Abstract
Higher closure rates of the open abdomen have been reported with negative pressure wound therapy (NPWT) than with other kinds of wound management. We have recently shown that NPWT decreases the blood flow in the intestinal wall, and that the blood flow could be restored by inserting a protective disc over the intestines. The aim of the present study was to investigate whether layers of Jelonet™ (Smith & Nephew) dressing (paraffin tulle gras dressing made from open weave gauze) over the intestines could protect the intestines from hypoperfusion. Midline incisions were made in ten pigs and were subjected to treatment with NPWT with and without four layers of Jelonet over the intestines. The microvascular blood flow was measured in the intestinal wall before and after the application of topical negative pressures of −50, −70 and −120 mmHg, using laser Doppler velocimetry. Baseline blood flow was defined as 100% in all settings. The blood flow was significantly reduced, to 61 ± 7% (P < 0·001), after the application of −50 mmHg using conventional NPWT, and to 62 ± 7% (P < 0·001) after the application of −50 mmHg with Jelonet dressings between the dressing and the intestines. The blood flow was significantly reduced, to 38 ± 5% (P < 0·001), after the application of −70 mmHg, and to 42 ± 6% (P < 0·001) after the application of −70 mmHg with Jelonet dressings. The blood flow was significantly reduced, to 34 ± 9% (P < 0·001), after the application of −120 mmHg, and to 38 ± 6% (P < 0·001) after the application of −120 mmHg with Jelonet dressings. The use of four layers of Jelonet over the intestines during NPWT did not prevent a decrease in microvascular blood flow in the intestinal wall.
Keywords: Intestinal wall, Laparostomy, Microvascular blood flow, Negative pressure wound therapy
INTRODUCTION
During the past 25 years, laparostomy has become a lifesaving intervention for surgical emergencies such as abdominal compartment syndrome, wound dehiscence, trauma and intra‐abdominal sepsis 1, 2, 3, 4. The primary goals of wound management in laparostomy include avoidance of mechanical contamination of the abdominal viscera, the active removal of exudates, estimation of third‐space fluid loss, control of infection and the prevention of intestinal fistulae (5). Various materials and techniques have been used for temporary closure of the abdominal cavity, including intravenous fluid bags, Gore‐tex®, Bogota bags and sandwiched gauze dressings, with various results 1, 6, 7. The major drawback of these techniques is the formation of extensive ventral hernias requiring later treatment. Negative pressure wound therapy (NPWT) involves the use of suction and an occlusive dressing (the vacuum pack method), to remove contaminated effluent from the peritoneal cavity. The use of airtight dressings and vacuum therapy to manage laparostomy has significantly improved care and increased the possibility of closure of laparostomies. However, the method has occasionally been associated with an increased risk of the development of intestinal fistulae and enteroatmospheric fistulae 8, 9, 10, 11, 12. The reason for this is unknown. The decrease in intestinal wall blood flow induced by NPWT has been suggested as a possible cause (13). However, the patient group is often extremely complex, and it is therefore possible that multiple reasons are behind the development of fistulae. We believe that the NPWT of laparostomy is a reliable form of treatment, but that it could be improved by increased knowledge of the mechanisms governing its effects. We have recently shown that NPWT in laparostomy induces a decrease in microvascular blood flow in the small intestinal wall lying close to the NPWT dressing. We also showed that the blood flow could be restored by inserting a protective, flexible, thin plastic disc between the intestines and the vacuum source. The decrease in blood flow was found to be greater with increasing negative pressure (13).
Jelonet™, or other kinds of paraffin gauze, are used in wound care to protect the underlying tissue during, for example, NPWT. In the present study, we examined whether four layers of Jelonet could protect the intestines from ischaemia during NPWT of laparostomy. We studied the microvascular blood flow using fibre‐optic laser Doppler probes. Changes in blood flow were measured in the small intestinal wall during exposure to NPWT at pressures of −50, −70 and −120 mmHg, with and without Jelonet dressings.
MATERIALS AND METHODS
Experimental animals
Ten domestic pigs of both genders with a median weight of 60 kg were used. The animals were fasted overnight but given free access to water. The investigation complied with the ‘Guide for the Care and Use of Laboratory Animals' as recommended by the US National Institutes of Health and published by the National Academies Press (1996).
Anaesthesia
Before commencing surgery, sodium thiopental (5 mg/kg), atropine (0·02 mg/kg) and pancuronium (0·5 mg/kg) were given intravenously. Intubation was performed with a Portex endotracheal tube (7·5 mm internal diameter, Medcompare, South San Francisco, CA). A servo‐ventilator (Siemens Elema 300A, Stockholm, Sweden) was used for mechanical ventilation throughout the experiments. The ventilator settings used were: minute volume = 100 ml/kg, FiO2 = 0·5, breathing frequency = 16 breaths/minute and positive end‐expiratory pressure = 5 cm H2O. Anaesthesia and muscular paralysis were maintained by continuous intravenous infusion of 8–10 mg/kg/hour propofol (Diprivan, AstraZeneca, Sweden), 0.15 mg/kg/hour fentanyl (Leptanal, Lilly, France) and 0·6 mg/ kg/hour pancuronium (Pavulon, Organon Teknika, Boxtel, The Netherlands).
Data acquisition
Heart frequency and ventilator parameters were recorded throughout the experiments.
Surgical procedure
A 30‐cm‐long midline incision was performed on each pig. The V.A.C.® abdominal dressing (KCI®, Inc., San Antonio, TX) was used. The visceral protective layer was cut to an approximate size of 35 cm wide and 35 cm long, extending into the paracolic gutters on both sides. A layer of polyurethane Granu Foam was placed on top of the visceral protective layer between the edges of the wound. The wound was covered with a self‐adhesive polyethylene drape, and a track pad was inserted through the drape (all from V.A.C., KCI), and then connected to a continuous vacuum source.
Microvascular blood flow was measured by laser Doppler velocimetry (Peri Flux System 5000, Perimed, Stockholm, Sweden) using a technique that quantifies the sum of the motion of the red blood cells in a specific volume. This method is applied extensively in plastic surgery procedures and uses a fibre‐optic probe carrying a beam of light. Light impinging on cells in motion undergoes a change in wavelength (Doppler shift), while light impinging on static objects remains unchanged. The magnitude and frequency distributions of the changes are directly related to the number and velocity of red blood cells. The information is collected by a returning fibre, converted into an electronic signal and analysed (14).
Laser Doppler probes were inserted into the intestinal wall of the ileac loop, sutured to the inner surface of the dressing centrally beneath the dressing and at the anterior abdominal wall. The experimental set‐up is shown in Figure 1. The locations of the probes were confirmed upon completion of the experiments.
Figure 1.
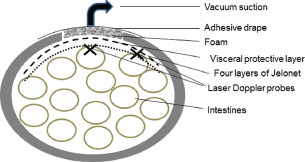
The figure shows a schematic of negative pressure wound therapy (NPWT) of the open abdomen with and without Jelonet, and the location of the laser Doppler probes (×). A loop of the intestinal wall in which the blood flow was measured was sutured to the inner surface of the dressing apically just beneath the dressing, or at the surface of the anterior abdominal wall. The locations of the probes were confirmed after each experiment.
Experimental protocol
The microvascular blood flow was measured continuously by the laser Doppler filament probes. Recordings were made before NPWT (baseline = 0 mmHg) and during exposure to NPWT at −50, −70 and −120 mmHg. The pressures were applied in random in order to prevent systematic effects, and baseline was restored between each pressure setting.
Jelonet
Jelonet (Smith & Nephew, London, UK) is sterile paraffin tulle gras dressing made from open weave gauze. The gauze has interlocking threads which minimises fraying when the dressing is cut. Jelonet is often used in wound care to protect the underlying tissue. Jelonet is soothing and low adherent, and allows the wound to drain freely into an absorbent secondary dressing.
Calculations and statistics
Laser Doppler velocimetry measurements were performed on ten pigs. The output was recorded continuously using the Peri Flux System 5000. Microvascular blood flow was expressed in terms of perfusion units (PU). Calculations and statistical analysis were performed using GraphPad 5.0 software (San Diego, CA). The Mann–Whitney test was used when comparing two groups, and the Kruskal–Wallis test with Dunn's test for multiple comparisons when comparing three groups or more. Significance was defined as P < 0·05 (*), P < 0·01 (**), P < 0·001 (***) and P > 0·05 (not significant, n.s.). The values given are the mean of ten measurements and the standard error on the mean (SEM).
RESULTS
Microvascular blood flow in the small intestinal loop located at the anterior abdominal wall
The microvascular blood flow was measured in the small intestinal loop sutured to the dressing at the anterior abdominal wall. The results are shown in Figure 2. Baseline blood flow was defined as 100% in all settings. The blood flow was significantly reduced, to 69 ± 10% (P < 0·001) after the application of NPWT at −50 mmHg. After the application of NPWT at −50 mmHg with four layers of Jelonet between the dressing and the intestines, the blood flow was also significantly reduced, to 72 ± 3% (P < 0·001). The blood flow was significantly reduced, to 44 ± 7% (P < 0·001) without Jelonet and to 48 ± 4% (P < 0·001) with four layers of Jelonet, after the application of NPWT at −70 mmHg. The blood flow was significantly reduced, to 33 ± 4% (P < 0·001) without Jelonet and to 41 ± 4% (P < 0·001) with four layers of Jelonet, after the application of −120 mmHg.
Figure 2.
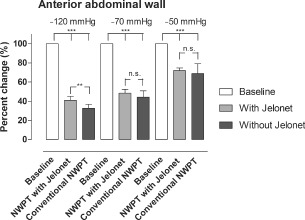
The microvascular blood flow in a small intestinal loop located at the surface of the anterior abdominal wall, relative to the baseline value, when exposed to topical negative pressures of −50, −70 and −120 mmHg. The results shown are the means of 10 measurements ± the standard error on the mean (SEM). Statistical analysis was performed using the Mann–Whitney test. Significance was defined as P < 0·05 (*), P < 0·01 (**), P < 0·001 (***) and P > 0·05 (not significant, n.s.).
Microvascular blood flow in the small intestinal loop located apically of the abdomen, beneath the dressing
The microvascular blood flow was also measured in the small intestinal loop of the abdomen sutured to the dressing, apical of the abdomen, just beneath the dressing. The results are shown in Figure 3. Baseline blood flow was defined as 100% in all settings. The blood flow was significantly reduced, to 61 ± 7% (P < 0·001) after the application of NPWT at −50 mmHg, and to 62 ± 7% (P < 0·001) after the application of −50 mmHg with four layers of Jelonet between the dressing and the intestines. The blood flow was significantly reduced, to 38 ± 5% (P < 0·001) without Jelonet and to 42 ± 6% (P < 0·001) with the Jelonet dressings, after the application of NPWT at −70 mmHg. The blood flow was significantly reduced, to 34 ± 9% (P < 0·001) without Jelonet and to 38 ± 6% (P < 0·001) with four layers of Jelonet, after the application of −120 mmHg.
Figure 3.
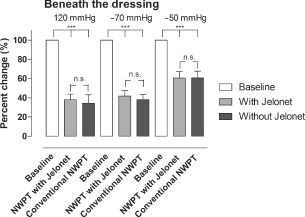
The microvascular blood flow in a small intestinal loop located centrally beneath the dressing of the abdomen, relative to the baseline value, when exposed to topical negative pressures of −50, −70 and −120 mmHg. The results shown are the means of 10 measurements ± the standard error on the mean (SEM). Statistical analysis was performed using the Mann–Whitney test. Significance was defined as P < 0·05 (*), P < 0·01 (**), P < 0·001 (***) and P > 0·05 (not significant, n.s.).
DISCUSSION
Higher closure rates of the abdomen have been reported with NPWT than with other techniques 5, 15, 16, 17, 18. However, the method has occasionally been associated with increased development of intestinal fistulae and enteroatmospheric fistulae 8, 9, 10, 11, 12. NPWT involves suction over a large polyurethane sponge under an occlusive dressing in the wound, providing constant medial traction of the abdominal fascia. The technique also allows the abdominal wall to move freely towards the midline without the interference of adhesions between the bowels and the abdominal wall. NPWT also allows efficient drainage, reducing the amount of peritoneal fluid and bacteria. NPWT has gained clinical acceptance because of excellent clinical results, but the basic mechanisms by which NPWT affects the laparostomy wound are still unclear. Investigations such as this may provide valuable knowledge allowing the treatment to be improved.
In earlier studies using NPWT on sternotomy wounds, it has been shown that NPWT induces an increase in the blood flow of the peristernal soft tissue (i.e. skeletal muscular and subcutaneous tissue), and also that the change is related to local effects, because the blood flow 4.5 cm from the wound edge was not affected by the negative pressure. The blood flow increased with increasing sub‐atmospheric pressure in both subcutaneous and skeletal muscular tissue (19). Interestingly, a zone of relative hypoperfusion was observed in the immediate proximity of the wound edge (19). This zone was larger at higher negative pressures. The size of the hypoperfused zone depended on the pressure applied, and expanded with increasing negative pressure (19). These physiological events may also take place in the intestinal wall during exposure to negative pressures, leading to a hypoperfusion zone in the intestinal wall that is in close contact with the NPWT dressing.
We have previously shown that the use of NPWT in laparostomy induces a decrease in the blood flow in the small intestinal wall lying close to the dressing. This decrease may indicate the relative zone of hypoperfusion found in other NPWT studies (13). We have also shown that the decrease in blood flow becomes greater with increasing negative pressure (13). Interestingly, the blood flow could be restored by inserting a protective thin plastic disc over the intestines (13). Macroscopic changes in the small intestines lying close to the NPWT dressing in laparostomy wounds over 24 and 48 hours were recently studied in 70‐kg pigs (20). Half of the animals were treated with a protective thin plastic disc over the intestines, while the other halves were treated with conventional NPWT for laparostomy. Slight petechial bleeding was seen in the small intestinal loops lying close to the dressing in both groups (20). The area of petechial bleeding was significantly larger after 24 hours, but especially after 48 hours, in the conventional NPWT group. In contrast, hardly any petechial bleeding was seen in the group treated with a protective disc over the intestines (20). The area of petechial bleeding may indicate signs of ischaemia.
In wound care, paraffin gauze is often used to protect underlying tissue or structures. In cardiac surgery, layers of paraffin gauze are placed over the heart during NPWT to prevent ingrowth of the heart into the NPWT foam (21). In laparostomy, paraffin gauze is sometime placed over the intestines in NPWT when no intestinal protective layer is used. The aim of the present study was to investigate whether layers of Jelonet over the intestines could protect the intestines from a zone of hypoperfusion, as found when using a thin protective plastic disc. We used paraffin gauze because this material is commonly used in wound care. We found that both conventional NPWT and NPWT with layers of Jelonet over the intestines induced a decrease in microvascular blood flow. We also found that the decrease in blood flow became greater with increasing negative pressure. Furthermore, four layers of Jelonet over the intestines were not sufficient to protect the intestines from hypoperfusion.
The cause of the decrease in blood flow may be the zone of hypoperfusion induced by NPWT, as mentioned above (19). It may however, also be induced by the minimal hernia created by NPWT in laparostomy. In cardiac surgery, NPWT is used in cases of poststernotomy mediastinitis. The foam is placed in the sternal split and the foam and vacuum source may come into direct contact with the heart (21). In magnetic resonance imaging studies in pigs, the heart was found to be drawn up towards the thoracic wall, and the right ventricle was seen to bulge into the space between the sternal edges (22). Placing multiple layers of paraffin gauze over the anterior portion of the heart did not prevent deformation of the heart. However, these events could be prevented by inserting a rigid plastic disc between the anterior part of the heart and the inside of the thoracic wall (22). When using NPWT in the treatment of laparostomy, it is possible that a similar mechanism occurs, i.e. bulging of the small intestines into the space between the wound edges, creating a hernia, which may in part explain the hypoperfusion seen in the underlying intestinal wall during NPWT in laparostomy.
CONCLUSIONS
In the present study, we show that applying NPWT to laparostomy wound in pigs induces hypoperfusion in the small intestinal wall lying close to the dressing. Inserting several layers of paraffin gauze, Jelonet, over the intestines does not protect the intestines from hypoperfusion.
REFERENCES
- 1. Schecter WP, Ivatury RR, Rotondo MF, Hirshberg A. Open abdomen after trauma and abdominal sepsis: a strategy for management. J Am Coll Surg 2006;203:390–6. [DOI] [PubMed] [Google Scholar]
- 2. Swan MC, Banwell PE. The open abdomen: aetiology, classification and current management strategies. J Wound Care 2005;14:7–11. [DOI] [PubMed] [Google Scholar]
- 3. Deenichin GP. Abdominal compartment syndrome. Surg Today 2008;38:5–19. [DOI] [PubMed] [Google Scholar]
- 4. Mughal MM, Bancewicz J, Irving MH. ‘Laparostomy’: a technique for the management of intractable intra‐abdominal sepsis. Br J Surg 1986;73:253–9. [DOI] [PubMed] [Google Scholar]
- 5. Stevens P. Vacuum‐assisted closure of laparostomy wounds: a critical review of the literature. Int Wound J 2009;6:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Navsaria PH, Bunting M, Omoshoro‐Jones J, Nicol AJ, Kahn D. Temporary closure of open abdominal wounds by the modified sandwich‐vacuum pack technique. Br J Surg 2003;90:718–22. [DOI] [PubMed] [Google Scholar]
- 7. Schachtrupp A, Fackeldey V, Klinge U, Hoer J, Tittel A, Toens C, Schumpelick V. Temporary closure of the abdominal wall (laparostomy). Hernia 2002;6:155–62. [DOI] [PubMed] [Google Scholar]
- 8. Bee TK, Croce MA, Magnotti LJ, Zarzaur BL, Maish GO, 3rd, Minard G, Schroeppel TJ, Fabian TC. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum‐assisted closure. J Trauma 2008;65:337–42; discussion 342–4. [DOI] [PubMed] [Google Scholar]
- 9. Becker HP, Willms A, Schwab R. Small bowel fistulas and the open abdomen. Scand J Surg 2007;96:263–71. [DOI] [PubMed] [Google Scholar]
- 10. Fischer JE. A cautionary note: the use of vacuum‐assisted closure systems in the treatment of gastrointestinal cutaneous fistula may be associated with higher mortality from subsequent fistula development. Am J Surg 2008;196:1–2. [DOI] [PubMed] [Google Scholar]
- 11. Trevelyan SL, Carlson GL. Is TNP in the open abdomen safe and effective? J Wound Care 2009;18:24–5. [DOI] [PubMed] [Google Scholar]
- 12. Rao M, Burke D, Finan PJ, Sagar PM. The use of vacuum‐assisted closure of abdominal wounds: a word of caution. Colorectal Dis 2007;9:266–8. [DOI] [PubMed] [Google Scholar]
- 13. Lindstedt S, Malmsjo M, Hansson J, Ingemansson R. Microvascular blood flow changes in the small intestinal wall during conventional negative pressure wound therapy and negative pressure wound therapy using a protective disc over the intestines in laparostomy. Ann Surg. In press. [DOI] [PubMed] [Google Scholar]
- 14. Zografos GC, Martis K, Morris DL. Laser Doppler flowmetry in evaluation of cutaneous wound blood flow using various suturing techniques. Ann Surg 1992;215:266–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppaniemi A, Olvera C, Ivatury R, D’Amours S, Wendon J, Hillman K, Wilmer A. Results from the international conference of experts on intra‐abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med 2007;33:951–62. [DOI] [PubMed] [Google Scholar]
- 16. Malbrain ML, De Laet I, Cheatham M. Consensus conference definitions and recommendations on intra‐abdominal hypertension (IAH) and the abdominal compartment syndrome (ACS)–the long road to the final publications, how did we get there? Acta Clin Belg Suppl 2007;1:44–59. [DOI] [PubMed] [Google Scholar]
- 17. Perez D, Wildi S, Demartines N, Bramkamp M, Koehler C, Clavien PA. Prospective evaluation of vacuum‐assisted closure in abdominal compartment syndrome and severe abdominal sepsis. J Am Coll Surg 2007;205:586–92. [DOI] [PubMed] [Google Scholar]
- 18. Svensson S, Monsen C, Kolbel T, Acosta S. Predictors for outcome after vacuum assisted closure therapy of peri‐vascular surgical site infections in the groin. Eur J Vasc Endovasc Surg 2008;36:84–9. [DOI] [PubMed] [Google Scholar]
- 19. Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum‐assisted closure therapy. Ann Thorac Surg 2005;79:1724–30; discussion 1730–1. [DOI] [PubMed] [Google Scholar]
- 20. Lindstedt S, Malmsjo M, Hansson J, Hlebowicz J, Ingemansson R. Macroscopic changes during negative pressure wound therapy of the open abdomen using conventional negative pressure wound therapy and NPWT with a protective disc over the intestines. BMC Surg 2011;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sjogren J, Malmsjo M, Gustafsson R, Ingemansson R. Poststernotomy mediastinitis: a review of conventional surgical treatments, vacuum‐assisted closure therapy and presentation of the Lund University Hospital mediastinitis algorithm. Eur J Cardiothorac Surg 2006;30:898–905. [DOI] [PubMed] [Google Scholar]
- 22. Malmsjo M, Petzina R, Ugander M, Engblom H, Torbrand C, Mokhtari A, Hetzer R, Arheden H, Ingemansson R. Preventing heart injury during negative pressure wound therapy in cardiac surgery: assessment using real‐time magnetic resonance imaging. J Thorac Cardiovasc Surg 2009;138:712–7. [DOI] [PubMed] [Google Scholar]


