Abstract
The aim of the therapy in open tibial fractures grade III was to cover the bone with soft tissue and achieve healed fracture without persistent infection. Open tibial fractures grade IIIC with massive soft tissue damage require combined orthopaedic, vascular and plastic–reconstructive procedures. Negative‐pressure wound therapy (NPWT), used in two consecutive cases with open fracture grade IIIC of the tibia diaphysis, healed extensive soft tissue defect with exposure of the bone. NPWT eventually allowed for wound closure by split skin graft within 21–25 days. Ilizarov external fixator combined with application of recombinant human bone morphogenetic protein‐7 at the site of delayed union enhanced definitive bone healing within 16–18 months.
Keywords: NPWT, open tibial fracture grade IIIC, rhBMP‐7; V.A.C.‐therapy
Introduction
Open tibial fractures Gustilo–Anderson grade IIIC represent a wide spectrum of injury to the bone and soft tissues. Most severe is segmental bone loss combined with circular soft tissue damage. Invasive infection together with the injury to blood vessels and nerves can be reason for limb loss. The aim of the therapy in open fractures was to achieve good soft tissue coverage and healing of the fracture without presence of infection 1. Some authors recommend as primary bone stabilisation a half pin external fixation or even plating, whereas other advocate primary or delayed intramedullary nailing even in types IIIB and IIIC 2, 3, 4, 5, 6. Radical debridement of the wound outside the zone of injury, skeletal stabilisation and early soft tissue coverage with a vascularised muscle flap are regarded as ideal management 7, 8. After bone fixation, usually multiple debridements are performed in order to prevent infection of the bone and achieve good soft tissue coverage over the bone. Waste and deep damage of skin and muscle are difficult to manage in orthopaedic ward without plastic–reconstructive department.
Negative‐pressure wound therapy (NPWT) states a potential method to manage soft tissue defect combined with high‐grade open fractures. Positive effect of subatmospheric pressure on healing of the infected wound in humans was first described by Kostiuchenko in 1986 9 and in 1991 by Davydov 10. In 1997, Argenta and Morykwas published the results of their own studies on wound healing under negative‐pressure conditions 11, 12. Negative pressure of 125 mmHg increases vascularity and blood flow by 400% in the wound, whereas negative pressure of 400 mmHg completely inhibits blood flow in the granulation tissue 13, 14. NPWT accelerates protein and collagen production, cell replication and reduces bacterial colonisation of the wound. It has been clinically and economically proven in the treatment of chronic wounds 15, 16. The Food and Drug Administration (FDA) approved NPWT in 1995 the treatment of acute and chronic post‐traumatic wounds. In 2000, indications for NPWT were extended to the use of skin graft, and in 2002 for the therapy of burn wounds. Open fractures combined with extensive soft tissue defect represent field of expected benefits from NPWT 17, 18. Hermetic dressing and container with filter for exsudate permits to keep the infection under control and allows patient to be mobile. The NPWT dressings are very demanding and time‐consuming when applied to the extremity stabilised with external fixator–multiple pins or K‐wires.
The reconstruction of consisting or potential bone defect is even important for extremity salvage in open tibial fracture grade III as the soft tissue condition. Lack of bone stock or prolonged bone healing decreases the chance to gain functional extremity in acceptable time. Osteogenic protein‐1 (OP‐1) is a natural protein inducing bone formation. Animal experiments have shown that local implantation of OP‐1 [synonym bone morphogenetic protein‐7(BMP‐7)] in combination with a collagen matrix stimulates repair of long bone defects and the formation of new bone in spinal fusion surgery 19. Clinical trials investigating OP‐1 applications have provided supportive evidence for the use of OP‐1 in the treatment of distal tibial fractures 20, open tibial fractures 21, tibial non‐unions 22, 23, 24 and atrophic long bone non‐unions 25. BMPs have been effective as a replacement or an adjunct in healing of graft for reconstruction of femur, tibia and fibula diaphyseal defects 26, 27, 28. First recombinant human BMP for clinical administration – rhBMP‐2 (dibotermin alfa) was approved by the FDA in 2004 with the name InFUSE (Medtronic, Minneapolis, MN.), and in 2003 in Europe with the name InductOs® (Wyeth Pharmaceuticals, Dublin, UK). The other clinically used BMP implant – rhBMP‐7 (eptotermin alfa) is available under the brand name Osigraft® (UK) or OP‐1 Putty (USA). The maximum recommended dose of rhBMP‐7 is 2 g as the effectiveness of higher doses has not been established. Positive effect of BMP appears to overweight over side‐effects such as heterotopic bone formation and antibody production against BMP itself and collagen carrier.
This article presents author's experience in the simultaneous use of NPWT and rhBMP‐7 in complex therapy of two consecutive cases with open tibial fractures grade III C with soft tissue defect and infected delayed bone union.
Materials and methods
NPWT was used at the Department of Orthopedics and Traumatology Medical University of Warsaw in 25 cases with acute and chronic wounds. In all cases, a V.A.C. System® (KCI, San Antonio, TX) was used with polyurethane foam having a pore size range of 400–600 µm. Two cases with open tibial fracture grade IIIC were treated with Ilizarov external fixation, NPWT and addition of one vial Osigraft® (Howmedica International S. de R.L. Division of Stryker Corporation, Raheen Industrial Estate, Limerick, Ireland). Each vial of Osigraft® contains 3·5 mg rhBMP‐7 as powder for suspension (3·5 mg of rh‐BMP‐7 in 1 g of vacuum‐dried collagen). In each case, suspension was prepared and rhBMP‐7 was activated by addition of 2·5 ml of sterile 0·9% sodium chloride solution.
Case 1
A 25‐year‐old woman sustained a comminuted open fracture grade IIIC of right tibia and closed proximal fibular fracture in 2006. Tibia was first stabilised with bridging external fixator (Figure 1). Soft tissues were damaged over the distal half of the leg involving anterior and posterior tibial vessels, peroneal and tibial nerve. Circulation on the foot was clinically sufficient and the foot was in good condition. Staged debridements were performed on days 5 and 8 after accident, which resulted in almost circular muscle defect with exception of lateral and posterior compartment over fibula. Removal of necrotic tibia fragments resulted in segmental diaphyseal defect of 14 cm (Figure 2). Digital subtraction angiography revealed maintained contrast flow in peroneal artery and lack of contrast blood flow in tibialis anterior and tibialis posterior artery. Patient refused amputation. NPWT was applied every 2–3 days after debridements (Figures 3 and 4). On day 21, wound was covered by split skin graft. Antibiotics were administered at the beginning of treatment, first intravenously, as pre‐emptive treatment with amoxicillin. After wound culture, clindamycin and ciprofloxacine were used. The use of antibiotics was discontinued during NPWT as no signs of invasive infection were present. After complete graft healing, reconstruction of segmental bone defect was performed by internal transport of ipsilateral fibula diaphysis, covered by soft tissue. Free vascularised bone with muscle flap was in author's opinion not possible because of presence of only one artery supplying the foot. After 3 months, the bridging fixator was exchanged into Ilizarov fixator. Osteotomy of fibula diaphysis was performed at two levels, separating fragment of 14‐cm fibula intended to be docked is tibia defect (Figure 5). Three K‐wires with bold tip were introduced from the lateral fibula cortex and attached to the traction system on the medial side of Ilizarov fixator. Traction was performed at a rate of 1 mm per day. Neither invasive local infection nor exposure of the distal tibial fragment occurred. After 3 and 5 weeks respectively, accessory modification of external frame configuration was performed. After 2 months, fibula fragment was placed in the tibia defect. Traction system was changed from three oblique to two transverse K‐wires, and after 5 months of therapy with Ilizarov fixator, bony fusion in distal docking site appeared (Figure 6). Malunion was still present at proximal docking site despite of the attempt of accessory traction of fibula. Twelve months after accident, rhBMP‐7 was introduced in the space between corresponding surfaces of tibia and fibula at the proximal docking site. Twelve months after stabilisation with Ilizarov fixator (16 months after accident), complete soft tissue healing and bony fusion of transported fibula in tibia defect were achieved. Clinical results after 5 years are shown in Figure 7A and B, and radiological outcome in Figure 8A and B.
Figure 1.
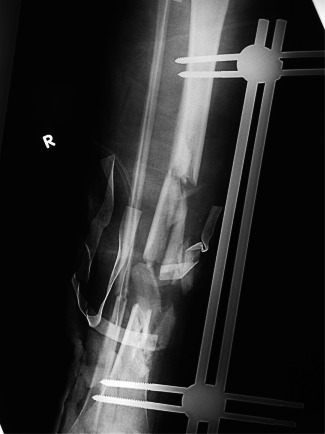
Radiograph of open tibia fracture grade IIIC after stabilisation of tibia with bridging external fixator.
Figure 2.
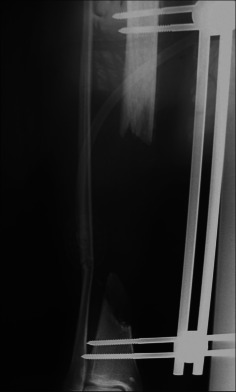
Antero‐posterior radiograph of segmental tibia defect.
Figure 3.
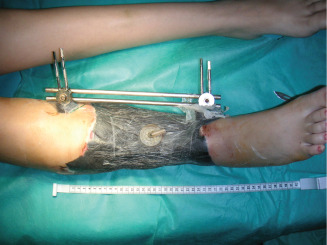
Application of VAC dressing.
Figure 4.
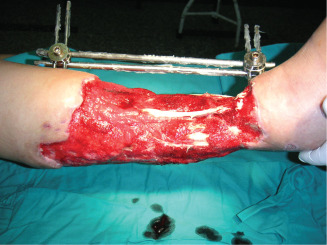
Appearance of the granulating tissue after NPWT.
Figure 5.
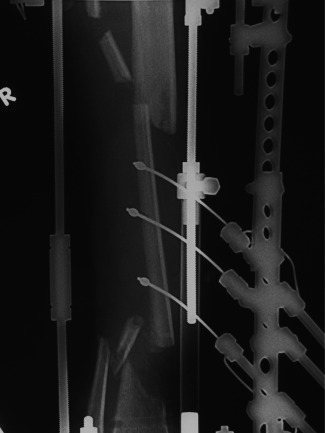
Radiograph showing osteotomy of fibula diaphyseal fragment intended to be docked within tibia defect – stage before final docking.
Figure 6.
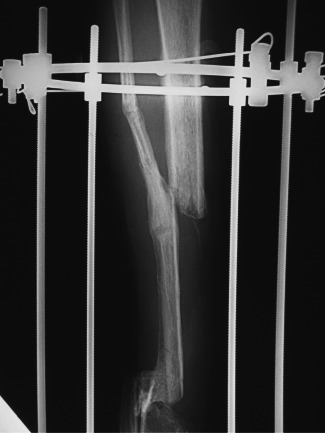
Radiograph showing fusion at distal docking site and non‐union at proximal docking site.
Figure 7.
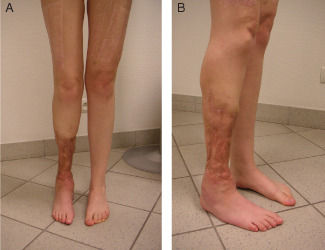
(A) and (B) Final appearance of the leg after 5 years.
Figure 8.
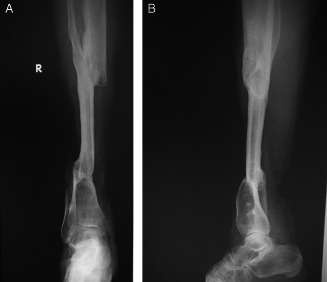
(A) and (B) Radiograph showing bone fusion of transported fibula in tibia defect.
Case 2
A 36‐year‐old man sustained open fracture grade IIIC of distal tibia diaphysis and open fibula fracture. Skin and muscles were completely necrotic on the medial side, but the muscles in the lateral and posterior compartments were spared. Anterior and posterior tibial vessels, and peroneal nerve were damaged. Fracture was stabilised with bridging external fixator. The infected wound was managed with NPWT, and after 25 days, covered with split skin graft. Initially, amoxicillin was administered intravenously and – after identification of Acinetobacter baumani in specimens from the wound, meropenem was given intravenously – according to the cultures, even during VAC therapy. The use of antibiotic was terminated after healing of skin graft. The course of therapy was complicated by osteomyelitis – medullary type. Reaming, brushing and irrigation of the medullary cavity were performed by local application of collagen sponge impregnated with 130 mg gentamicin and 2 G meropenem. During reaming of tibia fragments, the pins of external fixator were removed and then exchanged with new conical pins. Because of non‐union of tibia after 13 months, bridging external fixator was exchanged into Ilizarov fixator, and rhBMP‐7 was introduced in the space between proximal and distal tibia fragment, partially in the medullary cavity. Finally, fracture healed after 18 months.
Results
In case 1, bone reconstruction and fusion were finally obtained after 16 months and foot maintained in a nearly neutral position. Three years after accident, this young woman was married and pregnant. After 5 years, the patient was able to walk without crutches, using Toe‐Off orthosis and the foot was free from any trophic changes, despite only moderate sensory recovery, that is, protective skin sensation on the plantar surface of the foot. In case 2, bone union occurred after 18 months and the foot was finally in the 10° plantar flexion. After 5 years, the patient was ambulatory with crutches, using Toe‐Off orthosis and has maintained normal skin sensation on the plantar surface but diminished sensation on the dorsal surface of the foot. Both patients assessed subjectively the final result as satisfactory, taking into account the risk of limb loss. Both initially refused limb amputation, and after completing the therapy, they were convinced that the decision from their point of view was justified.
Discussion
The management of open tibial fractures grade IIIC is demanding due to the complexity of problems. Results are not always satisfactory and risk of limb loss remains still high. Although NPWT is an established method for wound therapy and BMPs have been used successfully in accelerating bone fusion in fractures, the use of both NPWT and rhBMP‐7 in the management of grade IIIC open tibial fractures has little support in the literature.
NPWT effectively removes purulent exsudate from wound and allows for quicker skin graft incorporation 18. This positive effect of NPWT on healing of traumatic wound has been proven in open tibial fractures grades IIIA and IIIB. Labler et al. 17 described the superior effect on healing of post‐traumatic wounds with the use of NPWT as compared with Epigard. NPWT reduced the frequency of deep infection in open tibial fractures IIIA or IIIB from 50% to 14% and delayed fracture healing from 25% to 7%.
Bone fusion without infection plays an equally important role as soft tissue condition in open tibial fractures grade IIIC for extremity salvage. Fracture healing can be improved by biophysical stimulation or by administration of biologically active substances, such as platelet‐rich plasma or bone grafts. The introduction of BMPs improved bone healing in fractures and during secondary reconstruction of bone defects. Actually, rhBMP‐2 is approved for the use in open fractures, whereas rhBMP‐7 currently represents an off‐label use. The BESTT study group showed positive effect of 1·5 mg/ml rhBMP‐2 addition on fracture healing in a clinical trial involving 450 tibial shaft fractures 28. The trial also included open tibial shaft fractures grade IIIA–B, treated with intramedullary fixation. The use of rhBMP‐2 was combined with lesser number of secondary interventions – 46% versus 26% compared with standard care including nail fixation and routine wound management. In the study by Swiontkowski et al. 21, clinical efficacy of rhBMP‐2 in the treatment of type‐IIIA and B open tibial fractures was reflected by decreased number of invasive accessory interventions from 28% to 9% and infections from 40% to 21%.
Application of rhBMP‐7 in fibular defects showed better bone formation and bridging of the segmental bone defects. RhBMP‐7 has also been effective as osteoinductive factor with the overall success rate of 82% in cases of fracture non‐unions, periproshtetic fractures, osteotomies, distraction osteogenesis, free fibular graft and joint arthrodesis 29. Nauth et al. 30 studied the use of rhBMP‐7 in Canadian trauma centres. Open tibial fractures were managed with initial debridement and intramedullary locking nail. The group of 124 tibia fractures included only few grade III fractures. Addition of 3·5 mg of rhBMP‐7 to the fracture site at the time of definitive wound closure was combined with lesser number of secondary interventions due to non‐union, decrease of pain after 12 months but have no influence on infection. In another study involving 20 distal tibia fractures (only two open) treated with hybrid external fixator, Ristiniemi 20 showed that the use of rhBMP‐7 posed faster bone fusion and return to work than in control group without rhBMP‐7.
Segmental bone defect accompanying open fracture can be treated by bone grafting alone or with addition of stimulating substances or bone substitutes. Alternative modality is distraction osteogenesis with external fixator or intramedullary nail. An important problem during bone transport is obtaining bone fusion at docking site. Effectiveness of rhBMP‐2 as adjunct in reconstruction of post‐traumatic segmental defects of tibia was proved by Jones 29. From 30 tibia fractures, 24 were grade IIIA or IIIB. Bone defect of 1–7 cm was managed either with autogenous bone graft or allogenic cancellous bone with rhBMP‐2. Reconstruction was performed at a median of 11 weeks after injury. The results regarding fracture healing, reinterventions or functional outcome were equivalent. But addition of rhBMP‐2 was superior over autogenous bone graft as regards intraoperative bleeding and avoiding the morbidity combined with graft harvesting.
The problem of cost‐effectiveness of therapy with NPWT and BMP has been proven in Level 1 studies 31. NPWT reduces costs of wound therapy, compared with conventional therapy, through combination of improved outcomes and reduced use of nursing resources (i.e. fewer dressing changes). But certainly – calculations made in one country may not be generalised in other countries. Economic aspects of the administration of BMPs in open fractures have also been investigated. Several studies have shown that clinical use of BMP‐2 and BMP‐7 is justified from the health economic point of view. In comparative study of BMP‐7 and autogenous bone graft, the treatment with BMP‐7 had the same effectiveness, but the cost of therapy with BMP‐7 was 6·78% higher owing to the price of BMP‐7 preparation 32. Alt et al. proved that additional use of rhBMP‐2 in grades IIIA and IIIB open tibial fractures is economically justified 33. It allows – at least in UK, Germany and France – for cost savings compared with intramedullary nailing and soft tissue management alone. Total savings from socio‐economic perspective in mentioned countries exceeded the price of rhBMP‐2.
The application of NPWT in presented cases posed wound healing within 21–25 days, allowed for skin grafting and thus definitive coverage of exposed bone. The use of rhBMP‐7 both at docking site in fibular transfer and at tibia non‐union in author's opinion improved and stimulated bone fusion, which was achieved after 16 and 18 months, respectively. Despite effectiveness of NPWT in reducing critical bacterial colonisation, the complexity of tissue damage in case 2 was cause of developing invasive bacterial infection of the bone, which if not recognised and not managed, might have lead to segmental osteomyelitis and potentially to amputation. The osteomyelitis – intramedullary type, in case 2 was effectively managed by temporary removal of pins, reaming of medullary cavity and local application of antibiotic carrier, according to protocol of therapy of orthopaedic device‐related infections 34.
An alternative to NPWT may be biological skin substitutes. They provide temporary or permanent coverage of the wound either as biosynthetic skin substitutes or cultured autologous engineered skin. Their advantage is the availability in large quantities and minimal risk of infection or immunological response, but their costs limit the use of these products 35. Growth factors and biologic wound products such as cytokines are also aimed to accelerate healing by augmenting or modulating inflammatory mediators, but are still under investigation.
In author's experience in case of open tibial fracture IIIC with extensive soft tissue defect, NPWT increases the chance of limb salvage, without microsurgical flaps, by stimulating granulation tissue, even on partially exposed bone. The microsurgical flaps have limited usefulness in case of damage of two from three arteries supplying the foot, or if the wound area exceeds the dimensions of potential free flap. NPWT offers relatively simple and effective method promoting healing of vast soft tissue defect accompanying open tibial fracture IIIC, especially in orthopaedic department without experience in microsurgical flaps.
Conclusion
The application of NPWT combined with the use of rhBMP‐7 and external fixation as a part of complex therapy in open tibial fracture IIIC with extensive soft tissue defect and bone defect or non‐union, can in selected cases help in salvage of supportive extremity, covered by healed soft tissue and free from infection. The prerequisites for optimal effect are maintained circulation to the non‐traumatised foot and protective sensation in the foot in functional position.
References
- 1. Behrens F, Searls K. External fixation of the tibia. Basic concepts and prospective evaluation. J Bone Joint Surg Br 1986;68:246–54. [DOI] [PubMed] [Google Scholar]
- 2. Okike K, Bhattacharyya T. Trends in the management of open fractures. J Bone Joint Surg Am 2006;88:2739–48. [DOI] [PubMed] [Google Scholar]
- 3. Bach AW, Hansen ST. Plates versus external fixation in severe open tibial shaft fractures: A randomized trial. Clin Orthop Relat Res 1989;241:89–94. [PubMed] [Google Scholar]
- 4. Tornetta P 3rd, Bergman M, Watnik N, Berkowitz G, Steuer J. Treatment of grade IIIB open tibial fractures: A prospective randomized comparison of external fixation and non‐reamed locked nailing. J Bone Joint Surg Br 1994;76:13–9. [PubMed] [Google Scholar]
- 5. Finkemeier CG, Schmidt AH, Kyle RF, Templeman DC, Varecka TF. A prospective, randomized study of intramedullary nails inserted with and without reaming for treatment of open and closed fractures of the tibial shaft. J Orthop Trauma 2000;14:187–93. [DOI] [PubMed] [Google Scholar]
- 6. Yokoyama K, Itoman M, Uchino M, Fukushima K, Nitta H, Kojima Y. Immediate versus delayed intramedullary nailing for open fractures of the tibial shaft: a multivariate analysis of factors affecting deep infection and fracture healing. Indian J Orthop 2008;42:410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br 2000;82:959–66. [DOI] [PubMed] [Google Scholar]
- 8. Hutson J, Dayicioglu D, Oeltjen J, Panthaki Z, Armstrong M. The treatment of Gustilo grade IIIB tibia fractures with application of antibiotic spacer, flap, and sequential distraction osteogenesis. Ann Plast Surg 2010;64:541–52. [DOI] [PubMed] [Google Scholar]
- 9. Kostiuchenko II, Kolker VA, Karlov VA. The vacuum effect in the surgical treatment of purulent wounds. Vestnik Khirurgii 1986;9:18–21. [PubMed] [Google Scholar]
- 10. Davydov YA, Larichev KG, Abramov AY. Concepts for clinical biological management of the wound process in the treatment of purulent wounds using vacuum therapy. Vestnik Khirugii 1991;2:132–5. [Google Scholar]
- 11. Morykwas MJ, Argenta LC, Shelton‐Brown El, McGuirt W. Vacuum‐assisted closure: a new method for wound control end treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 12. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control end treatment: clinical experience. Ann Plast Surg 1997;38:563–77. [PubMed] [Google Scholar]
- 13. Zoch G. Das Prinzip der vakuumunterstutzten Wundbehandlung. Eur Surg 2003;35(Suppl):3–5. [Google Scholar]
- 14. Sibbald RG, Mahoney J, V.A.C.® Therapy Canadian Consensus Group. A consensus report on the use of vacuum‐assisted closure in chronic, difficult‐to‐heal wounds. Ostomy Wound Manage 2003;49:52–66. [PubMed] [Google Scholar]
- 15. Kaufman WSM, Pahl DW. Vaccum‐assisted closure therapy: wound care and nursing implications. Dermatol Nurs 2003;15:317–25. [PubMed] [Google Scholar]
- 16. Stannard J. Complex orthopaedic wounds: prevention and treatment with negative pressure wound therapy. Orthop Nurs 2004;23:1–6. [PubMed] [Google Scholar]
- 17. Labler L, Keel M, Trentz O. VAC‐Wundverband zur temporaren Weichteildeckung bei drittgradig offenen Frakturen der unteren Extremitat. Eur Surg 2003;35:(Suppl):10–11. [Google Scholar]
- 18. Stannard J, Singanamala N, Volgas DA. Fix and flap in the era of vacuum suction devices: What do we know in terms of evidence based medicine?. Injury 2010;41:780–6. [DOI] [PubMed] [Google Scholar]
- 19. Chen D, Zhao M, Mundy G. Bone morphogenetic proteins. Growth Factors 2004;22:233–41. [DOI] [PubMed] [Google Scholar]
- 20. Ristiniemi J, Flinkkilä T, Hyvönen P, Lakovaara M, Pakarinen H, Jalovaara P. RhBMP‐7 accelerates the healing in distal tibial fractures treated by external fixation. J Bone Joint Surg 2007;89‐B:265–72. [DOI] [PubMed] [Google Scholar]
- 21. Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A, Kregor PJ, Nordsletten L, Paiement G, Patel A. Recombinant Human Bone Morphogenetic Protein‐2 in Open Tibial Fractures: A Subgroup Analysis of Data Combined from Two Prospective Randomized Studies. J Bone Joint Surg Am 2006;88:1258–1265. [DOI] [PubMed] [Google Scholar]
- 22. Desmyter S, Goubau Y, Benahmed N, de Wever A, Verdonk R. The role of Bone Morphogenetic Protein‐7 (Osteogenic Protein‐1®)) in the treatment of tibial fracture non‐unions. An overview of the use in Belgium. Acta Orthop Belg 2008;74:534–7. [PubMed] [Google Scholar]
- 23. Dimitriou R, Dahabreh Z, Katsoulis E, Matthews SJ, Branfoot T, Giannoudis PV. Application of recombinant BMP‐7 on persistent upper and lower limb non‐unions. Injury 2005;36(4 Suppl):51–9. [DOI] [PubMed] [Google Scholar]
- 24. Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein‐1 (bone morphogenetic protein‐7) in the treatment of tibial nonunions. J Bone Joint Surg 2001;83‐A(Suppl pt 2):151–8. [PMC free article] [PubMed] [Google Scholar]
- 25. Giannoudis P, Tzioupis C. Clinical applications of BMP‐7: the UK perspective. Injury 2005;36(3 Suppl):47–50. [DOI] [PubMed] [Google Scholar]
- 26. Cook SD, Barrack RL, Santman M, Patron LP, Salkeld SL, Whitecloud TS. Strut allograft healing to the femur with recombinant human osteogenic protein‐1. Clin Orthop 2000;381:47–57. [DOI] [PubMed] [Google Scholar]
- 27. Geesink R, Hoefnagels N, Bulstra S. Osteogenic activity of OP‐1® bone morphogenetic protein (BMP‐7) in a human fibular defect. J Bone Joint Surg 1999;81‐B:710–8. [DOI] [PubMed] [Google Scholar]
- 28. Govender S, Csimma C, Genent HK, Valentin‐Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Börner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Rüter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. BMP‐2 Evaluation in Surgery for Tibial Trauma (BESTT) Study Group. Recombinant human bone morphogenetic protein‐2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 2002;84‐A:2123–34. [DOI] [PubMed] [Google Scholar]
- 29. Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX. BMP‐2 Evaluation in Surgery for Tibial Trauma‐Allgraft (BESTT‐ALL) Study Group. Recombinant human BMP‐2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg 2006;88‐A:1431–41. [DOI] [PubMed] [Google Scholar]
- 30. Nauth A, Ristiniemi J, McKee MD, Schemitsch E. Bone morphogenetic proteins in open fractures: past, present and future. Injury, Int J Care Injured 2009;40:27–31. [DOI] [PubMed] [Google Scholar]
- 31. Birke‐Sorensen H, Malmsjo M, Rome P, Hudson D, Krug E, Berg L, Bruhin A, Caravaggi C, Chariker M, Depoorter M, Dowsett C, Dunn R, Duteille F, Ferreira F, Martinez JMF, Grudzien G, Ichioka S, Ingemansson R, Jeffery S, Lee C, Vig S, Runkel N, Martin R, Smith J. Evidence‐based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer) ‐steps toward an international consensus. J Plast Reconstr Aesthet Surg 2011;64:1–16. [DOI] [PubMed] [Google Scholar]
- 32. Dahabreh Z, Calori GM, Kanakaris NK, Giannoudis PV. A cost analysis of treatment of tibial fracture nonunion by bone grafting or bone morphogenetic protein‐7. Int Orthop 2009;33:1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alt V, Donell ST, Chhabra A, Bentley A. A health economic analysis of the use of rhBMP‐2 in Gustilo‐Anderson grade III open tibial fractures for the UK, Germany and France. Injury, Int J Care Injured 2009;40:1269–75. [DOI] [PubMed] [Google Scholar]
- 34. Górecki A, Babiak I. Infection of joint prosthesis and local drug delivery. In: Kienapfel H, Kühn K‐D, editors The infected implant. Berlin‐Heidelberg: Springer‐Verlag GmbH, 2009:19–26. [Google Scholar]
- 35. Murphy PS, Evans GD. Advances in wound healing: a review of current wound healing products. Plast Surg Int 2012;2012:190436. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]


