Abstract
Venous ulcers are characterised by longstanding and recurrent loss of skin integrity. Once occurred, healing is slow and recurrence is high because of inappropriate conditions of the wound bed. This study involves 20 patients with chronic venous ulcers at least 6 weeks of duration treated with negative pressure wound therapy (NPWT). Patients underwent a radical debridement of all devitalised tissues in the first operation. After adequate haemostasis, silver‐impregnated polyurethane foam was applied. Once the wounds were determined to be clean and adequate granulation tissue formation was achieved, split‐thickness skin grafts were applied. Black polyurethane foam was applied over them. All wounds completely healed without the need for further debridement or regrafting. The mean number of silver‐impregnated foam dressing changes prior to grafting was 2·9 (one to eight changes). The mean number of NPWT foam changes was 2·6 after skin grafting (two to five changes). Two patients who did not use conservative treatments for chronic venous insufficiency (CVI) after discharge from the hospital had recurrence of venous ulcers in the follow‐up period. Application of NPWT provides quick wound‐bed preparation and complete graft take in venous ulcer treatment.
Keywords: Chronic venous insufficiency, Chronic wound, Negative pressure wound therapy, Skin graft, Venous ulcer
Venous ulcers are characterised by a longstanding and recurrent loss of skin integrity. They have irregular borders, are surrounded by a brown hyperpigmentation, and are classically located at the lateral or medial malleolus of a swollen leg 1, 2. They are expensive to treat and adversely impact patients' quality of life 1, 2, 3. The condition restricts an individual's social function and work life and affects up to 1% of the adults in developed countries 4, 5, 6. In people over the age of 65, this rate increases to 4% (7).
Ulceration is a severe clinical manifestation of chronic venous insufficiency (CVI). The prevalence of CVI ranges from 2% to 7% in the population, and CVI is responsible for about 70% of chronic ulcers of the lower limb 7, 8. CVI triggers a cascade of events leading to blood reflux from deep to superficial veins via perforating vessels, and consequently chronic venous hypertension develops 9, 10. Venous hypertension interferes with the capillary haemodynamic balance and disrupts the skin's microcirculation. Increased pressure in the venous end of the capillaries allows the large molecules and cells to escape into the interstitial fluid (11). Extracapillary fibrinogen forms pericapillary cuffs to act as a barrier and inhibits dermal collagen production (12). Red and white blood cell accumulation and plugging of the capillaries lead to tissue ischaemia 12, 13. Leucocytes secrete transforming growth factor (TGF)‐β1 to activate fibroblasts; however, TGF‐β1 is bounded by α 2‐macroglobulin, which leaks from the vessels because of venous hypertension (14). Moreover, it is shown that matrix metalloproteases are highly elevated in CVI. All these unfavourable factors lead to tissue breakdown and create a non healing environment 15, 16.
Once occurred, healing rates may be as slow as 22% at 12 weeks, and recurrence rates may be as high as 69% in 12 months because of inappropriate conditions of the wound bed 17, 18. Treatment of venous ulcers should start with efforts to decrease venous hypertension and regulate venous flow such as extremity elevation, compression bandaging, Unna boot application and vascular surgical intervention 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. Various techniques, wound dressing products, devices, cultured cells and growth factors have been proposed to improve healing and decrease the recurrence of venous ulcers 3, 19, 20, 30, 31, 32, 33, 34, 35, 36, 37, 38.
This study describes the effective use of negative pressure wound therapy (NPWT) for the treatment of recalcitrant and large venous ulcers in a series of 20 patients. NPWT was applied for the preparation of the wound bed prior to grafting and for improving the take of meshed grafts later as well.
PATIENTS AND METHODS
From August 2009 to December 2010, 20 patients with chronic venous ulcers of at least 6 weeks of duration were treated with NPWT in the Okmeydani Training and Research Hospital Plastic and Reconstructive Surgery Clinic (Table 1). Detailed written informed consent was obtained from all subjects. There were 18 male and 2 female patients with a mean age of 54·1 years. Bedridden patients, patients with diabetic or ischaemic ulcers, patients with arterial insufficiency of the lower extremity (ankle/brachial index <0·9), obese patients, and patients with existing deep venous thrombosis were not included in the study. Wound dimensions ranged between 4 × 5 cm and 25 × 35 cm.
Table 1.
Patient characteristics
| Patient | Age | Sex | Duration of the VI (years) | Duration of the existing ulcer (months) | Ulcer dimensions (cm) | Calf circumference on admittance (cm) | Calf circumference in grafting operation (cm) | Complication |
|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | 20 | 36 | 7 × 10 | 40 | 35 | NONE |
| 2 | 63 | M | 2 | 12 | 5 × 10 | 39 | 34 | NONE |
| 3 | 76 | M | 50 | 246 | 16 × 15 | 43 | 37 | Recurrence |
| 4 | 16 | M | 5 | 24 | 4 × 5 | 41 | 35 | NONE |
| 5 | 68 | F | 30 | 2 | 8 × 5 | 38 | 33 | NONE |
| 6 | 30 | M | 25 | 48 | 10 × 12 | 46 | 41 | NONE |
| 7 | 59 | M | 39 | 48 | 15 × 8 | 35 | 30 | NONE |
| 8 | 40 | M | 15 | 84 | 25 × 35 | 38 | 33 | NONE |
| 9 | 65 | M | 42 | 210 | 10 × 5 | 45 | 39 | Recurrence |
| 10 | 55 | M | 30 | 22 | 10 × 12 | 37 | 34 | NONE |
| 11 | 58 | F | 22 | 50 | 15 × 10 | 43 | 38 | NONE |
| 12 | 52 | M | 28 | 56 | 20 × 12 | 46 | 40 | NONE |
| 13 | 60 | M | 26 | 100 | 5 × 12 | 35 | 29 | NONE |
| 14 | 50 | M | 24 | 146 | 14 × 16 | 33 | 27 | NONE |
| 15 | 48 | M | 28 | 41 | 8 × 12 | 35 | 29 | NONE |
| 16 | 65 | M | 40 | 64 | 9 × 12 | 38 | 32 | NONE |
| 17 | 48 | M | 20 | 92 | 10 × 12 | 41 | 36 | NONE |
| 18 | 67 | M | 15 | 40 | 13 × 15 | 37 | 33 | NONE |
| 19 | 45 | M | 22 | 64 | 7 × 8 | 38 | 34 | NONE |
| 20 | 55 | M | 30 | 258 | 6 × 7 | 35 | 33 | NONE |
VI, venous insufficency.
All patients received standardised conservative treatment for their leg ulcers upon admission (elevation, compressing bandaging and dressings). To document the presence of venous insufficiency, patients underwent a continuous wave Doppler evaluation of deep veins and perforators of both legs. Calf circumference was measured upon admission and on every dressing change at the mid‐point of a line drawn between the lateral malleolus and fibular head. Multiple incisional biopsies for pathological examination and tissue samples for bacterial culture were obtained. IV antibiotics (cefazolin) were started empirically. Antibiotic regimens were changed considering the culture results. Vancomycin was administered for meticilline‐resistant Staphylococcus aureus (MRSA) and Ciprofloxacin was administered for P. aeruginosa in appropriate doses upon the results of antibiogram tests.
Patients underwent a radical debridement of all chronic and infected granulation tissue in the first operation. All necrotic and unhealthy materials were excised tangentially down to clean and bleeding tissues. After adequate haemostasis, silver‐impregnated polyurethane foam (Granufoam Silver, KCI, San Antonio, TX) was applied and the pump was set to a continuous negative pressure of 125 mm Hg. Dressings were changed on alternate days, and the amounts of discharge and calf circumference were noted (1, 2, 3, 4). Once the wounds were determined to be clean by clinical assessment and adequate granulation tissue formation was achieved, meshed split‐thickness skin grafts were applied. The grafts were fixed with skin staples, and standard black polyurethane foam (Granufoam, KCI, San Antonio, TX) was applied over them with a continuous negative pressure of 75 mm Hg. The NPWT dressings were changed on the 3rd day and every other following day. NPWT application was discontinued when it was considered that the grafts had completely taken (Figures 5, 6, 7, 8, 9
Figure 1.
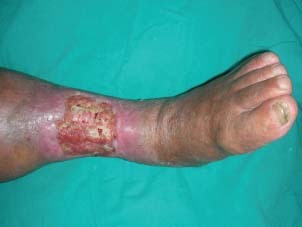
A large venous ulcer at the anterior aspect of the left cruris.
Figure 2.
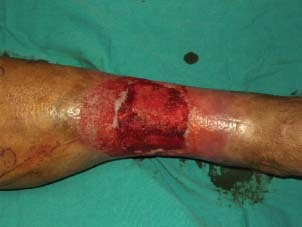
The wound was clean and granulated after debridement and two sessions of silver impregnated foam application.
Figure 3.
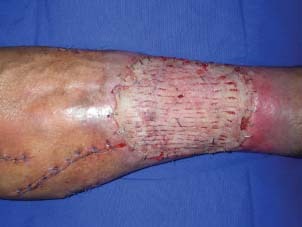
The graft considered to be completely taken on postoperative 5th day.
Figure 4.
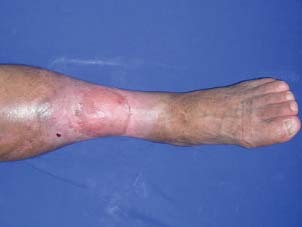
Appearance of the patient on postoperative 6th month.
Figure 5.
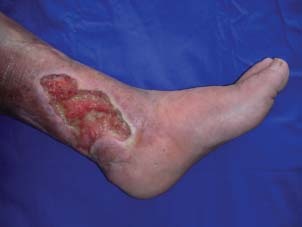
Large venous ulcer at the medial aspect of the left cruris.
Figure 6.
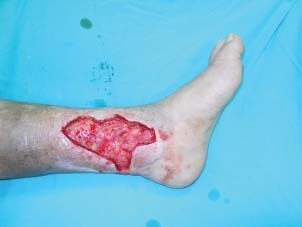
The wound was radically debrided down to health tissues.
Figure 7.
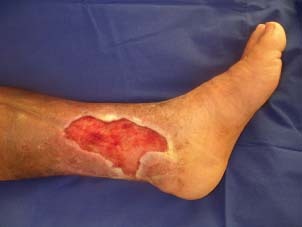
The wound was clean and granulated after silver impregnated foam application for 5 days.
Figure 8.
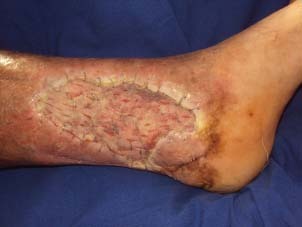
The graft seemed completely taken on postoperative 5th day.
Figure 9.
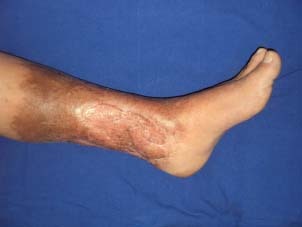
The appearance of the patient 1‐year after grafting.
The patients were examined on monthly follow‐up visits after discharge and their accordance with the preventive methods were checked.
RESULTS
Patients were followed between 6 and 22 months (mean: 17·4 months).
The mean duration of venous insufficiency in the selected patient group was 25·6 (2–50 years) years, and the mean duration of the existing ulcer was 91·1 months (2–246 months) (Table 1).
The mean calf circumference was measured as 39·1 cm (33–46 cm) on admittance. The mean calf circumference rapidly decreased to 34·1 cm (27–41 cm) after NPWT application to prepare the wound for grafting (Table 1).
While 16 wounds were infected with P. aeruginosa, 7 wounds had a mixed infection of P. aeruginosa and MRSA. S. aureus was the only microorganism isolated from two patients (Table 2).
Table 2.
Isolated microorganism and number of dressing changes
| Patient | Isolated microorganism | Number of dressing changes prior to grafting | Number of dressing changes after grafting | Days of hospital stay |
|---|---|---|---|---|
| 1 | P | 2 | 2 | 15 |
| 2 | P | 2 | 2 | 12 |
| 3 | P + MRSA | 8 | 3 | 43 |
| 4 | MRSA | 2 | 2 | 13 |
| 5 | P | 1 | 3 | 15 |
| 6 | P + MRSA | 1 | 2 | 10 |
| 7 | P | 5 | 3 | 24 |
| 8 | P + MRSA | 4 | 3 | 18 |
| 9 | P + MRSA | 2 | 5 | 15 |
| 10 | S | 1 | 2 | 14 |
| 11 | MRSA | 2 | 3 | 12 |
| 12 | P | 3 | 4 | 21 |
| 13 | P + MRSA | 4 | 2 | 18 |
| 14 | P | 3 | 2 | 13 |
| 15 | P | 2 | 3 | 16 |
| 16 | P | 6 | 2 | 19 |
| 17 | P + MRSA | 3 | 2 | 12 |
| 18 | S | 2 | 3 | 21 |
| 19 | P + MRSA | 4 | 2 | 15 |
| 20 | P | 2 | 2 | 13 |
P, Pseudomonas aeruginosa; S, Staphylococcus aureus; MRSA, meticilline‐resistant Staphylococcus aureus.
Pathologic examination of tissue biopsies showed chronic inflammation with layers of fibrin deposits surrounding capillaries and increased number of dermal capillaries. There was no sign of malignant differentiation in any case.
Only one silver‐impregnated foam application for 2 days was enough in three patients for preparing the wound for skin grafting. The mean number of silver‐impregnated foam dressing changes prior to grafting was 2·9 (one to eight changes). The mean number of NPWT foam changes was 2·6 after skin grafting (two to five changes) (Table 2). The mean duration of hospital stay was 16·9 days (10–43 days).
All wounds completely healed without the need for further debridement or regrafting. The NPWT dressings were well tolerated by the patients. None of the patients complained about the pain limiting the application of negative pressure.
Two patients who did not use conservative treatments for CVI after discharge from the hospital had recurrence of venous ulcers in the follow‐up period.
DISCUSSION
Topical application of negative pressure has been shown to improve wound healing in a number of settings from acute traumatic injuries to chronic and infected wounds 39, 40, 41. Moreover, it has been used to prepare wound beds for grafting or flap closure (42).
Controlled experiments in animals indicated that the reduction of the interstitial fluid by means of NPWT significantly enhances the wound healing and oedema. In our opinion, NPWT has substantial contribution in the rapid decrease of the calf circumference in our patients. It is assumed that the reduction of interstitial oedema facilitates the local blood circulation and thus reduces the mechanical limitation of the capillary flow (43). In addition, increased granulation tissue, decreased bacterial levels and increased cell growth have been noticed in NPWT‐treated wounds 44, 45.
Chronic venous ulcers are commonly colonised by bacteria such as P. aeruginosa and S. aureus (46). Chronic infection prolongs healing and may increase ulcer size over time. Decreasing the bacterial counts is of paramount importance in the wound‐bed preparation for skin grafting. Using an acute wound swine model that was treated with NPWT, Morkywas et al. showed a marked decrease in bacterial counts of quantitative cultures (43). Gerry et al. presented two infected venous ulcer cases where they used silver‐impregnated NPWT foam effectively in wound‐bed preparation for grafting (47). They placed the silver‐impregnated foam to secure the grafts as well. Despite its advantages in treating contaminated wounds, silver has been shown to be cytotoxic to human keratinocytes (48). Therefore, we used the silver‐impregnated foam prior to grafting to clear the wound and replaced it with cheaper black NPWT foam to secure the graft and drain the exudates.
NPWT has been shown to increase the graft take and graft quality by changing the passive process of inosculation into an active one and removing the blood and exudates that may prevent the graft from adhering 42, 44. In their recent study of patients with chronic leg ulcers, Körber et al. showed complete healing in 92·9% of meshed grafts in which they had applied negative pressure to secure the grafts in spite of 67·4% graft take in the control group (49). However, they reported significantly lower graft take rates (71·4%) in patients with venous ulcers. Although our study does not contain a control group, we showed that 100% graft take can be accomplished in patients with venous ulcers. Thanks to the NPWT, the graft is adhered to its bed strictly by the negative pressure, and interstitial oedema resulting from the venous insufficiency is effectively removed.
Despite NPWT dressings and devices being more expensive than other wound‐care products, cost‐benefit analyses show lower treatment costs. In their randomised controlled trial, Vuerstack et al. showed significant shorter wound‐preparation time and faster complete healing compared with the control group (50). They emphasised that higher treatment costs of the control group were created by higher personnel costs and longer hospitalisation time due to slower healing.
CONCLUSION
This study describes the effective and efficient use of NPWT in the treatment of chronic and recalcitrant venous ulcers. Although chronically colonised wounds are often refractory to definitive treatment, we achieved 100% wound closure with split‐thickness skin grafts in 20 patients. Application of NPWT provided quick wound‐bed preparation and complete graft take in venous ulcer treatment. Further controlled and randomised trials are needed to show the benefits of NPWT in the treatment of venous ulcers.
REFERENCES
- 1. Etufugh CN, Phillips TJ. Venous ulcers. Clin Dermatol 2007;25:121–30. [DOI] [PubMed] [Google Scholar]
- 2. Van Gent WB, Wilschut ED, Wittens C. Management of venous ulcer disease. BMJ 2010;341:1092–96. [DOI] [PubMed] [Google Scholar]
- 3. Ruckley C. Socioeconomic impact of chronic venous insufficiency and leg ulcers. Angiology 1997;46:67–9. [DOI] [PubMed] [Google Scholar]
- 4. Leclere FM, Puechguiral IR, Rotteleur G, Thomas P, Mordon SR. A prospective randomized study of 980 nm diode laser‐assisted venous ulcer healing on 34 patients. Wound Rep Reg 2010;18:580–85. [DOI] [PubMed] [Google Scholar]
- 5. Ghauri ASK, Nyamekye I, Grabs AJ, Farndon JR, Whyman MR, Poskitt KR. Influence of a specialized leg ulcer service and venous surgery on the outcome of venous leg ulcers. Eur J Endovasc Surg 1998;16:38–244. [DOI] [PubMed] [Google Scholar]
- 6. Fowkes FGR. Epidemiology of chronic venous insufficiency. Phlebology 1996;11:2–5. [Google Scholar]
- 7. Callam MJ. Epidemiology of varicose veins. Br J Surg 1994;81:167–73. [DOI] [PubMed] [Google Scholar]
- 8. Berqvist D, Lindholm C, Nelzen O. Chronic leg ulcers: the impact of venous disease. J Vasc Surg 1999;29:752–5. [DOI] [PubMed] [Google Scholar]
- 9. Abbade LPF, Lastoria S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol 2005;44:449–56. [DOI] [PubMed] [Google Scholar]
- 10. Olivencia JA. Pathophysiology of venous ulcers: surgical implications review and update. Dermatol Surg 1999;25:880–5. [DOI] [PubMed] [Google Scholar]
- 11. Browse NL, Burnard KG. The cause of venous ulceration. Lancet 1982;2:243–5. [DOI] [PubMed] [Google Scholar]
- 12. Falanga V. Venous ulceration. J Dermatol Surg Oncol 1993;19:764–71. [DOI] [PubMed] [Google Scholar]
- 13. Scott H, Coleridge‐Smith P, Scurr J. Histologic study of white blood cells and their association with lipodermosclerosis and venous ulceration. Br J Surg 1991;78:210–1. [DOI] [PubMed] [Google Scholar]
- 14. Highley HR, Ksander GA, Gerhardt CO, Falanga V. Extravasation of macromolecules and possible trapping of transforming growth factor‐beta in venous ulceration. Br J Dermatol 1995;132:79–85. [DOI] [PubMed] [Google Scholar]
- 15. Raffeto JD, Khalil RM. Matrix metalloproteinases in venous tissue remodeling and varicose vein formation. Curr Vasc Pharmacol 2008;6:158–72. [DOI] [PubMed] [Google Scholar]
- 16. Wysocki AB, Staiano‐Coico L. Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol 1993;101:64–8. [DOI] [PubMed] [Google Scholar]
- 17. Moffatt CJ, Franks PJ, Bosanquet OM., Brown P, Greenhalgh RM, McCollum CN. Community clinics for leg ulcers and impact on healing. BMJ 1992;305:1389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monk BE, Sarkany I. Outcome of treatment of venous stasis ulcers. Clin Exp Dermatol 1982;7:397–400. [DOI] [PubMed] [Google Scholar]
- 19. Patel NP, Labropoulos N, Pappas PJ. Current management of venous ulceration. Plas Reconstr Surg 2006;117(7 Suppl):254S–60S. [DOI] [PubMed] [Google Scholar]
- 20. Raffetto JD, MArston WA. Venous ulcer: what is new? Plas Reconstr Surg 2011;127(1 Suppl):279S–88S. [DOI] [PubMed] [Google Scholar]
- 21. Smith PC. Daflon 500 mg and venous leg ulcer: new results from a meta analysis. Angiology 2005;56(1 Suppl):33S–9S. [DOI] [PubMed] [Google Scholar]
- 22. Milic DJ, Zivic SS, Bogdanovi DC, Jovanovic MM, Jankovic RJ, Milosevic ZD, Stamenkovic DM, Trenkic MS. The influence of different sub‐bandage pressure values on venous leg ulcer healing when treated with compression therapy. J Vasc Surg 2010;51:655–61. [DOI] [PubMed] [Google Scholar]
- 23. Falanga V, Fujitani RM, Diaz C, Hunter G, Jorizzo J, Lawrence PF, Lee BY, Menzoian JO, Tretbar LL, Holloway GA, Hoballah J, Seabrook GR, McMillan DE, Wolf W. Systemic treatment of venous leg ulcers with high doses of pentoxifylline: efficacy in a randomized, placebo controlled trial. Wound Repair Regen 1999;7:208–13. [DOI] [PubMed] [Google Scholar]
- 24. Belcaro G, Cesarone MR, Ericchi BM, Ledda A, Di Renzo A, Stuard S, Dugall M, Pellegrini L, Rohdewald P, Ippolito E, Ricci A, Cacchio M, Ruffini I, Fano F, Hosoi M. Venous ulcers: microcirculatory improvement and faster healing with local use of Pycnogenol. Angiology 2005;56:699–705. [DOI] [PubMed] [Google Scholar]
- 25. Word R. Medical and surgical therapy for advanced chronic venous insufficiency. Surg Clin N Am 2010;90:1195–214. [DOI] [PubMed] [Google Scholar]
- 26. Neequaye SK, Douglas Ad, Hofman D, Wolz M, Sharma R, Cummings R, Hands L. The difficult venous ulcer: Case series of 177 ulcers referred for vascular surgical opinion following failure of conservative management. Angiology 2009;60:492–495. [DOI] [PubMed] [Google Scholar]
- 27. TenBrook Jr JA , Iafrati MD, O’Donnell Jr TF , Wolf MP, Hoffman SN, Pauker SG, Lau J, Wong JB. Systemic review of outcomes after surgical management of venous disease incorporating subfascial endoscopic perforator surgery. J Vasc Surg 2004;39:583–9. [DOI] [PubMed] [Google Scholar]
- 28. Chong TW, Bott MJ, Kern JA, Peeler BB, Tribble CG, Harthun NL. Subfascial endoscopic perforator vein surgery (SEPS) for the treatment of venous ulcers. Ostomy Wound Manage 2005;51:26–91. [PubMed] [Google Scholar]
- 29. Nelzen O, Fransson I. True long‐term healing and recurrence of venous leg ulcers following SEPS combined with superficial venous surgery: a prospective study. Eur J Vasc Endovasc Surg 2007;34:605–12. [DOI] [PubMed] [Google Scholar]
- 30. Raju S, Fredericks R. Valve reconstruction procedures for non obstructive venous insufficiency: rationale, techniques, and results in 107 procedures with two‐to eight‐year follow up. J Vasc Surg 1988;7:301–10. [DOI] [PubMed] [Google Scholar]
- 31. Top H, Benlier E, Aygit AC, Kiyak M. Distally based sural flap in treatment of chronic venous ulcers. Ann Plast Surg 2005;55:160–5. [DOI] [PubMed] [Google Scholar]
- 32. Steed DL. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg 2006;117(7 Suppl):143S–9S. [DOI] [PubMed] [Google Scholar]
- 33. Martin LK, Kirsner RS. Use of a meshed bilayered cellular matrix to treat a venous ulcer. Adv Skin Wound Care 2002;15:260–2. [DOI] [PubMed] [Google Scholar]
- 34. Omar AA, Mavor AID, Jones AM, Homer‐Vanniasinkam S. Treatment of venous leg ulcers with dermagraft. Eur J Vasc Endovasc Surg 2004;27:666–72. [DOI] [PubMed] [Google Scholar]
- 35. Saap LJ, Donohue K, Falanga V. Clinical classification of bioengineered skin use and its correlation with healing of diabetic and venous ulcers. Dermatol Surg 2008;30:1095–100. [DOI] [PubMed] [Google Scholar]
- 36. Renner R, Simon JC. Mathematical modeling of venous ulcer healing rates after implantation of keratinocytes: new ways to predict the efficacy of wound healing after regenerative methods. Wound Repair Regen 2010;18:624–8. [DOI] [PubMed] [Google Scholar]
- 37. Tawfick W, Sultan S. Does topical wound oxygen (TWO2) offer and improved outcome over conventional compression dressing (CCD) in the management of refractory venous ulcers (RVU)? A parallel observational comparative study. Eur J Vasc Endovasc Surg 2009;38:125–132. [DOI] [PubMed] [Google Scholar]
- 38. Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard‐to‐heal venous ulcers. Wound Repair Regen 1999;7:201–7. [DOI] [PubMed] [Google Scholar]
- 39. Labanaris AP, Polykandriotis E, Horch RE. The effect of vacuum‐assisted closure on lymph vessels in chronic wounds. J Plast Reconstr Aesth Surg 2009;62:1068–75. [DOI] [PubMed] [Google Scholar]
- 40. Bhattacharrya T, Mehta P, Smith RM, Pomanac B. Routine use of wound vacuum assisted closure does not allow coverage delay for open tibial fractures. Plast Reconstr Surg 2008;121:1263–6. [DOI] [PubMed] [Google Scholar]
- 41. Evans D, Land L. Topical negative pressure dressing for treating chronic wounds: a systemic review. Br J Plast Surg 2001;54:238–42. [DOI] [PubMed] [Google Scholar]
- 42. Moisidis E, Heath T, Brooer C, Ho K, Deva AK. A prospective, blinded, randomized controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004;114:917–22. [DOI] [PubMed] [Google Scholar]
- 43. Morkywas MJ, Argenta LC, Shelton‐Brown EI, Mc Guirt W. Vacuum‐assisted closure, a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–562. [DOI] [PubMed] [Google Scholar]
- 44. Argenta LC, Morkywas MJ, Marks MW, DeFranzo AJ, Molnar JA, David LR. Vacuum assisted closure: state of clinic art. Plast Reconstr Surg 2006;117(7 Suppl):127S–42S. [DOI] [PubMed] [Google Scholar]
- 45. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: Microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–1096. [DOI] [PubMed] [Google Scholar]
- 46. Halbert AR, Stacey MC, Rohr JB, Jopp‐McKay A. The effect of bacterial colonization on venous ulcer healing. Australias J Dermatol 1992;33:75–80. [DOI] [PubMed] [Google Scholar]
- 47. Gerry R, Kwei S, Bayer L, Breuing KH. Silver‐impregnated vacuum‐assisted closure in the treatment of recalcitrant venous stasis ulcers. Ann Plast Surg 2007;59:58–62. [DOI] [PubMed] [Google Scholar]
- 48. Paddle‐Ledinek JE, Nasa Z, Cleland HJ. Effect of different wound dressings on cell viability and proliferation. Plast Reconstr Surg 2006;117(7 Suppl):110S–8S. [DOI] [PubMed] [Google Scholar]
- 49. Körber A, Franckson T, Grabbe S, Dissemond J. Vacuum assisted closure device improves the take of mesh grafts in chronic leg ulcer patients. Dermatology 2008;216:250–6. [DOI] [PubMed] [Google Scholar]
- 50. Vuerstack JDD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JCJM. State‐of‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (VAC) with modern wound dressings. J Vasc Surg 2006;44:1029–37. [DOI] [PubMed] [Google Scholar]


