Abstract
Negative pressure wound therapy (NPWT), which was introduced as a commercial product (V.A.C.® Therapy, KCI USA, Inc., San Antonio, TX) less than 20 years ago, has revolutionised the treatment of complex wounds. Indicated for wide variety of wound types, NPWT is an adjunctive therapy that can be used safely in a range of care settings. Current research indicates that there are four primary NPWT mechanisms of action: macrodeformation, microdeformation, fluid removal and environmental control of the wound. The interaction of the primary mechanisms results in secondary effects through cell signalling (e.g. granulation tissue formation, cell proliferation and modulation of inflammation). Better understanding of the mechanisms of action also provides insight into future directions for NPWT research that could create better solutions for patients with complex wounds.
Keywords: Negative pressure, NPWT, Subatmospheric pressure, VAC, Wound healing
Introduction
Complex wounds are a growing medical problem across all nations. Ageing population, increasing prevalence of type II diabetes mellitus along with increasing rates of obesity all contribute to the rapid incidence of chronic wounds. Armed conflicts and the ability to safely perform complex surgeries add to the number of challenging acute wounds 1. In the USA, the market for advanced wound care products is now approximately $4·4B in size 2 and is expected to increase at an annual rate of 7·3% through 2016 3. These costs do not include hospitalisations, diagnostic tests or physician fees. The cost of healing a diabetic foot ulcer has been estimated to be between $9000 for a simple ulcer, $17 000 for an infected ulcer and $45 000 for a full amputation 4. Clearly, with sustained growth rates in this area and the significant amount of suffering that complex wounds cause patients, better solutions are necessary.
Traditionally, cotton gauze has been the mainstay of wound care and continues to be used frequently today. For simple wounds, gauze serves as an inexpensive product that absorbs exudate and keeps wounds clean and covered. Winter reported in 1962 that wounds kept moist healed at a faster rate than those exposed to air 5. This single observation changed the field of wound care and was largely responsible for the nearly 1500 products available for clinicians to use today. The concepts of keeping the wound clean, moist and adequately debrided have become principal axioms of wound care professionals.
The introduction of flaps, including free tissue transfer, has allowed surgeons to close even the most complex wounds; however, the surgery is specialised, can have high complication rates and leaves scars at the tissue harvest site. Methods that can stimulate the innate ability of wounds to heal would be very valuable to patients.
The physics of negative pressure wound therapy
On Earth we live within a pressurised atmosphere of about 760 mmHg at sea level. In modern jet airplanes, cabins are pressurised to about 635 mmHg to allow passengers to be comfortable and to have adequate oxygen for metabolism. The barometric pressure goes down as altitude increases and very often mountaineers have used supplemental oxygen to assist in their assent to Earth's highest peaks. The behaviour of gases within our atmosphere can be roughly approximated by the ideal gas equation. This equation states that: P × V = n × R × T, where P is the pressure, V the volume, n the number of moles of gas, R the universal gas constant and T is the temperature (degree Kelvin). As V, n, R and T are always positive scalars, P must always be a positive value. In an absolute vacuum, such as outer space, pressure is 0 mmHg. Therefore, negative pressure is a misnomer but is commonly referred to in the literature and relates to gauge pressure or the difference between the applied pressure and the atmospheric pressure where it is applied. However, a chest tube connected to 20 mmHg suction at sea level would have an absolute pressure of 740 mm or a gauge pressure of −20 mmHg.
Closed suction drainage system has been an important adjunct to surgical procedures to facilitate mass transport of liquids from the body. By using this system in the chest, air can be evacuated from the pleural space to allow a lung to expand after a pneumothorax. Closed suction can also be used to drain the stomach contents, which helps to treat small bowel obstruction. The level of suction used must be carefully regulated. For example, surgeons have identified that suction levels that exceed 40 mmHg in the chest can cause damage to the lung. High levels of suction can even remove tissues from the body (e.g. in a liposuction procedure).
Argenta and Morykwas confronted a large number of complex wounds in the early 1990s and endeavoured to develop a better method of treatment 6, 7. They wondered if there was a way to distribute suction to wounds to help draw the edges together and designed several prototypes that facilitated wound healing. The one that has had substantial clinical success consists of an open‐pore polyurethane foam that is placed in the wound, covered by a semi‐occlusive dressing, and connected by a tube to a vacuum source. They called this method negative pressure wound therapy (NPWT). Others have marketed similar devices in which alternative interface materials are used. Another descriptor that more accurately describes the physics of what is happening is the microdeformational wound therapy (MDWT) 8.
The device that Argenta and Morykwas described has revolutionised the treatment of many complex wounds currently, and has become the most common device used to treat these types of wounds in acute care settings in the USA. Growth has also occurred in nursing facilities, home care settings and internationally.
Indications for use
NPWT is commonly used under the trademark V.A.C.® Therapy (KCI USA, Inc., San Antonio, TX). Table 1 lists indicated wound types treated with NPWT 9. These indications cover many of the complex wounds (e.g. Figure 1) encountered in a typical tertiary referral center in the USA. Table 2 provides a summary of contraindications for NPWT 9.
Table 1.
NPWT (V.A.C.® Therapy) indicated wound types 9
| Chronic woundsa |
| Acute woundsa |
| Traumatic woundsa |
| Subacute wounds |
| Dehisced wounds |
| Partial‐thickness burns |
| Ulcers (such as diabetic, pressure or venous insufficiency)a |
| Flaps and graftsa |
NPWT, negative pressure wound therapy.
Some data from randomised controlled clinical trials.
Figure 1.
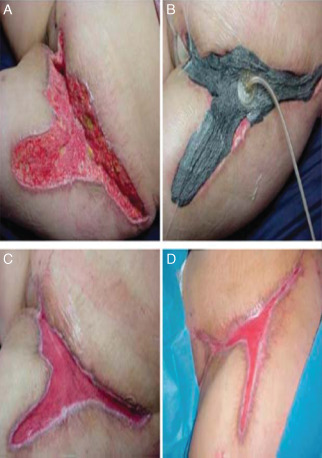
A 34‐year‐old patient with third‐degree burn to her groin and lower abdomen area was treated with negative pressure wound therapy for a 10‐week period. (Photos reprinted with permission from Lauren R. Bayer PA‐C.)
Table 2.
NPWT (V.A.C.® Therapy) contraindications 9
|
NPWT, negative pressure wound therapy.
Refer to Warnings section in the V.A.C.® Therapy Clinical Guidelines for osteomyelitis information 9.
After debridement of necrotic tissue and complete removal of eschar, V.A.C.® Therapy may be used.
Because of the induced proliferation resulting from NPWT, there is a concern that applying the therapy to an area with a known malignancy might potentiate malignancy growth. In our experience, using NPWT devices on wounds that are not adequately debrided, that have necrotic tissue or eschar or have untreated osteomyelitis is not effective. Care must be taken when working around enteric fistulas, but with isolation and careful monitoring it is possible to successfully use NPWT in certain cases 9.
Mechanisms of action
Several NPWT mechanisms of action have been proposed. Because it is essentially a mechanical device, we believe that there are four primary mechanisms of action and potentially multiple secondary actions occurring through cell signalling effects (Figure 2).
Figure 2.
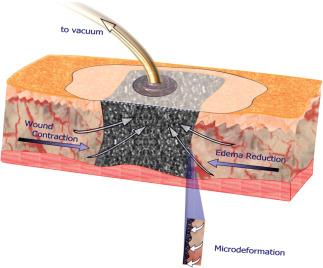
Negative pressure wound therapy combines suction with a foam dressing and a semi‐occlusive drape to keep the wound moist, facilitate fluid removal and stimulate healing at both macrocellular and microcellular levels 1. (Reprinted with permission from Elsevier.)
Primary effects
Macrodeformation: The open‐pore foam draws the wound edges together, depending on the mobility of tissues surrounding the wound 1. For example, in an obese patient with an open abdominal wound, NPWT will often bring the edges close to approximation. In contrast, for a large scalp wound, there will be little deformation of the surrounding tissues.
Microdeformation: We proposed that deformation of the wound surface at a microscopic level stretches cells, facilitating division and proliferation 10.
Fluid removal: In many wounds the surrounding tissues are oedematous, and NPWT has the capacity to remove large amounts of fluid from the extracellular space 6, 11.
Environmental control of the wound: NPWT provides an insulated, warm and moist environment 12.
Secondary effects
Granulation tissue formation: The new NPWT user is impressed with the robust granulation tissue response that occurs as a result of application. There are likely several contributing primary mechanisms including microdeformation, which induces localised hypoxia near the wound surface that upregulates the HIF‐1alpha‐VEGF pathway 13.
Cell proliferation: At least three of the primary mechanisms are likely to contribute to proliferation, including microdeformation 10, fluid removal 11 and maintenance of a warm and moist wound environment 1.
Modulation of inflammation: Inflammation is a critical response to injury, and we are just beginning to learn how this is modulated by NPWT. For example, in mast cell‐deficient mice, granulation tissue response has been shown to be muted, suggesting that mast cells are critical for NPWT success 14.
Change in neuropeptides: We have previously shown in a mouse model that NPWT upregulates neurotransmitters 15.
Change in bacterial levels: Various studies have shown both increased and decreased bacterial levels following the use of NPWT 1, 16.
Additional precautions
There are several important precautions that need to be employed, learnt from years of experience with NPWT. The first is to place only one piece of foam into a single wound, when possible. When more than one piece of foam is placed, clinicians must carefully document the number of foam pieces and develop a mechanism to communicate this number to the next person changing the dressing. In a few instances, wounds have not healed because of retained foam.
When using NPWT around exposed blood vessels and organs, a thick layer of natural tissue or multiple layers of non‐adherent dressing must be placed over the vessels or organs. There have been case reports of bleeding while using NPWT; therefore, such wounds should be carefully monitored while these patients are in the hospital. We also minimise the amount of time that NPWT is used in these wounds.
As previously mentioned, NPWT is not a substitute for adequate debridement or reconstructive techniques such as a skin graft or flap by a well‐trained surgeon. We have seen instances where surgeons have unnecessarily delayed definitive closure in the hope that NPWT will ultimately close a large wound. As with any other wound therapy, if the wound is not progressing, it is incumbent to reassess and consider other therapies.
Care must be taken when using NPWT immediately after a large debridement or when a patient is anticoagulated. The reticulated open‐cell foam (ROCF) dressing is designed to facilitate fluid removal when connected to suction. If bleeding occurs, suction should be stopped to re‐explore the wound or to remove the device and pack the wound with gauze to control bleeding.
Future directions
With continued experience using NPWT, we will be able to determine optimal wound types, foam size, kinetics of application and type of interface material to apply to specific wounds (Figure 3).
Figure 3.
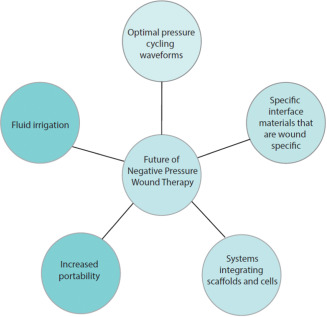
Future directions for negative pressure wound therapy.
The addition of fluids including antiseptics and antibiotics has been proposed and may be a way to extend the usage of NPWT, particularly when bone, tendon or biomaterials are involved. The optimal methods to deliver this therapy still need to be clarified.
Now that a theory has been developed for the mechanisms of action of this class of devices, surgeons will likely innovate to be able to deliver precise deformations of the wound surface. Kane et al. developed a microfabricated silicone device that can be precisely made and could allow suction to be transmitted to the wound. The addition of a perfusion circuit could also deliver cells, cytokines and fluids such as antibiotics 17.
Much work is being done now on scaffolds. Most studies suggest that the NPWT is synergistic with the use of these devices and can potentiate close contact with the wound and minimise complications 18.
Finally, a large amount of work is being done on a variety of stem cells including those of the epidermis and dermis as well as adipose‐derived stem cells that have a large degree of plasticity. In future, treatment of many complex wounds will likely involve some combination of a mechanical device such as NPWT, a dermal scaffold and specific cells.
Acknowledgements
Dr. David P Orgill presented as a faculty member during the 2013 International Surgical Wound Forum (ISWF), an annual educational event sponsored by Kinetic Concepts, Inc. (KCI). His article is part of a KCI‐funded educational supplement based on faculty presentations at 2012 and 2013 ISWF sessions related to wound care strategies with a focus on use of negative pressure wound therapy with instillation (i.e. VA.C. Instill® Wound Therapy and V.A.C. VeraFlo™ Therapy, KCI, San Antonio, TX). KCI assisted with editorial review of the manuscript. Lauren R Bayer states no conflict of interest or financial relationship with KCI.
Orgill DP, Bayer LR. Negative pressure wound therapy: past, present and future.
References
- 1. Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanisms of action of vacuum assisted closure: more to learn. Surgery 2009;146:40–51. [DOI] [PubMed] [Google Scholar]
- 2.2011 Wound Care Product Manufacturing in the US: Market Research Report. 2011. URL http://www.ibisworld.com/industry/wound‐care‐product‐manufacturing.html [accessed on 1 March 2013]
- 3.2012 Wound Care Product Manufacturing in the US: Market Research Report. 2012. URL http://www.ibisworld.com/industry/wound‐care‐product‐manufacturing.html [accessed on 1 March 2013]
- 4. Kruse I, Edelman S. Evaluation and treatment of diabetic foot ulcers. Clin Diabetes 2006;24:91–3. [Google Scholar]
- 5. Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293–4. [DOI] [PubMed] [Google Scholar]
- 6. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 7. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 8. Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, Orgill DP. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 2006;56:418–22. [DOI] [PubMed] [Google Scholar]
- 9.V.A.C. therapy clinical guidelines: a reference source for clinicians. San Antonio: Kinetic Concepts, Inc. URL http://www.kci1.com/KCI1/vactherapyformsandbrochures [accessed on 1 August 2010]
- 10. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 11. Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum‐assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117:121S–6S. [DOI] [PubMed] [Google Scholar]
- 12. Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum‐assisted closure device. Plast Reconstr Surg 2008;122:786–97. [DOI] [PubMed] [Google Scholar]
- 13. Erba P, Ogawa R, Ackermann M, Adini A, Miele LF, Dastouri P, Helm D, Mentzer SJ, D'Amato RJ, Murphy GF, Konerding MA, Orgill DP. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg 2011;253:402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Younan GJ, Heit YI, Dastouri P, Kekhia H, Xing W, Gurish MF, Orgill DP. Mast cells are required in the proliferation and remodeling phases of microdeformational wound therapy. Plast Reconstr Surg 2011;128:649e–58e. [DOI] [PubMed] [Google Scholar]
- 15. Younan G, Ogawa R, Ramirez M, Helm D, Dastouri P, Orgill DP. Analysis of nerve and neuropeptide patterns in vacuum‐assisted closure‐treated diabetic murine wounds. Plast Reconstr Surg 2010;126:87–96. [DOI] [PubMed] [Google Scholar]
- 16. Moues CM, Vos MC, Van Den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum‐assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004;12:11–7. [DOI] [PubMed] [Google Scholar]
- 17. Kane BJ, Younan G, Helm D, Dastouri P, Prentice‐Mott H, Irimia D, Chan RK, Toner M, Orgill DP. Controlled induction of distributed microdeformation in wounded tissue via a microchamber array dressing. J Biomed Mater Res A 2010;95:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molnar JA, DeFranzo AJ, Hadaegh A, Morykwas MJ, Shen P, Argenta LC. Acceleration of Integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg 2004;113:1339–46. [DOI] [PubMed] [Google Scholar]


