, Smith S. Use of a biothesiometer to measure individual vibration thresholds and their variation in 519 non‐diabetic subjects. Br Med J (Clin Res Ed) 1984;288:1793–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young MJ, Breddy JL, Veves A, Boulton AJM. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care 1994;17:557–60. [DOI] [PubMed] [Google Scholar]
- 16. Baker JD. Assessment of peripheral arterial occlusive disease. Crit Care Nurs Clin North Am 1991;3:493–8. [PubMed] [Google Scholar]
- 17. Jude E, Gibbons J. Identifying and treating intermittent claudication in people with diabetes. Diabet Foot J 2005;8:84–92. [Google Scholar]
- 18. Schomig M, Ritz E, Standl E, Allenberg J. The diabetic foot in the dialyzed patient. J Am Soc Nephrol 2000;11:1153–9. [DOI] [PubMed] [Google Scholar]
- 19. Boulton AJ. The pathogenesis of diabetic foot problems: an overview. Diabet Med 1996;13 Suppl 1:S12–16. [PubMed] [Google Scholar]
- 20. Beckert S, Sundermann K, Wolf S, Konigsrainer A, Coerper S. Haemodialysis is associated with changes in cutaneous microcirculation in diabetes mellitus. Diabet Med 2009;26:89–92. [DOI] [PubMed] [Google Scholar]
- 21. Hinchliffe RJ, Kirk B, Bhattacharjee D, Roe S, Jeffcoate W, Game F. The effect of haemodialysis on transcutaneous oxygen tension in patients with diabetes‐a pilot study. Nephrol Dial Transplant 2006;21: 1981–3. [DOI] [PubMed] [Google Scholar]
- 22. Rith‐Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at high risk for lower‐extremity amputation in a primary health care setting. A prospective evaluation of simple screening criteria. Diabetes Care 1992;15:1386–9. [DOI] [PubMed] [Google Scholar]
- 23. Pirzada NA, Morgenlander JC. Peripheral neuropathy in patients with chronic renal failure. A treatable source of discomfort and disability. Postgrad Med 1997;102:249–50, 255–7, 261. [DOI] [PubMed] [Google Scholar]
- 24. USRDS. United States Renal Data System Annual Report. Washington DC: Government Printing Office, 2000. [Google Scholar]
- 25. O'Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999‐2000. Circulation 2004;109:320–23. [DOI] [PubMed] [Google Scholar]
- 26. DeLoach SS, Mohler ER III. Peripheral arterial disease: a guide for nephrologists. Clin J Am Soc Nephrol 2007;2:839–46. [DOI] [PubMed] [Google Scholar]
- 27. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care 1999;22:1036–42. [DOI] [PubMed] [Google Scholar]
- 28. Peters EJ, Lavery LA. Effectiveness of the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care 2001;24:1442–7. [DOI] [PubMed] [Google Scholar]


