Abstract
The Woundcare for Epidermolysis bullosa (WEB) project aims to enable people with Epidermolysis bullosa (EB), their carers and clinicians to co‐produce wound care products to meet their needs. EB is an inherited disorder causing extensive, painful skin blistering and wounds. It is relatively rare, with approximately 300 000 patients worldwide, but it incurs high costs (up to £50 000 per month on products alone). During the course of four workshops, adults with EB, their carers and specialist nurses gave detailed accounts of their experiences with pre‐sized, pre‐shaped dressings, including the need to patchwork individual dressings over large areas of broken skin. Five themes were identified from the workshop data relating to the limitations of existing products for EB wounds: dressing fit, stability, comfort, temperature and exudate. Novel designs were generated from these themes and although the intention was to develop the designs iteratively with the workshop participants, issues arose necessitating the interim use of surrogates. Our account of the design process is given, presenting the arguments for and against the use of surrogates, with suggestions for incorporating surrogate input into product development in a way that does not undermine the integrity of patient experiences or the co‐production process.
Keywords: Co‐production, Epidermolysis bullosa, Novel wound dressing retention garment, Surrogates, User experience
Introduction
The purpose of this paper is to report the use of human surrogates in the design of novel products for people with Epidermolysis bullosa (EB), and to discuss the implications for using surrogates in the development of externally worn medical devices more generally. EB is a group of rare genetic skin fragility disorders, which cause extensive skin blistering, wounds, pain and discomfort (1). The population of patients with EB is small affecting approximately 1:17 000 live births. In the UK, there are an estimated 4000–5000 people with the condition; with around 300 000 worldwide. The adult population comprises a small percentage of the total population (2). For example, at the UK specialist centre for adults with EB (St John's Institute, St Thomas' Hospital London), there are approximately 160 adult patients registered with the specialist EB service.
In the UK, the cost of wound care to the health services is estimated to be between £2–3 billion, 78% of this is nursing time and 22% wound care products (3). The costs of EB wound care have not been quantified. However, with the advent of home deliveries of wound care products in the UK data on the provision and costs of wound care products to EB patients is being made available to service providers. One patient with EB and whole body skin breakdown can use £50 000 worth of dressing in 1 month; this figure excludes care costs (Pillay 2010, personal communication).
The use of products and time spent on dressing changes by EB patients, their carers and clinicians, without their wound care needs actually being met, led to the Woundcare for Epidermolysis bullosa (WEB) project to address these needs through the design and development of novel products 1, 4.
The WEB project is a qualitative participatory case study that draws on methods and approaches widely used in the design sciences. The study aims to design novel wound care solutions to overcome the limitations of conventional wound dressings and retention garments experienced by patients with EB, their informal carers and specialist nurses. The intention in WEB was to develop the novel products based exclusively on direct patient, carer and clinician experience and input. However, certain obstacles arose which resulted in the use of surrogates (principally the research assistant on the project and the designer). The definition of a ‘surrogate’ adopted in the study follows those identified in a literature review by Shah and Robinson (5) who support the use of surrogates as a potentially useful approach to ascertaining end user requirements, whilst also advocating a critical case by case approach to ensure that end users interests are best served in the process. Surrogates were defined therein as any healthcare professional and/or caregiver who acts formally on behalf of the healthcare end user concerning the end user's health care.
The WEB project builds on a two‐stage consultation with adults with EB and their wound care needs, undertaken in two Engineering and Physical Sciences Research Projects: Woundcare Research for Appropriate Products (WRAP) project and the Multidisciplinary Assessment of Technologies for Healthcare (MATCH) project. In WRAP, the consultation took place during the course of validating a clinical note‐making system. Three participants with EB contributed their experiences to assist the design and validation of clinical indicators 4, 6. In MATCH, four workshops were conducted, which generated the substantive accounts of the limitations of existing dressing products that the WEB project draws on to support the process of co‐designing the novel products (paper submitted for publication). Additionally, WEB developed patient recorded outcome measures for EB in a separate audit study, the findings from which will be published elsewhere (7).
In MATCH groups of between 6 and 20 patients with EB and their carers attended the four workshops, together with 6–8 EB clinical nurse specialists. Explicit accounts were generated by the workshop participants of their individual and shared difficulties in dressing extensive areas of the body with current dressings, which are pre‐sized and shaped. Routinely they use numerous (20–50) dressings, patch‐worked, to cover large areas of broken skin. The patch‐worked dressings are then layered with pads and secured with tape and bandages to keep them in place. Clothing, normal movement and daily activities result in these ad hoc systems slipping and falling off causing further damage, pain and embarrassment from soiling and odour. For some, routine dressing changes can take between 3 and 7 hours daily or on alternate days (4) (paper submitted for publication).
The paper gives an account of the use of surrogates in the WEB project to progress the design and development of novel products for EB woundcare, presenting arguments for and against their use, and offers suggestions for incorporating surrogate input into medical device development, without damaging the integrity of user participation.
Methodology and methods
A participatory research design was adopted. Participatory research is described as a collaborative co‐governance research approach whereby researchers, those affected by the subject of research and who may take forward the outputs of research, work together in a framework within which individuals are able to be self‐determining and are valued for their knowledge and contribution 8, 9. This approach suited the aims of WEB, which are to collaborate with people with the condition EB, the people who participate closely in supporting these individuals (lay carers and professional nurse specialists), to capture their problems with current wound dressings and to work with people who can provide solutions to the current limitations in dressing performance for EB (the designer and manufacturer).
The model of user engagement in medical device design and development, which underpins the WEB project, developed from four studies identified during the literature review undertaken as part of the MATCH study 10, 11. Essentially it is a ‘user‐centred’ design methodology which takes account of the particular milestones that need to be achieved for a new medical device to be accessible to patients through health services supply chains 12, 13, 14, 15.
Key assumptions of this model are that the privacy of the patients, the end users of the devices, must be protected while their needs drive the design and development of novel products. Their needs should not be compromised by vested interests and pressures to conform to existing purchasing, manufacturing and design processes (11).
It comprises five key aspects of product development:
Identification of patients' needs, whilst protecting patients' privacy.
Development of design concepts and prototypes from these needs.
Proof of concept testing and finalisation.
Evaluation of clinical performance and costs of finalised products.
Engagement of manufacturers early in the process to ensure a route to market and patient access to the products they have helped to develop.
Phase four, evaluation of clinical performance and costs of the final products, is in two phases. The first is to confirm that the novel materials and designs meet patient specifications for comfort, fit and stability. The second is to evaluate them against standard products using an n = 1 experimental case study design developed from the UK Medical Research Council guidance on the designs of complex interventions 15, 16, 17. The outcome measurement tool for this study is the TELER® system of patient recorded outcomes measurement, which was applied to wound care by Grocott et al. (18) and developed further in the WRAP study (1). In the WEB audit study, it has been applied to EB in a novel digital pen and paper format (7). The findings from this and the audit study will be reported in subsequent publications. Manufacturer engagement (aspect five) has been intrinsic to the development of the garment from the start of the WEB project and is ongoing.
Study aims and objective
The specific aim of the WEB project reported here is to develop a novel dressing retention garment as part of a two‐layer system for EB wound care, comprising a disposable primary wound contact layer and the secondary reusable retention garment. During the course of the MATCH project, the participants with EB and the clinical nurse specialists prioritised the development of a reusable retention garment, to be followed by novel designs and materials for a disposable wound contact layer. The grounds were that the garments would take a shorter time to develop than the wound contact layer. They considered that new approaches to keep dressings in place would improve the performance of their current wound contact dressings. The nurse specialists also proposed that economic benefits could be accrued by not disposing of retention bandages at each dressing change, by dressings that remain in situ between planned dressing changes, thereby reducing the demand for wound care products and time taken for dressing interventions. However, some patients cautioned that should the garment layer become very soiled with wound exudate, they would dispose of it rather than recycle via washing (19).
The novel dressing system under development comprises a non‐invasive, external device that needs to be worn by the patients, and fit in with activities of daily living. It therefore seemed logical and appropriate that adults with EB, the end users of the system, should participate thoroughly in the iterative processes of design development, refinement and finalisation. There were however limits to their engagement in the WEB study, which led to the use of surrogates.
Challenges to the project and the need for surrogate testing
With the necessary ethical approval in place, the four workshops were conducted with adults with EB, their carers and clinical nurse specialists. The data obtained were analysed thematically and shared in their anonymised form with the design consultant and the manufacturing company to make progress in the design and development of the new novel garments. The methodology and findings from these workshops have been submitted for publication (19). Before progress in the WEB project could be made on prototype testing, further user input was required to determine if the garments met basic comfort, fit, stability and temperature control needs.
Following a successful application for further ethical approval to obtain this input from the participants through garment fittings and revisions by the design consultant, there were delays in gaining local research and development (R&D) approvals to proceed with this aspect of the study. The iterative development of the novel garments proved challenging for the R&D and Medicines and Healthcare Regulatory Agency to decide at what stage the garments required Conformité Européenne (CE) marking. The decision was made to apply for the mark prior to finalisation of the designs to provide a safe guard for the participants and the ethics committee and R&D. However, the extra paperwork, changes to the protocol and a resubmission to the ethics council had the potential to delay the developmental work.
The study ran on tight timelines and a small budget. Delays in fitting, refining and finalising the garments with the participants, due to the resubmission for ethical and R&D review, was slowing down the flow of the garment development. This posed real threats, particularly in terms of the design consultant being unable to continue with the project and the momentum with the manufacturing company because of competing demands on their time and resources. In addition, the project had raised expectations and there was discernable pressure placed on the researchers for solutions to the daily problems of EB wound care. The EB nurse specialists had shown the patients the prototypes and the informal feedback was very positive, but also accompanied with pleas for urgent access to the products.
Garment development up to this point had been a team approach. The design consultant would bring new samples to meetings with the clinical nurses and academics, and the team would consider them against the set criteria established by the end user group. These included considerations of designs to accommodate disabilities arising from EB and the need for sustainable stretch and recovery, softness and stability. When the project delays occurred, the research assistant took the initiative and proposed, and convinced, the lead investigator of the value of surrogate testing against the end user criteria. The design consultant had established the process of generating meaningful data in the design portfolio she developed that set in motion the prototype development with the company. The research assistant, aided by members of her family, and the design consultant pushed this development forward in the surrogate testing activity.
The idea of using surrogates was seen as a practical means of informing the ongoing development of the garments, and momentum of the project on the basis of the knowledge acquired via the supporting projects. At no point were these data considered a replacement for end user input. The selective use of surrogates speeded up the process of testing and refining prototypes, with iterative consultation with the clinical nurse specialist group, prior to final testing, refinement and validation with the end user group. The latter is being conducted in further studies with the necessary ethical approval and research and development permissions.
Method of surrogate testing
Five factors were identified through analysis of the workshop data (paper submitted for publication) as interrelated – and observable – criteria of improvement of wound dressing retention garments. Although interrelated, there were discrete activities involved in refining the prototype designs to achieve optimal functioning on the five factors:
Fit
Stability
Comfort
Temperature
Exudate Control
It was hypothesised that any difficulties experienced by a person with healthy skin in relation to the above criteria would be enhanced for a person with compromised skin.
Using the same method and products as the EB participants were using for wound care, topical treatments (emollients) were applied and covered with primary and secondary dressings, then either secured with bandages or tubular fastenings, were held on and covered by the novel garments to determine stability.
Observational notes and photographs were taken by the research assistant and designer as they tested the prototypes to record how well the garments met the stated criteria, together with design aspects that were unsuitable. The photographs and notes were complied as a series of documents charting the progression of the garments, and distributed to the team. 1, 2, 3, 4, 5 summarise the systematic process undertaken by the principal surrogate to test and refine the prototype designs. 1, 2, 3, 4 provide visual examples of the testing undertaken by the surrogates.
Table 1.
Criterion 1: fit
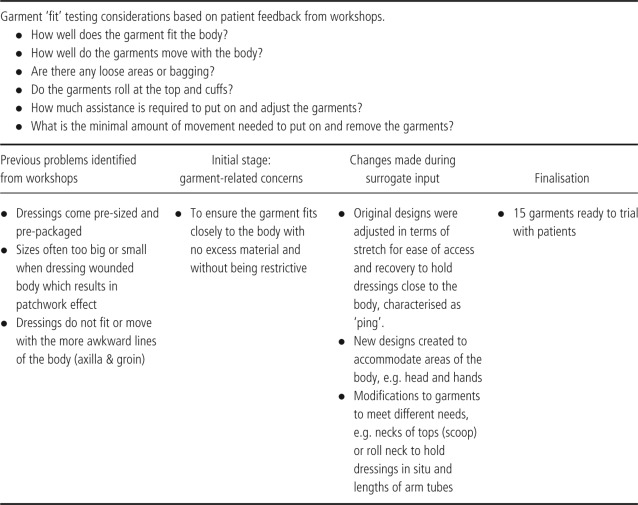
Table 2.
Criterion 2: stability
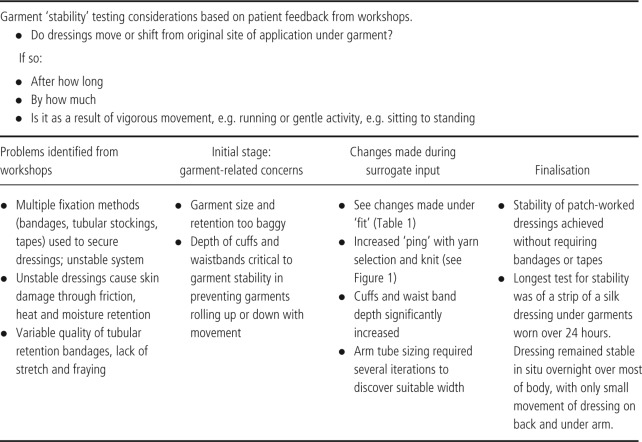
Table 3.
Criterion 3: temperature
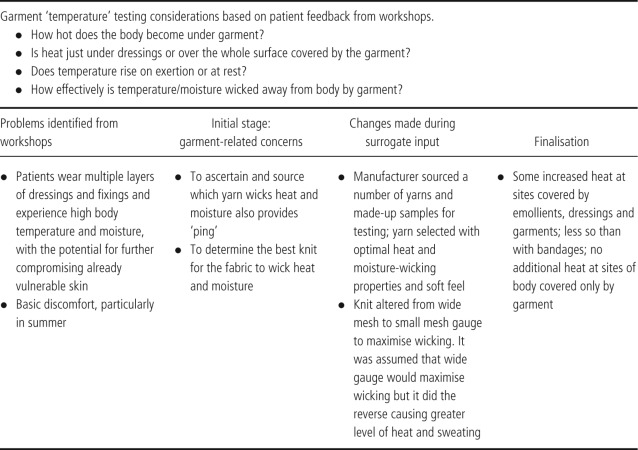
Table 4.
Criterion 4: comfort
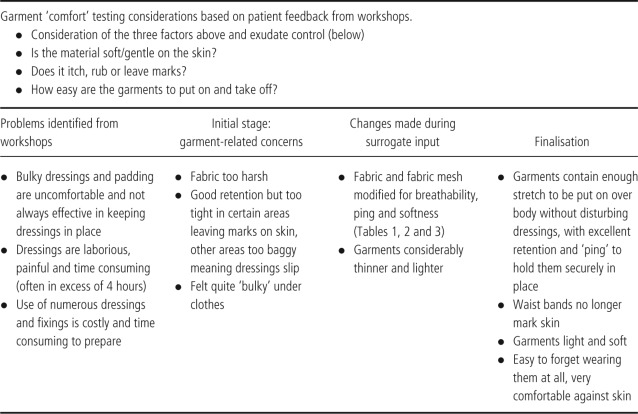
Table 5.
Criterion 5: exudate control
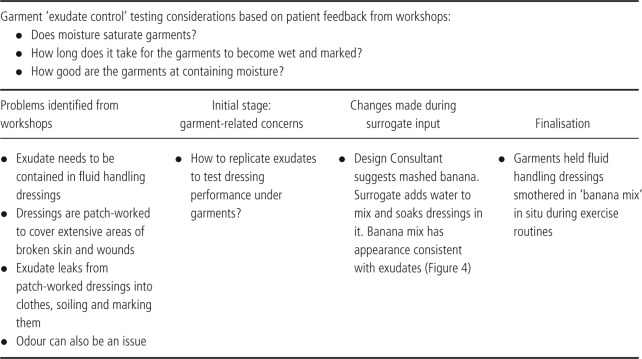
Figure 1.
Early design of an arm tube with bagging at the cuff, cuff too short and tube not long enough.
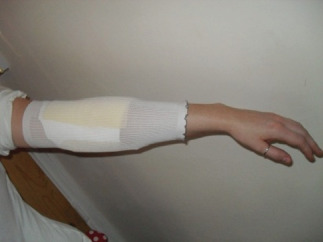
Figure 2.
‘Freestanding’ dressing on thigh under garment with no bandage or fixing support; insufficient ‘ping’ to hold dressing in situ.
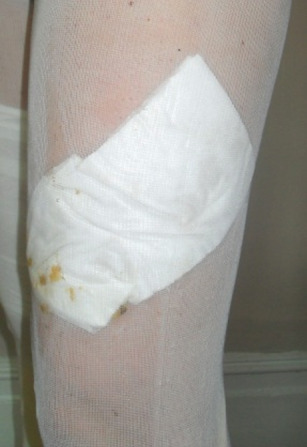
Figure 3.
Shows the side of legging that have stretched to tearing point.
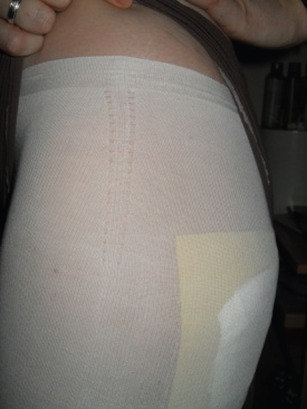
Figure 4.

Exudate substitute: banana and water mix.
The design consultant refined the designs according to the feedback, communicated this feedback to the manufacturers, and worked with them to alter and return revised prototypes to be retested through the above process. The manufacturer has computerised machinery and is therefore able to respond quickly to prototype revision. The fine details of the designs and the manufacturing process need to be protected as it constitutes valuable intellectual property, and will not therefore be disclosed here.
With regard to fit and garment and dressing stability, changes in the designs were made when initial ideas for garments proved difficult to use. For example, an early design included a single leg garment that came to the top of the thigh. This proved unstable and rolled down, as well as leaving marks on the skin. The latter in a person with EB is very likely to result in skin blisters. In response to this, the design consultant created a new leg tube with a deep waistband; she also designed various lengths so an individual can dress either the whole single leg or just the top portion (lower leg tubes were already in process). This resolved the lack of stability, rolling and marking. In addition, an arm tube needed to be redesigned and manufactured to prevent bagging, to achieve optimal stretch and recovery (ping) and to achieve the right length (Table 1; 1, 2, 3).
With regard to comfort and temperature particular attention was paid to the softness and avoidance of friction together with participants' experiences of warm weather, which aggravates the symptoms of EB such as itchiness. Increased environmental heat, coupled with the usual multiple layers of cream and dressings leads to an occlusive environment where the skin cannot breath. A range of different knits and yarns were tested, and the selection was refined to an exquisitely soft, highly stretchable knit fabric, which conveys heat and moisture by capillary action, and is very light to wear. This fabric is several steps on from an earlier version in the same yarn but knitted in a mesh‐like structure, with the aim to maximise moisture and heat loss. The surrogate feedback in this example was vital as the mesh structure turned out to trap heat, becoming intolerably hot with exercise, and the exact opposite of what is required (3, 4).
With regard to the exudate control criterion a series of ‘wet’ tests were undertaken. A banana and water mix, recommended by the design consultant, was applied to dressings and put on the skin to replicate EB wound exudate, which worked remarkably well (Figure 4).
Once dressed and with a garment in place, normal outer clothing were worn and the system was tested for between 45 minutes and 4 hours. This was based on the information disclosed by the participants that they are faced with a frequent issue of dressing slippages, which can occur remarkably quickly after the dressing change. Accordingly 4 hours was seen to be a good period of time to address stability, temperature, comfort, fit and seepage (Table 5).
During the surrogate tests, activities requiring various degrees of exertion were undertaken to test the garments as thoroughly as possible in regards to the set criteria. These included cardio exercises, driving and running, as well as consideration for sitting to standing and vice versa, and basic hygiene needs.
While the above examples were highly constructive steps to perfecting the garments, as with all stages of this project, the garment designs will not be finalised or commercialised until they have been fitted and approved by the EB group. This is being done in a two‐phase study, with ethics and R&D approvals and permissions.
Phase one objective – finalisation of novel garments.
Fitting of the garments over conventional dressings with the design consultant to judge their fit, stability and comfort.
Refinement of the garments (e.g. knit and sizing) as needed by the designer and manufacturer.
Phase two objective – clinical investigation of novel garments.
Clinical investigation of the end user experiences and clinical performance of the novel garments, and the overall costs of wound care.
The data from phase two will be used by the manufacturer to support applications for entry into health services supply chains and access by the end users.
Discussion and conclusions
The novel garments have been designed by and for adults with EB. However, depending on the strength of the clinical evaluation data, our hypothesis is that they will also meet the needs of individuals with EB who have not participated in the validation study, given the evidence and our understanding that they have similar problems with wound dressings (4). In addition, there are commonalities across all patients with advanced skin breakdown in terms of covering the skin and retaining dressings in situ with natural movement. The development of products to meet the needs of EB patients may therefore also have a more generic application to patients with extensive wounds of different aetiologies who require wound coverings, e.g. malignant infiltration of the skin, burns and major trauma. This generalisation will need to be tested empirically and the anticipation is that prior to such testing some design, sizing and manufacturing refinements may need to be made that are specific to these different patient groups.
The processes of obtaining ethical and R&D permissions and approvals, together with registration of medical devices were, on one hand, barriers to progress in the WEB study. On the other hand had the team been granted approval to develop, iteratively, the novel designs with the end user group, the end users may have been overburdened with requests to try on the various iterations of the garment, and provide the level of feedback required by the design consultant and the manufacturer. Instead the surrogates, who were knowledgeable about the problems with EB wound care via their participation in the earlier workshops undertook this role. In this study therefore, surrogates with day‐to‐day contact with the clinical and patient problem contributed to new device development.
The use of surrogates in the WEB study is consistent with the findings of Shah and Robinson (5) in terms of acting on behalf of the device users, without losing sight of the fact that users should have the final say on the design and presentation of the novel devices. In the MATCH literature review by Bridgelal Ram et al. (11) a short report was identified which illustrates clearly the negative effects of not involving end users appropriately in both design and purchasing. An orthopaedic surgeon in a small state in northern India discovered that amputees were not wearing their prosthetic limbs because the limbs were of a western design and did not fit with the rural lifestyle (20). He involved the amputees in designing and manufacturing their prosthetics from local products with the support of local artisans, a process which resulted in the highly successful global business, the Jaipur limb (21).
Other studies included in the review indicated that user recruitment raised ethical and safety issues, making it necessary to use surrogates for a sample population in particular situations. One example is the use of physical models such as anthropomorphic test dummies, for prototype testing wheelchair occupant restraint systems. This was followed by testing, by healthy volunteers. At the point when the safety of devices has been established, it would seem logical to test such appliances with the end users for whom the device is intended (11).
A recent investigation into the manufacturers' perspectives on using formal methods of engaging users, for example, human factors engineering methods, in the design and development of medical devices found that they were reluctant to do so (22). They predicted lengthy processes, including delays in obtaining ethical approval. Most notably, which the WEB study challenges, was their view that senior healthcare practitioners and patients are rarely seen to be able to provide valuable input into the medical device design and development process.
Coercion to act as surrogates is a potential ethical issue in a project of this kind, largely because of pressure to maintain project momentum and deliver milestones. However, in this study, the research assistant had to persuade the lead investigator, who was very convinced by the research conducted in MATCH of the need to work directly with end users, of the value of surrogate testing in WEB. The design consultant saw this type of testing as part and parcel of her work.
Overall, the use of surrogates may enable the early stages of novel product development to progress quickly from first to second design, however true the representation of patient wishes or choices in the final product is a key concern 23, 24. While the use of surrogates in some cases is unavoidable, and may prove highly beneficial, it is evident (for instance in the example given above) that there are circumstances where failure to consult the end users of medical devices on their needs, results in poor design and thereby the effectiveness and usability of the products.
The final endorsement of our use of surrogates in the WEB study will of course come with the forthcoming clinical evaluation study. In the meantime, the use of surrogates provided a solution to challenges around timescales and patient access and allowed the research team to maintain momentum within a process of prototype design. It also gave detailed insights into the issues facing the patient group in terms of living with wound dressings. Drawing from the experience of the WEB project, the conclusions are that surrogates can provide vital contributions to medical device design and development. However, they need to be knowledgeable of the day‐to‐day problems that the proposed medical devices are designed to overcome. In addition, surrogates should not be viewed as a complete substitute for direct end user involvement in the various stages of medical devices development. This includes identifying gaps in product availability, defining flaws in existing devices, and ensuring that novel devices are fit for purpose prior to product finalisation and commercialisation.
The manufacturer will take forward the outputs of the project, in terms of new products, to the market place to enable seamless provision of successful products to the people with EB and their lay and professional carers, through health services supply chains. As an academic institution provided the funding for the development of the new products, ownership of the intellectual property arising resides with the institution. The manufacturer has an exclusive licence to manufacture and market the new products in recognition of their significant ‘in kind’ contributions made to develop the new products. Overall reciprocity in terms of respecting each participant's knowledge, particular expertise and contribution has been an underlying project goal along with the model of engaging end users of medical devices in their design and development.
The WEB study is specifically dedicated to improving the quality of wound care for EB. However, there are potential wider generalisations, both for the model of user engagement and the novel dressing retention garments. The model can be viewed as one of knowledge transfer through a user‐centred model of device development and validation, located within a clinical academic setting. The methodologies and tools to accomplish this work have developed systematically from the related projects cited in this paper. With the focus on generating novel designs and products from patients experiences it also shares characteristics with experience‐based co‐design (25), whereby the end user experiences of unmet needs can be turned into design solutions through a collaborative and iterative process of product development. This involves engaging the end users in the design, development, proof of concept, and clinical and cost effectiveness validation of novel products. Once established this model can be self‐sustaining through inward investment from the manufacturers who draw on the findings from the design process, protection of intellectual property and design rights, and licences to manufacture.
References
- 1. Pillay E. Epidermolysis bullosa. Part 1: causes, presentation and complications. Br J Nurs 2008;17:292–6. [DOI] [PubMed] [Google Scholar]
- 2. DebRA. 2011. URL http://www.debra.org.uk/genetic‐research.html [accessed on 24 June 2011].
- 3. Posnet J, Franks P. The burden of chronic wounds in the UK. Nurs Times 2008;104:44–5. [PubMed] [Google Scholar]
- 4. Campling N, Grocott P, Cowley S. Disconnection: the user voice within the wound dressing supply chain. J Nurs Manage 2008;16:204–13. [DOI] [PubMed] [Google Scholar]
- 5. Shah SG, Farrow A, Robinson I. The representation of healthcare end users' perspectives by surrogates in healthcare decisions: a literature review. Scand J Caring Sci 2009;23:809–19. [DOI] [PubMed] [Google Scholar]
- 6. Browne N, Grocott P, Cowley S, Cameron J, Dealey C, Keogh A, Vowden K, Vowden P. Woundcare research for appropriate products (WRAP): validation of the TELER method involving users. Int J Nurs Stud 2004;41:559–71. [DOI] [PubMed] [Google Scholar]
- 7. Grocott P, Blackwell R, Pillay E, Young R. Digital TELER: clinical note‐making and patient outcome measures [serial on the Internet]. Wounds Int 2011;2. URL http://woundsinternational.com/article.php?issueid=336&contentid=122&articleid=10058&page=1 [accessed on 5 March 2012]. [Google Scholar]
- 8. Reason P. The practice of co‐operative inquiry. Syst Pract Act Res 2002;15:169–76. [Google Scholar]
- 9. Heron J, Reason P. A participatory inquiry paradigm. Qual Inq 1997;3:274–94. [Google Scholar]
- 10. Grocott P, Weir H, Bridgelal Ram M. A model of user engagement in medical device development. Int J Health Care Qual Assur 2007;20:484–93. [DOI] [PubMed] [Google Scholar]
- 11. Bridgelal Ram M, Campling N, Grocott P, Weir H. A methodology for a structured survey of the healthcare literature related to medical device users. Evaluation 2008;14:49–73. [Google Scholar]
- 12. Cooper R, Kleinschmidt E. An investigation into the new product process: steps, deficiencies and impact. J Prod Innov Manage 1986;3:71–85. [Google Scholar]
- 13. Sculpher M, Drummond M, Buxton M. The iterative use of economic evaluation as part of the process of health technology assessment. J Health Serv Res Policy 1997;2:26–30. [DOI] [PubMed] [Google Scholar]
- 14. Sheredos S, Cupo M. The department of veterans affairs rehabilitation research and development service's technology transfer process. Technol Disabil 1997;7:25–9. [Google Scholar]
- 15. Medical Research Council. A framework for development and evaluation of RCTs for complex interventions to improve health. URL http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC003372 [accessed on 5 March 2012].
- 16. Medical Research Council. Developing and evaluating complex interventions: new guidance. URL http://www.mrc.ac.uk/complexinterventionsguidance [accessed on 10 October 2010].
- 17. Grocott P, Campling N. A methodology for evaluating wound care products in complex chronic wounds. Wounds UK 2009;5:28–34. [Google Scholar]
- 18. Grocott P, Cowley S, Richardson A. Solving methodological challenges using a theory‐driven evaluation in the study of complex clinical care. Evaluation 2002;8:306–421. [Google Scholar]
- 19. Grocott P, Blackwell R, Weir H, Pilley E. Living in dressings at bandages: findings from workshops with people with epidermolysis bullosa. Int Wound J. In press. [DOI] [PMC free article] [PubMed]
- 20. Sethi P. Appropirate technolgy for rehabilitation aids in developing countries. Ann Nat Acad Med Sci (India) 1982;18:34–42. [Google Scholar]
- 21. Project TRJL. Jaipur limb. The rotary jaipur limb project. URL http://www.rotaryjaipurlimb.co.uk/ [accessed on 5 March 2012].
- 22. Money AG, Barrett J, Kuljis J, Craven MP, Martin JL, Young T. The role of the user within the medical device design and development process: medical device manufacturers' perspectives [serial on the Internet]. BMC Med Inform Decis Mak 2011;15. URL http://www.biomedcentral.com/1472‐6947/11/15 [accessed on 5 March 2012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castle N. Are family members suitable proxies for transitional care unit residents when collecting satisfaction information? Int J Qual Health Care 2005;17:439–45. [DOI] [PubMed] [Google Scholar]
- 24. Bate P, Robert G. Experience‐based design: from redesigning the system around the patient to co‐designing services with the patient. Qual Saf Health Care 2006;15:307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bate P, Robert G. Bringing user experience to healthcare improvement. Oxford: Radcliffe Publishing, 2007. [Google Scholar]


