Abstract
As a dermal scaffold, artificial dermal substitutes allow the body to accomplish its own tissue regeneration through infiltration of cells and neovascularisation. However, they show not only rather lower take rates compared to autologous skin grafts alone, but they also require more time for sufficient vascular ingrowth to overlay the skin graft. To accelerate this overlaying, we applied vacuum‐assisted closure negative‐pressure settings over the artificial dermis: Terudermis® and Pelnac® grafts. Fourteen patients with complex tissue defects were treated, including bone exposure in two cases, tendon exposure in seven cases and soft tissue defects in five cases. Nine cases had combined wound infections. The time interval between the first artificial dermis graft and the second split‐thickness skin graft over it was 7·64 days on average. Dermal substitutes took place completely in all cases and there were no graft failures.
Keywords: Artificial dermal substitutes, Pelnac®, Terudermis®, Vacuum‐assisted closure
INTRODUCTION
Various artificial skin substitutes have been developed to restore functional and aesthetic integrity in patients with burns, trauma and cancer. They have proved to be a useful aid in many reconstructive surgical procedures. Among them, AlloDerm® (Life Cell Inc., The Woodlands, TX), Integra® (Integra Life Sciences Corp., Plainsborg, NJ), SureDerm® (Hans Biomed Corp., Seoul, Korea), Terudermis® (Terumo Corp., Tokyo, Japan) and Pelnac® (Gunze Corp., Osaka, Japan) have been widely used in resurfacing complex tissue defects 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11. These substitutes serve as scaffolding for the ingrowth of fibroblasts and endothelial cells and provide good dermal regeneration templates. Although only AlloDerm® can be applied in a one‐stage operation with split‐thickness skin grafting over the wound bed simultaneously, most artificial dermises require a second‐stage skin graft and still have a lower take rate because of mechanical weakness, infection and haematoma formation (4).
Many surgeons have proposed the use of negative‐pressure wound therapy to improve split‐thickness skin graft take 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 and donor‐site healing (25). Huckfeldt et al. (26) applied a continuous microcurrent to improve the skin graft take and Molnar et al. (27) and Kim and Hong (9) reported that the vacuum‐assisted closure (VAC) system accelerated the incorporation of Integra® and AlloDerm® over the wound bed. VAC is known to help remove deleterious substances from the wound, relieve oedema and stimulate cell proliferation, thereby promoting granulation and inhibiting chronic inflammation. Furthermore, VAC also seems to assist in neovascularisation of skin grafts and tissue‐engineered skin substitutes. However, investigators have not yet shown the effects of subatmospheric pressure as an adjunct to optimise incorporation of Terudermis® and Pelnac®, which are commonly used products in Korea and Japan 1, 2, 3, 10, 11. We report the effect of subatmospheric pressure on various complex wound defects that would otherwise have required more extensive surgery.
PATIENTS AND METHODS
After obtaining Institutional Review Board approval, we retrospectively reviewed patient records. Between January 2007 and July 2009, 14 complex tissue defects were reconstructed using artificial dermis (Terudermis® and Pelnac®) under subatmospheric negative‐pressure therapy (Table 1). Terudermis® consists of the bottom layer, which is made of a low antigenic atelocollagen that is produced from a calf hide collagen eliminated the telopeptide by a preparation of protease, and the silicone top layer that prevents infection from outside and controls permeability of water such as exudation. Pelnac® is composed of an inner sponge layer of collagen from a pig and an outer layer of silicone. Split‐thickness skin grafts were applied over the surviving artificial dermis as a second‐stage operation. The VAC (KCI Inc., San Antonio, TX) was then replaced and again maintained at continuous subatmospheric pressure (125 mmHg).
Table 1.
Patients' summary
| No. | Age | Sex | Cause of defect | Size (cm2) | Wound site | Exposed in wound | Infection | Time until STSG (days) | Dermal substitute |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | Burn | 20 | Hand | Tendon | × | 8 | TD |
| 2 | 84 | F | Trauma | 9 | Foot | Tendon | PA | 8 | TD |
| 3 | 15 | M | Burn | 36 | Ankle | Tendon | × | 8 | TD |
| 4 | 43 | F | Necrotising fasciitis | 768 | Leg | Tendon | PA | 7 | PN |
| 5 | 9 | F | Trauma | 84 | Thigh | Soft tissue | × | 8 | TD |
| 6 | 36 | M | Trauma | 30 | Trunk | Soft tissue | MRSA | 7 | PN |
| 7 | 19 | M | PBSC | 72 | Foot, ankle | Tendon | × | 8 | PN |
| 8 | 5 | F | PBSC | 99 | Foot, ankle | Tendon | PA | 8 | PN |
| 9 | 73 | M | Trauma | 25 | Leg | Bone | PA | 7 | PN |
| 10 | 63 | F | Wound infection | 45 | Groin | Bone | MRSA | 7 | PN |
| 11 | 68 | F | Trauma | 68 | Trunk | Soft tissue | × | 8 | PN |
| 12 | 52 | M | Trauma | 30 | Foot | Tendon | MRSA | 9 | PN |
| 13 | 73 | M | Trauma | 60 | Foot | Soft tissue | MRSA | 7 | PN |
| 14 | 64 | F | Trauma | 42 | Leg | Soft tissue | MRSA | 7 | PN |
F, female; M, male; MRSA, methicillin‐resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa; PBSC, postburn scar contracture; PN, Pelnac®; STSG, split thickness skin graft; TD, Terudermis®.
In all cases, the wounds were repeatedly debrided until the graft bed was deemed clinically appropriate. Complete excision was performed on necrotic and inflammatory tissues and meticulous haemostasis was achieved to prevent seroma and haematoma formation. Terudermis® or Pelnac® were then grafted onto the region according to wound size and a polyurethane foam was applied over a layer of silicone upon the replaced artificial dermis. The region was sealed with a transparent dressing to avoid air leaks. After this, a tube that was previously attached to the transparent dressing was connected to the VAC and constant negative pressure (125 mmHg) was applied to the region. No splint apparatus was placed in the graft region, even if there was a joint nearby, because the VAC itself worked as a splint apparatus. The dressing was replaced every 4 days and the graft was observed through tissue analysis after obtaining a 3‐mm punch biopsy. The growth of blood vessels from the recipient bed onto the artificial dermis was considered to represent a successful take of the artificial dermis. After the Terudermis® and Pelnac® were deemed clinically incorporated through histological confirmation, the silicone layer was removed and the take rate was estimated with visual inspection. Split‐thickness skin grafts were applied over the Terudermis® and Pelnac® and the VAC was replaced and again maintained at continuous subatmospheric pressure. The VAC was removed after 7 days and skin graft survival was assessed.
RESULTS
There were seven male and seven female patients, with a mean age of 47·0 years (range, 5–84 years) (Table 1). Complex tissue defects were caused by trauma in eight patients, burn in two patients, wound infections in two patients and release of burn scar contractures in two patients. Defects occurred in the lower extremities in ten patients, trunk in three patients and hand in one patient. There were five patients with soft tissue defects, seven with tendon exposure and two with bone exposure. Nine patients exhibited bacterial infections on wound culture: five methicillin‐resistant Staphylococcus aureus and four Pseudomonas aeruginosa. The average tissue defect size was 99·1 cm2 (range: 9–768 cm2).
There were no haematomas or seromas and no signs of infection. No artificial dermises or split‐thickness skin grafts was lost. Two kinds of artificial dermis – Terudermis® and Pelnac®– survived completely within 9 days and had their survival confirmed pathologically. The average interval between initial artificial dermis grafting and the follow‐up split‐thickness skin grafting was 7·64 days (range: 7–9 days). Patients achieved good cosmetic results and stable coverage of their defects.
CASE REPORTS
Patient 2
An 84‐year‐old woman with diabetes mellitus and hypertension sustained a crush injury when a heavy object fell on the dorsum of her left foot (Figure 1). She underwent serial debridements and treatment with a VAC until she had a clean wound with early granulation. After removal of the non viable tissue at the wound site, the resulting wound measured approximately 3 × 3 cm in size and the second extensor digitorum longus was exposed. Wound culture performed at the patient's first visit to our clinic showed P. aeruginosa infection.
Figure 1.
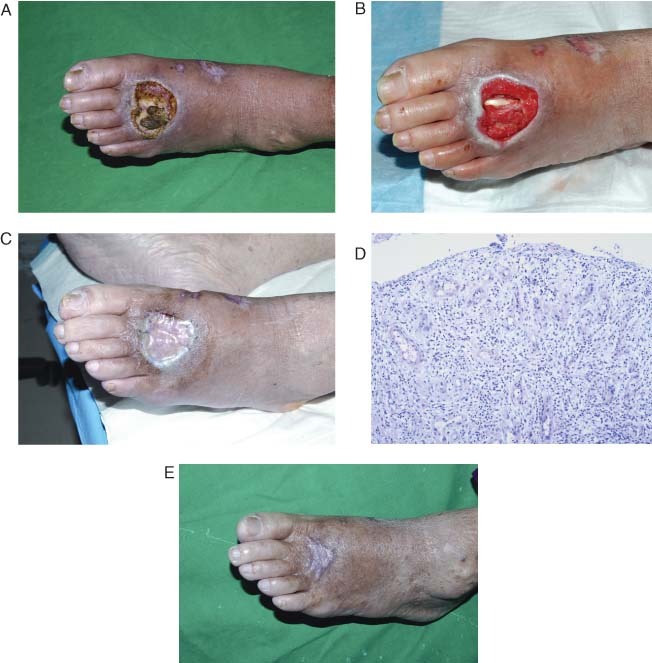
Patient 2 (Table 1). (A) An 84‐year‐old female patient incurred trauma to the dorsum of her left foot. (B) The VAC was applied until healthy granulation tissue was seen. (C) Terudermis® was grafted onto the wound bed. (D) Histological examination of the dermal substitute Terudermis® on the fifth day after surgery showed successful neovascularisation (haematoxylin‐ and eosin‐stained section, ×100). (E) Two months after surgery, the wound showed good resurfacing with split‐thickness skin grafting.
A 3 × 3 cm sheet of Terudermis® was grafted directly onto the lesion with application of VAC. Tissue analysis of the artificial dermis from the wound bed at the fifth postoperative day showed revascularisation of the graft. Blood vessels were observed growing vertically to the surface of the wound. Three days later, a split‐thickness skin graft of 0·011‐inch thickness was applied. VAC was applied continuously for seven more days to achieve acceptable results. The grafts showed a complete take 2 months after surgery.
Patient 4
A 43‐year‐old woman developed necrotising fasciitis in her right lower leg (Figure 2). After the fasciotomy, the patient was transferred to our department for coverage of her soft tissue defects. The wound culture showed P. aeruginosa infection. The patient underwent serial debridements. VAC treatment was applied until she had a clean wound with healthy granulation tissue. Multiple tendons, including the peroneus of the right lower leg, were exposed with a wound defect 64 × 12 cm in size.
Figure 2.
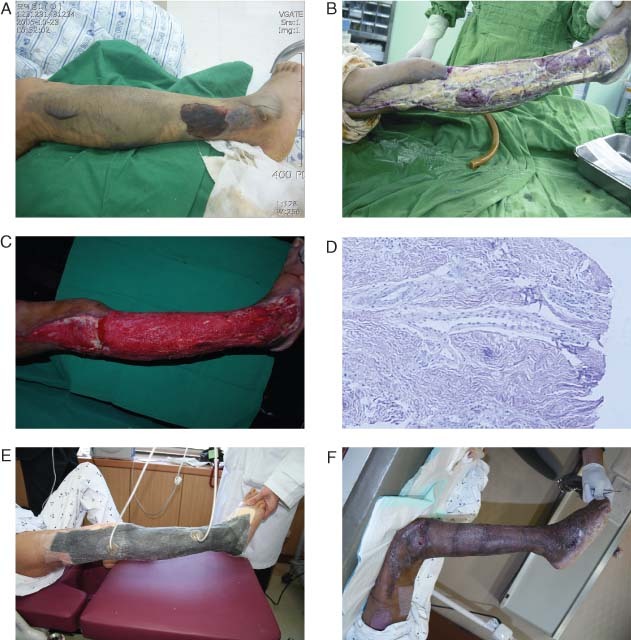
Patient 4 (Table 1). (A, B) A 43‐year‐old female patient had necrotising fasciitis on her right leg resulting in complex tissue defects involving multiple tendons. The defect was 64 × 12 cm in size. Pseudomonas aeruginosa infection was identified through microbiological culture. (C) After serial debridements, a VAC was applied until healthy granulation tissue was visible. Then Pelnac® was grafted on the healthy granulation tissues. This shows the sixth day after Pelnac® grafting. (D) A punch biopsy performed at the same time showed neovascularisation of the Pelnac® graft. Blood vessels had grown up to the wound surface (haematoxylin‐ and eosin‐stained section, ×100). (E) Split‐thickness skin grafting was performed on the seventh day after the Pelnac® graft was placed. The VAC was applied again to accelerate the graft take. (F) The split‐thickness graft took completely without any complications in 2 months.
Pelnac®, an artificial dermis, was grafted directly onto the peroneus tendons and surrounding tissues (graft size, 20 × 5 cm). VAC was applied again simultaneously over the Pelnac® graft. On the sixth postoperative day, punch biopsy confirmed revascularisation of the wound surface and visual inspection showed graft survival. A split‐thickness skin graft, 0·011 inches thick, was performed the next day over the Pelnac® and the whole wound defect. VAC was continued for 7 more days and the autologous skin graft took completely within the following 7 days. The patient was satisfied with the final results.
DISCUSSION
With the development of multiple allografts, the need for flap surgery, a more aggressive and complicated procedure, has been reduced. These allografts allow bone, tendon and other vital, although poorly vascularised, structures to be successfully covered with vascularised tissues without the use of a flap. Many reports have indicated the value of dermal regeneration templates for trauma, burns, cancer, scar release and reconstruction of donor‐site defects where appropriate vascular systems are not available 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11. However, patients usually need a second‐stage operation with skin grafting later, and there is still a high possibility of graft loss because of complications and inappropriate care. Heimbach et al. (4) reported that Integra® grafts had a survival rate of 76·8% in burn patients, and Machens et al. (5) reported that the take rate was much higher ventrally than dorsally in burn patients. Sheridan et al. (6) noted the poorest results in areas such as the axilla, groin, buttock and posterior thigh, where autografts also show their poorest outcomes. The main reasons behind the loss of artificial dermis even before epidermal grafting included infection, haematoma formation, seroma formation and improper mobilisation; these factors also cause loss in skin grafts. We believe that once the bioartificial dermal replacement can be integrated as neodermis, the epidermis take rate might increase as well. Dermal matrices are generally known to become vascularised in 2–4 weeks and they eventually remodel into a dermal equivalent 4, 5. Manufacturers recommend waiting at least 2 weeks before a skin graft is laid over the dermal matrix. Cells from the epidermal autograft grow and form a confluent stratum corneum, thereby closing the wound and reconstituting a functional dermis and epidermis.
With respect to trials aimed at improving the take rate of artificial dermis, Kim and Hong (9) and Molnar et al. (27) applied subatmospheric pressure for the acceleration of AlloDerm® and Integra® incorporation into complex tissue defects. They showed that the VAC system could help increase the graft take in difficult wounds by minimising haematoma and seroma formation, optimising contact with the wound bed and eliminating shear forces 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 30, 31, 32. The VAC system also reduces the need for additional splinting in flexion crease areas because the sponge becomes firm when subatmospheric pressure is applied, making movement impossible (27). In cases of wound infection, micromechanical forces to the wound site not only evacuate interstitial fluid and cellular debris, but also reduce oedema, helping to control wound infection. Negative pressure may also have antibacterial properties through unknown mechanisms 33, 34. Therefore, the mean time for clinically assessed vascularisation of the artificial dermis is shortened with increased incorporation. However, among the various biological artificial dermis products, Terudermis® and Pelnac®, which are commonly used in South Korea and Japan 1, 2, 3, 10, 11, have not yet been shown scientifically to have such an effect. In this series, we were able to secure an increased rate of incorporation for Terudermis® and Pelnac® over the wound bed and shorten the time interval between artificial dermal grafting and epidermal grafting by applying the VAC system.
The VAC device was developed for controlled continuous or intermittent subatmospheric pressure of 125 mmHg, because negative‐pressure wound therapy decreases perfusion beneath negative‐pressure wound therapy dressings (32). Although more than 700 articles have been published on the expansion of indications for vacuum therapy 29, 30, 31, 32, 35, 36, 37, 38, 39, 40, we only found a few reports indicating that it could accelerate the incorporation of artificial dermis, AlloDerm® or Integra®, with onlay skin grafts 9, 27.
The major concerns regarding the use of artificial skin are the waiting period, the prevention of infection and the need for meticulous wound care (7). Ko et al. (10) successfully placed a split‐thickness skin graft over the exposed tibia after 14–18 days of Terudermis® engraftment. Lee et al. (11) reported that an average period of 15·1 days was required for engraftment to cover the radial forearm free flap donor sites. In our series, Terudermis® and Pelnac® were engrafted successfully over an average of 7·64 days without any complications. This period was shorter than the 2–3 weeks recommended by the manufacturing company. Histological examination confirmed engraftment in our study with complete neovascularisation through engraftment of artificial dermis at 5–8 days. Therefore, the use of VAC with Terudermis® and Pelnac® decreases the waiting period, minimises the risk of infection and simplifies wound care, eliminating these concerns. These findings support the idea that a certain amount of negative‐pressure therapy contributes to the beneficial effects of artificial dermis and skin engraftment over complex wound beds.
In conclusion, successful split‐thickness skin engraftment over artificial Terudermis® and Pelnac® dermis grafts may be accelerated with subatmospheric pressure therapy in complex tissue defects. This corresponds to previous reports describing AlloDerm® and Integra® engraftment in conjunction with VAC treatment. Our report indicates that VAC may help reduce the period of hospitalisation and the associated medical costs, as well.
ACKNOWLEDGEMENT
The authors have no financial or personal relationships with other people or organisations that might inappropriately influence their work.
REFERENCES
- 1. Suzuki S, Kawaki K, Ashoori F, Morimoto N, Nishimura Y, Ikada Y. Long‐term follow‐up study of artificial dermis composed of outer silicone layer and inner collagen sponge. Br J Plast Surg 2000;53:659–66. [DOI] [PubMed] [Google Scholar]
- 2. Yurugi S, Hatoko M, Kuwahara M, Tanaka A, Lioka H, Niitsuma K. Usefulness and limitations of artificial dermis implantation for posttraumatic deformity. Aesthetic Plast Surg 2002;26:360–4. [DOI] [PubMed] [Google Scholar]
- 3. Lee JW, Jang YC, Oh SJ. Use of artificial dermis for radial forearm flap donor site. Ann Plast Surg 2005;55:500–2. [DOI] [PubMed] [Google Scholar]
- 4. Heimbach DM, Warden GD, Luterman A, Jordan MH, Ozobia N, Ryan CM, Voigt DW, Hickerson WL, Saffle JR, DeClement FA, Sheridan RL, Dimick AR. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil 2003;24:42–8. [DOI] [PubMed] [Google Scholar]
- 5. Machens HG, Berger AC, Mailaender P. Bioartificial skin. Cells Tissues Organs 2000;167:88–94. [DOI] [PubMed] [Google Scholar]
- 6. Sheridan RL, Hegarty M, Tompkins RG, Burke JF. Artificial skin in massive burns: result to ten years. Eur J Plast Surg 1994;17:91–3. [Google Scholar]
- 7. Chou TD, Chen SL, Lee TW, Chen SG, Cheng TY, Lee CH, Chen TM, Wang HJ. Reconstruction of burn scar of the upper extremities with artificial skin. Plast Reconstr Surg 2001;108:378–84. [DOI] [PubMed] [Google Scholar]
- 8. Lee LF, Porch JV, Spenler CW, Garner WL. Integra in lower extremity reconstruction after burn injury. Plast Reconstr Surg 2008;121:1256–62. [DOI] [PubMed] [Google Scholar]
- 9. Kim EK, Hong JP. Efficacy of negative pressure therapy to enhance take of 1‐stage allodermis and a split‐thickness graft. Ann Plast Surg 2007;58:536–40. [DOI] [PubMed] [Google Scholar]
- 10. Ko JH, Lee JW, Jang YC, Oh SJ. Reconstruction of the tibial exposure by using Terudermis® in extensive deep burned patients on lower extremities. J Korean Burn Soc 2003;6:164–8. [Google Scholar]
- 11. Lee JS, Lee JW, Burm JS, Jang YC, Ha JW. Coverage of donor site defect of radial forearm free flap by using Terudermis®. J Korean Soc Plast Reconstr Surg 2001;28:357–61. [Google Scholar]
- 12. Blackburn JH II, Boemi L, Hall WW, Jeffords K, Hauck RM, Banducci DR, Graham WP. III Negative‐pressure dressing as a bolster for skin grafts. Ann Plast Surg 1998;40:453–7. [DOI] [PubMed] [Google Scholar]
- 13. Molnar JA, DeFranzo AJ, Marks MW. Single‐stage approach to skin grafting the exposed skull. Plast Reconstr Surg 2000;105:174–7. [DOI] [PubMed] [Google Scholar]
- 14. Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004;114:917–22. [DOI] [PubMed] [Google Scholar]
- 15. Sposato G, Molea G, Di Caprio G, Scioli M, La Rusca I, Ziccardi P. Ambulant vacuum‐assisted closure of skin‐graft dressing in the lower limbs using a portable mini‐VAC device. Br J Plast Surg 2001;54:235–7. [DOI] [PubMed] [Google Scholar]
- 16. Rozen WM, Shahbaz S, Morsi A. An improved alternative to vacuum‐assisted closure (VAC) as a negative pressure dressing in lower limb split thickness skin grafting: a clinical trial. J Plast Reconstr Aesthet Surg 2008;61:334–7. [DOI] [PubMed] [Google Scholar]
- 17. Scherer L, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device. A method of securing skin grafts and improving graft survival. Arch Surg 2002;137:930–4. [DOI] [PubMed] [Google Scholar]
- 18. Senchenkov A, Petty PM, Knoetgen J III, Moran SL, Johnson CH, Clay RP. Outcomes of skin graft reconstructions with the use of vacuum assisted closure (VAC®) dressing for irradiated extremity sarcoma defects. World J Surg Oncol 2007;5:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinfeld AB, Kelley P, Yuksel E, Tiwari P, Hsu P, Choo J, Hollier LH. Circumferential negative‐pressure dressing (VAC) to bolster skin grafts in the reconstruction of the penile shaft and scrotum. Ann Plast Surg 2005;54:178–83. [DOI] [PubMed] [Google Scholar]
- 20. Llanos S, Danilla S, Barraza C, Armijo E, Pineros JL, Quintas M, Searle S, Calderon W. Effectiveness of negative pressure closure in the integration of split thickness skin grafts. A randomized, double‐masked, controlled trial. Ann Surg 2006;244:700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Körber A, Franckson T, Grabbe S, Dissemond J. Vacuum assisted closure device improves the take of mesh grafts in chronic leg ulcer patients. Dermatology 2008;216:250–6. [DOI] [PubMed] [Google Scholar]
- 22. Stokes TH, Follmar KE, Silverstein AD, Weizer AZ, Donatucci CF, Anderson EE, Erdmann D. Use of negative‐pressure dressings and split‐thickness skin grafts following penile shaft reduction and reduction scrotoplasty in the management of penoscrotal elephantiasis. Ann Plast Surg 2006;56:649–53. [DOI] [PubMed] [Google Scholar]
- 23. Carson SN, Overall K, Lee‐Jahshan S, Travis E. Vacuum‐assisted closure used for healing chronic wounds and skin grafts in the lower extremities. Ostomy Wound Manage 2004;50:52–8. [PubMed] [Google Scholar]
- 24. Blackburn JH II, Boemi L, Hall WW, Jeffords K, Hauck RM, Banducci DR. Negative‐pressure dressings as a bolster for skin grafts. Ann Plast Surg 1998;40:453–7. [DOI] [PubMed] [Google Scholar]
- 25. Genecov DG, Schneider AM, Morykwas MJ, Parker D, White WL, Argenta LC. A controlled subatmospheric dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg 1998;40:219–25. [DOI] [PubMed] [Google Scholar]
- 26. Huckfeldt R, Flick AB, Mikkelson D, Lowe C, Finley PJ. Wound closure after split‐thickness skin grafting is accelerated with the use of continuous direct anodal microcurrent applied to silver nylon wound contact dressings. J Burn Care Res 2007;28:703–7. [DOI] [PubMed] [Google Scholar]
- 27. Molnar JA, DeFranzo AJ, Hadaegh A, Morykwas MJ, Shen P, Argenta LC. Acceleration of Integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg 2004;113:1339–46. [DOI] [PubMed] [Google Scholar]
- 28. Banwell PE, Musgrave M. Topical negative pressure therapy: mechanisms and indications. Int Wound J 2004;1:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 30. Kairinos N, Voogd AM, Botha PH, Kotze T, Kahn D, Hudson DA, Solomons M. Negative‐pressure wound therapy II: negative‐pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg 2009;123:601–12. [DOI] [PubMed] [Google Scholar]
- 31. Argenta LC, Morykwas MJ, Marks MW, DeFranzo AJ, Molnar JA, David LR. Vacuum‐assisted closure: state of clinic art. Plast Reconstr Surg 2006;117 (Suppl):127S–42S. [DOI] [PubMed] [Google Scholar]
- 32. Morykwas KJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum‐assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117 (Suppl):121S–6S. [DOI] [PubMed] [Google Scholar]
- 33. Venturi ML, Attinger CE, Mesbahi AN. Mechanisms and clinical applications of the vacuum‐assisted closure (VAC) device: a review. Am J Clin Dermatol 2005;16:185–94. [DOI] [PubMed] [Google Scholar]
- 34. Hanasono MM, Skoracki RJ. Securing skin grafts to microvascular free flaps using the vacuum‐assisted closure (VAC) device. Ann Plast Surg 2007;58:573–6. [DOI] [PubMed] [Google Scholar]
- 35. DeFranzo AJ, Pitzer K, Molnar JA, Marks MW, Chang MC, Miller PR, Letton RW, Argenta LC. Vacuum‐assisted closure for defects of the abdominal wall. Plast Reconstr Surg 2008;121:832–9. [DOI] [PubMed] [Google Scholar]
- 36. Mendonca DA, Papini R, Price PE. Negative‐pressure wound therapy: a snapshot of the evidence. Int Wound J 2006;3:261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erdmann D, Wong MS, Lettieri SC, Levin LS, Gunn LA. Use of the vacuum‐assisted closure in the treatment of enterocutaneous fistulas: a follow‐up. Plast Reconstr Surg 2007;15:1092. [DOI] [PubMed] [Google Scholar]
- 38. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–77. [PubMed] [Google Scholar]
- 39. Ichioka S, Watanabe H, Sekiya N, Shibata M, Nakatsuka T. A technique to visualize wound bed microcirculation and the acute effect of negative pressure. Wound Repair Regen 2008;16:460–5. [DOI] [PubMed] [Google Scholar]
- 40. Schneider A, Morykwas M, Argenta L. A new reliable method of securing skin grafts to the difficult recipient bed. Plast Reconstr Surg 1998;102:1195–8. [DOI] [PubMed] [Google Scholar]


