Abstract
Negative pressure therapy has been successfully applied to clean, closed incisions in patients at high‐risk for wound complications. Using laser Doppler flowmetry, we evaluated peristernal perfusion after cardiac surgery via median sternotomy, assessing the influence of mammary artery harvesting and the impact of negative pressure therapy. Twenty adult patients underwent median sternotomy for cardiac surgery followed by routine closure. Negative pressure was applied at 125 mm Hg for 4 days postoperatively in patients with increased risk for wound complications (n = 10, negative pressure group); standard dressings were applied to control incisions postoperatively (n = 10). Presternal perfusion was determined at baseline and daily for 4 days postoperatively using laser Doppler flowmetry. Results within and between groups were compared with analysis of variance. No wound complications were encountered in either group. Perfusion increased among the patients who underwent negative pressure therapy and decreased among the controls (P = 0·004). Mammary artery harvesting reduced peristernal perfusion by 25·7% in the controls, but negative pressure increased perfusion by 100% after mammary harvesting (P = 0·04). Negative pressure therapy increased perfusion relative to controls and compensated for reduced perfusion rendered by mammary artery harvesting, providing additional support for ‘well wound therapy’ in high‐risk patients.
Keywords: Negative pressure, Sub‐atmospheric pressure, Surgery, Thoracic, Wound healing
INTRODUCTION
Median sternotomy is the most versatile surgical approach for performing cardiac surgery, allowing access to all important structures of the heart, great vessels and both pulmonary hila (1). However, there is a well‐defined incidence of sternal wound complications, which can be lethal in cases of deep sternal wound infection or mediastinitis 2, 3, 4, 5, 6, 7. Although several mechanisms for sternal wound complications are proposed, it is widely accepted that reduced sternal perfusion by virtue of internal mammary artery (IMA) harvesting for use as a vascular conduit in coronary artery revascularisation is an important cause of sternal non healing and infection 1, 2, 3, 4, 5, 6, 8. Augmenting sternal perfusion in the postoperative setting may improve outcomes in high‐risk patients.
We recently reported our experience with negative pressure therapy (NPT) as applied to the clean, closed sternotomy incision following median sternotomy for cardiac surgery (9). This form of ‘well wound therapy’ appears to improve wound healing and to prevent incisional complications after sternotomy and other wounds in high‐risk patients 9, 10, 11. However, physiologic data to support these clinical observations and to document improved wound healing characteristics with NPT are lacking.
The present evaluation assessed peristernal perfusion after median sternotomy for cardiac surgery using laser Doppler flowmetry (LDF), which has been used extensively for monitoring wound perfusion including sternal perfusion 12, 13, 14, 15, 16. In addition, the effect of NPT on peristernal blood flow was assessed across varying degrees of residual native sternal perfusion, rendered by IMA harvesting for coronary artery revascularisation.
METHODS
Following local Institutional Review Board approval for retrospective comparison and reporting of two study groups, records of 20 adult male patients who underwent cardiac surgery via median sternotomy were reviewed. IMA harvesting for coronary artery surgery was performed as a pedicle: the accompanying mammary veins and surrounding chest wall muscle and fascia were mobilised with the artery (8). The study cohort was divided into two groups: the treatment group (NPT group, n = 10) had NPT instituted upon the clean, closed sternotomy incision. Application of NPT in this group was based on higher risk profiles for sternal wound complications and has been described previously (9). The control group (n = 10) had standard dressings applied after routine sternal closure and were considered not to have increased risk for sternal wound complications. Sternal closure techniques were identical in each group and consisted of sternal wire circlage to re‐approximate the sternal halves followed by layered re‐approximation of the presternal soft tissues. The skin was re‐approximated with either skin staples or by an absorbable, subcuticular suture at the surgeon's discretion.
The protocol for NPT application and duration has been previously reported (9). Briefly, a narrow strip of non adherent gauze was placed directly on the closed incision, followed by a strip of Granufoam Silver® foam (Kinetic Concepts, Inc. San Antonio, TX). An occlusive, transparent, plastic dressing covered the foam. Negative pressure was applied to the dressing and maintained at −125 mm Hg continuously. Typically, a single dressing change was performed 2 days after surgery to allow for wound inspection and supportive device (chest tubes or temporary pacing wires) removal.
Wounds were monitored postoperatively by the primary surgical team for complications including dehiscence or infection. Wound complications were considered to occur if inpatient hospital readmission was required for wound care or medical therapy directed at cellulitis, drainage, dehiscence or infection was required. When necessary, wound infection was classified as described by Jones et al. for sternal wound infection (17).
Data acquisition and management
Initial LDF measurements were obtained after induction of general anesthesia and prior to the surgical incision (Laserflo BPM2, Vasamedics, St Paul, MN). LDF assessment was performed with 10‐second averaging and a P‐430 right‐angle probe, as has been reported (12). LDF data are unit less, although some have reported the data in ‘perfusion units' or ‘LDF units' 12, 13. After surgical completion, 4–6 hours were allowed for re‐warming and for physiologic stabilisation prior to recording the initial postoperative LDF. Importantly, NPT was not applied to the incision in the NPT group until initial postoperative LDF assessment was made. Subsequently, daily LDF recordings were made for a total of 4 days (Figure 1).
Figure 1.
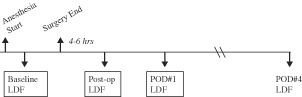
The data acquisition time line of the study shows baseline laser Doppler flowmetry (LDF) values were recorded immediately after anesthetic induction and prior to surgical incision. Initial postoperative LDF values were obtained 4–6 hours after surgery, and daily LDF values were recorded on the first 4 postoperative days.
LDF data were recorded at each time point on a diagram as depicted in Figure 2. As the technique of LDF measurement assesses a relatively small volume of tissue (approximately 1 mm3), data were collected at four separate stations on each side of the anterior chest, relative to the midline sternotomy incision. Baseline LDF and daily postoperative LDF values were averaged across the four stations per hemisternum at each time point, which was felt to most accurately reflect perfusion to each side of the sternum. Furthermore, each hemisternum was evaluated separately as perfusion was thought to be impacted independently by the state of the ipsilateral IMA. For example, if the left IMA was harvested and the right IMA was intact, it is assumed that perfusion to each hemisternum is inherently different, based on IMA harvesting.
Figure 2.
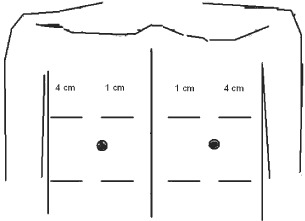
Each dataset was recorded on the template depicted. As laser Doppler flowmetry (LDF) assesses a relatively small volume of tissue (1 mm3), data were collected at four stations per hemisternum relative to the midline sternotomy incision. Average values for each hemisternum were determined for each data collection point. Baseline values for each hemisternum and the maximum postoperative LDF recording were also noted for each hemisternum.
Maximum postoperative LDF values were noted for each sternal half in each patient. Mean baseline and maximum postoperative LDF data were used to determine the percent change in LDF as depicted in Equation (1).
 |
(1) |
Data are presented as mean ± standard deviation unless otherwise noted. Data within and between groups were analysed using analysis of variance (ANOVA), and statistical significance was considered at <0·05.
RESULTS
The composition of both groups with respect to surgical procedure is shown in Table 1. In the NPT group, six patients underwent coronary artery revascularisation using a single IMA, while four patients underwent bilateral IMA harvesting, introducing differing degrees of postoperative sternal perfusion. Among controls, eight patients underwent coronary artery surgery using a single IMA, and two patients had procedures which did not require IMA harvesting. All cases were completed successfully, and all patients were discharged from the hospital in good condition. Importantly, no patients in either group experienced sternal wound complications as defined previously.
Table 1.
Composition of study groups relative to surgical procedure performed
| Procedure performed | NPT group (n = 10) | Control group (n = 10) |
|---|---|---|
| CAB/single IMA | 6 | 8 |
| CAB/both IMA | 4 | 0 |
| Other procedure (no CAB/IMA) | 0 | 2 |
CAB, coronary artery bypass; IMA, internal mammary artery; NPT, negative pressure therapy.
Baseline LDF and maximum postoperative LDF for both groups are shown in Figure 3. There was no difference in baseline peristernal perfusion between groups (NPT group baseline LDF 3·12 ± 2·4 versus control group baseline LDF 3·76 ± 2·4, P = 0·33). Postoperatively, peristernal perfusion improved in the NPT group (baseline LDF 3·02 ± 2·4 versus max postoperative LDF 3·88 ± 2·6, P = 0·13; Figure 3), whereas perfusion worsened in the control group (baseline LDF 3·76 ± 2·4 versus max postoperative LDF 2·86 ± 1·8, P = 0·09, Figure 3). When data were analysed in terms of percent change in LDF across the study timeline, the difference between the two groups for percent change in LDF was statistically significant (NPT group 100 ± 150% versus control group −12·7 ± 70%; P = 0·004, ANOVA, Figure 4).
Figure 3.
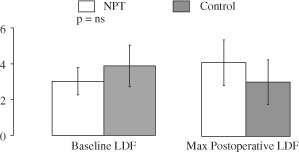
Mean baseline and maximum postoperative laser Doppler flowmetry (LDF) values for the negative pressure therapy (NPT) group (white bars) and the control group (grey bars) are shown. Increased LDF among the NPT group and decreased LDF among controls approached but did not reach statistical significance (P = 0·09).
Figure 4.
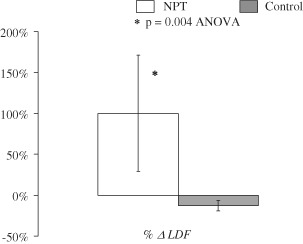
When mean baseline and maximum postoperative laser Doppler flowmetry (LDF) data were analysed as percent change in LDF (% ΔLDF), the difference in postoperative presternal perfusion was significant. Perfusion increased by 100% when wounds were treated with NPT, whereas perfusion decreased by over 12% relative to baseline among controls.
LDF data were also analysed with respect to IMA harvesting and whether or not NPT was applied, allowing assessment of 20 sternal halves in each group (Table 2). When the IMA was intact, NPT (n = 6) improved perfusion (% change LDF) by 101 ± 66%, whereas perfusion decreased by 1·99 ± 20% among controls (n = 12). The difference between these two groups approached statistical significance (P = 0·08 ANOVA, Table 2). When the IMA was harvested, perfusion was reduced by 25·7 ± 22% among controls (n = 8) but was increased by 100 ± 43% with NPT (n = 14). The difference in these two groups was statistically significant (P = 0·037 ANOVA, Table 2).
Table 2.
Impact of negative pressure therapy on presternal perfusion with respect to internal mammary artery (IMA) harvesting
| Status of IMA Intact | Harvested | ||
|---|---|---|---|
| Negative pressure therapy used | Yes | 101 ± 66% (n = 6) | 100 ± 43% (n = 14) |
| No | −1·99 ± 20% (n = 12) | −25·7 ± 22% (n = 8) |
P = 0·037; ANOVA.
DISCUSSION
Sternal wound complications following cardiac surgery are costly, morbid and lethal 2, 3, 4, 5, 6. Despite advancements in most aspects of perioperative care, rates of sternal wound complications, including mediastinitis, following adult cardiac surgery have varied little over the past 30 years 2, 3, 4, 5, 6. Several different factors have been implicated in the development of sternal wound infection, most consistently obesity and diabetes mellitus 5, 6. In addition, using the IMA as a conduit for coronary artery bypass, which has significantly improved short‐ and long‐term results of the procedure 18, 19, has been shown to impact sternal wound healing, particularly when both IMAs are used 18, 20. In fact, most models or hypotheses regarding etiologies of mediastinitis invoke decreased sternal perfusion induced by IMA harvesting, in addition to various patient characteristics 8, 21, 22, 23, 24, 25. Therefore, improving perfusion to the sternum rendered ischemic by IMA harvesting is very appealing, but a few such treatment modalities exist.
The present evaluation assessed peristernal perfusion after median sternotomy and under various degrees of reduced native sternal perfusion as a result of IMA harvesting. These data show that after median sternotomy and IMA harvesting, peristernal perfusion is significantly reduced and recovers little in the time‐frame studied. However, NPT increases peristernal perfusion compared with controls regardless of the status of the ipsilateral IMA (Table 2), providing a rare piece of physiologic evidence for the efficacy of NPT and supports the use of NPT as a form of ‘well wound therapy’, particularly in patients at high‐risk for sternotomy complications. These findings are clinically important and relevant, implying that NPT can augment peristernal soft tissue perfusion made relatively ischemic by IMA harvesting.
Over the past several years, several groups have gained experience with application of NPT to clean, closed incisions as a mechanism to support wound healing physiology 9, 10, 11. This approach appears to be effective in preventing wound complications, is easily applied, and well‐tolerated. In addition, it appears to be a cost‐effective strategy when applied to patients at higher risk for wound complications 9, 11. For example, as previously reported, based on estimated costs of NPT for 4 days ($490) and the known impact on hospital costs of poststernotomy infection required to treat sternal infection (26), prevention of one case of mediastinitis per 100 cases wound provide a cost advantage to the hospital system (9). In order to maintain this fiscal advantage, we have applied NPT to cardiac surgical patients determined objectively to be at increased risk for sternal wound infection, including obese diabetic patients and in those undergoing bilateral IMA harvesting for coronary artery bypass surgery (7). However, the results of the present study may argue for wider application of NPT by showing physiologic advantages that may benefit a broader cohort of patients.
Previous studies suggest that NPT improves sternal perfusion when applied to the open sternotomy incision, but it is unclear if this occurs via augmented flow through the intact IMA or its branches 14, 15, 16. Negative pressure application appears to improve wound microvascular blood flow particularly in muscular tissue (27), which corroborates the findings of the present study. In the context of chest surgery, improved soft tissue perfusion as shown in the present study is certainly clinically relevant because at least one‐half of cases of suspected deep sternal wound infection are actually superficial infections without penetration of the pectoralis fascia 28, 29. In fact, one prospective study found superficial sternal infection to be three times as common as deep sternal infection (30). Furthermore, Fokin et al. have proposed that substrate diffusion through peristernal and soft tissues may be an important mechanism for residual sternal perfusion after IMA harvesting until collateral blood supply to the sternum is well‐established 20, 25. On the basis of this mechanism, NPT may indeed augment sternal and/or periosteal perfusion via improved peristernal ‘diffusion’ through improved soft tissue perfusion as shown in the present study.
The present study has several limitations, including wide variability of individual LDF values. Several explanations exist for this. For example, patients recovered from surgery at differing rates and expressed differing degrees of physiologic recovery at the time of LDF data acquisition. Temperature differences, hemodynamic perturbations, presence of vasoactive medications and intravascular volume status could have affected LDF measurements. To overcome inherent data variability, multiple measurements were taken along each hemisternum at each data collection timepoint. Averaging the individual values at each of the four stations per hemisternum was felt to most accurately represent the aggregate perfusion of each hemisternum and account best for the potential physiologic differences previously noted. Scanning laser Doppler assessment, which provides a broader image and quantitative information concerning regional perfusion could have helped to overcome the heterogeneity of individual LDF data (31), but this was not available for the present study. In addition, subtle differences in IMA harvesting techniques or in IMA anatomical variations could have influenced residual sternal perfusion (22). For example, skeletonised or semi‐skeletonised harvesting of IMA has been shown to preserve sternal perfusion relative to pedicled IMA harvesting (23) while pedicled IMA harvesting reduces retrosternal blood flow (24). Finally, sternal circlage may have also impacted local peristernal perfusion by interruption or reduced flow through IMA perforators or other branches. Once again, this should be overcome by use of mean LDF values at each time point.
These data lack randomisation, but this should have favoured the control group. For example, patients in the control group had reduced risk factors for sternal complications after cardiac surgery, including decreased incidence of diabetes and obesity, and theoretically had better residual sternal perfusion following surgery by virtue of reduced rates IMA harvesting and no cases of bilateral IMA harvesting.
In conclusion, assessment of peristernal perfusion following median sternotomy for cardiac surgery using LDF, showed that applying NPT to the clean, closed incision results in improved perfusion relative to baseline conditions and controls. Importantly, NPT appears to compensate for the reduced peristernal blood flow induced by ipsalateral IMA harvesting. These data provide further support for the use of NPT in patients undergoing cardiac surgery via median sternotomy who are at high risk for sternal healing complications based on their comorbidities or the procedure performed.
DISCLAIMER
The views expressed are those of the authors and do not represent official policy of the Department of Defense, the Department of Veteran Affairs, or the US Government.
REFERENCES
- 1. Dalton ML, Connally SR, Sealy WC. Julian's reintroduction of Milton's operation. Ann Thorac Surg 1992;53:532–3. [DOI] [PubMed] [Google Scholar]
- 2. Hollenbeak CS, Murphy DM, Koenig S, Woodward RS, Dunagan WC, Fraser VJ. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest 2000;118:397–402. [DOI] [PubMed] [Google Scholar]
- 3. Karra R, McDermott L, Connelly S, Smith P, Sexton DJ, Kaye KS. Risk factors for 1‐year mortality after postoperative mediastinitis. J Thorac Cardiovasc Surg 2006;132:537–43. [DOI] [PubMed] [Google Scholar]
- 4. Braxton JH, Marrin CAS, McGrath PD, Ross CS, Morton JR, Norotsky M, Charlesworth DC, Lahey SJ, Clough RA, O’Connor GT. Mediastinitis and long‐term survival after coronary artery bypass graft surgery. Ann Thorac Surg 2000;70: 2004–7. [DOI] [PubMed] [Google Scholar]
- 5. Milano CA, Kesler K, Archibald N, Sexton DJ, Jones RH. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long‐term survival. Circulation 1995;92:2245–51. [DOI] [PubMed] [Google Scholar]
- 6. Olsen MA, Lock‐Buckley P, Hopkins D, Polish LB, Sundt TM, Fraser VJ. The risk factors for deep and superficial chest surgical‐site infections after coronary artery bypass graft surgery are different. J Thorac Cardiovasc Surg 2002;124:136–45. [DOI] [PubMed] [Google Scholar]
- 7. Fowler VG Jr, O’Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation 2005;112 Suppl 1:I–358–65. [DOI] [PubMed] [Google Scholar]
- 8. Berdajs D, Zund G, Turina MI, Genoni M. Blood supply of the sternum and its importance in internal thoracic artery harvesting. Ann Thorac Surg 2006;81:2155–9. [DOI] [PubMed] [Google Scholar]
- 9. Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG. Does negative pressure wound therapy have a role in preventing post‐sternotomy wound complications? Surg Innov 2009;16:140–6. [DOI] [PubMed] [Google Scholar]
- 10. Stannard JP, Robinson JT, Anderson ER, McGwin G, Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. J Trauma 2006;60:1301–6. [DOI] [PubMed] [Google Scholar]
- 11. Stannard JP, Atkins BZ, O’Malley J, Singh H, Bernstein B, Fahey M, Masden D, Attinger CE. Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage 2009;55:58–66. [PubMed] [Google Scholar]
- 12. Heller L, Levin LS, Klitzman B. Laser Doppler flowmeter monitoring of free‐tissue transfers: blood flow in normal and complicated cases. Plast Reconstr Surg 2001;107:1739–45. [DOI] [PubMed] [Google Scholar]
- 13. Yuen JC, Feng Z. Monitoring free flaps using the laser Doppler flowmeter: five‐year experience. Plast Reconstr Surg 2000;105:55–61. [DOI] [PubMed] [Google Scholar]
- 14. Petzina R, Gustafsson L, Mokhtari A, Ingemansson R, Malmsjo M. Effect of vacuum‐assisted closure on blood flow in the peristernal thoracic wall after internal mammary artery harvesting. Eur J Cardiothorac Surg 2006;30:85–9. [DOI] [PubMed] [Google Scholar]
- 15. Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum‐assisted closure therapy. Ann Thorac Surg 2005;79:1724–31. [DOI] [PubMed] [Google Scholar]
- 16. Petzina R, Ugander M, Gustafsson L, Engblom H, Hetzer R, Arheden H, Ingemansson R, Malmsjö M. Topical negative pressure therapy of a sternotomy wound increases sternal fluid content but does not affect internal thoracic artery blood flow: assessment using magnetic resonance imaging. J Thorac Cardiovasc Surg 2008;135: 1007–13. [DOI] [PubMed] [Google Scholar]
- 17. Jones G, Jurkiewicz MJ, Bostwick J, Wood R, Bried JT, Culbertson J, Howell R, Eaves F, Carlson G, Nahai F. Management of the infected median sternotomy wound with muscle flaps. Ann Surg 1997;225:766–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grover FL, Johnson RR, Marshall G, Hammermeister KE. Impact of mammary grafts on coronary bypass operative mortality and morbidity. Ann Thorac Surg 1994;57:559–68. [DOI] [PubMed] [Google Scholar]
- 19. Cameron A, Davis KB, Green G, Schaff HV. Coronary bypass surgery with internal thoracic artery grafts‐effects on survival over a 15‐year period. N Engl J Med 1996;334:216–9. [DOI] [PubMed] [Google Scholar]
- 20. Grossi EA, Esposito R, Harris LJ, Crooke GA, Galloway AC, Colvin SB, Culliford AT, Baumann FG, Yao K, Spencer FC. Sternal wound infections and use of internal mammary artery grafts. J Thorac Cardiovasc Surg 1991;102: 342–6. [PubMed] [Google Scholar]
- 21. Fokin AA, Robicsek F, Masters TN, Fokin A Jr, Reames MK, Anderson JE Jr. Sternal nourishment in various conditions of vascularization. Ann Thorac Surg 2005;79:1352–7. [DOI] [PubMed] [Google Scholar]
- 22. de Jesus RA, Acland RD. Anatomic study of the collateral blood supply of the sternum. Ann Thorac Surg 1995;59:163–8. [DOI] [PubMed] [Google Scholar]
- 23. Lorbergoym M, Medalion B, Bder O, Lockman J, Cohen N, Schachner A, Cohen AJ. 99mTc‐MDP bone SPECT for the evaluation of sternal ischaemia following internal mammary artery dissection. Nucl Med Commun 2002;23:47–52. [DOI] [PubMed] [Google Scholar]
- 24. Knobloch K, Lichtenberg A, Pichlmaier M, Mertsching H, Krug A, Klima U, Haverich A. Microcirculation of the sternum following harvesting of the left internal mammary artery. Thorac Cardiovasc Surg 2003;51:255–9. [DOI] [PubMed] [Google Scholar]
- 25. Medalion B, Katz MG, Lorberboym M, Bder O, Schachner A, Cohen AJ. Decreased sternal vascularity after internal thoracic artery harvesting resolves with time: an assessment with single photon emission computed tomography. J Thorac Cardiovasc Surg 2002;123:508–11. [DOI] [PubMed] [Google Scholar]
- 26. Loop FD, Lytle BW, Cosgrove DM, Mahfood S, McHenry MC, Goormastic M, Stewart RW, Golding LA, Taylor PC. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49:179–87. [DOI] [PubMed] [Google Scholar]
- 27. Wackenfors A, Sjogren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum‐assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 2004;12:600–6. [DOI] [PubMed] [Google Scholar]
- 28. Parisian Mediastinitis Study Group. Risk factors for deep sternal wound infection after sternotomy: a prospective, multicenter study. J Thorac Cardiovasc Surg 1996;111:1200–7. [DOI] [PubMed] [Google Scholar]
- 29. Lucet JC. Surgical site infection after cardiac surgery: a simplified surveillance method. Infect Control Hosp Epidemiol 2006;27:1393–6. [DOI] [PubMed] [Google Scholar]
- 30. Lu JCY, Grayson AD, Jha P, Srinivasan AK, Fabri BM. Risk factors for sternal wound infection and mid‐term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg 2003;23:943–9. [DOI] [PubMed] [Google Scholar]
- 31. Arnold F, He CF, Jia CY, Cherry GW. Perfusion imaging of skin island flap blood flow by a scanning laser‐Doppler technique. Br J Plast Surg 1995;48:280–7. [DOI] [PubMed] [Google Scholar]


