Abstract
Venous leg ulcer slough is unpleasant to the patient and difficult to manage clinically. It harbours infection, also preventing wound management materials and dressings from supporting the underlying viable tissues. In other words, slough has significant nuisance value in the tissue viability clinic. In this study, we have sought to increase our knowledge of slough by building upon a previous but limited analysis of this necrotic tissue. In particular, slough has been probed using Western blotting for the presence of proteins with the capacity to engage microbial surface components recognising adhesive matrix macromolecules. Although the samples were difficult to resolve, we detected fibrinogen, fibronectin, IgG, collagen, human serum albumin and matrix metalloproteinase‐9. Furthermore, the effect of a maggot‐derived debridement enzyme, chymotrypsin 1 on macromolecules in slough was confirmed across seven patient samples. The effect of chymotrypsin 1 on slough confirms our thesis that this potential debridement enzyme could be effective in removing slough along with its associated bacteria, given its observed resistance to intrinsic gelatinase activity. In summary, we believe that the data provide scientists and clinicians with further insights into the potential molecular interactions between bacteria, wound tissue and Lucilia sericata in a clinically problematic yet scientifically interesting wound ecosystem.
Keywords: Bacterial adhesins, Insect serine proteinase, Matrix metalloproteinase, Venous leg ulcer
Introduction
In the UK alone, the cost to the National Health Service of treating chronic, non‐healing wounds such as pressure ulcers, venous leg ulcers (VLUs) and diabetic foot ulcers is estimated to be approximately £2·3–3·1 billion per year 1. This represents a significant financial burden, and the cost looks set to rise, as it has been suggested that the number of non‐healing surgical wounds may increase in the future, because of factors such as the growing complexity of surgery, hospital‐acquired infections and an increasing senior population. A feature of non‐healing wounds is necrotic tissue, which is claimed to support bacterial growth, inhibit the penetration of antibiotics, prevent the formation of granulation tissue and subsequent re‐epithelialisation and interfere with wound contraction 2, 3, 4. To aid wound management, necrotic tissue is often removed, using a procedure termed debridement 4, 5. This may take the form of surgical debridement, or involve the use of topical agents which contain enzymes [for example Santyl or Debrase 6, 7]. Biosurgery using larvae from the greenbottle fly Lucilia sericata has also been shown to be an effective method of wound debridement 8. Debridement in this instance is mediated by an insect serine proteinase (ISP), chymotrypsin 1 9.
Bacteria in the form of biofilms can inhabit necrotic tissue and slough 10, 11, 12, and microbial surface components recognising adhesive matrix macromolecules (MSCRAMMs) play an important role in the initial attachment of bacteria prior to biofilm formation. For example, Pseudomonas aeruginosa agglutinin 1 (PA‐1 L) binds to mannose and galactose residues present on both fibronectin and fibrinogen 13. Similarly, Staphylococcus aureus expresses fibronectin‐binding proteins that bind to both fibronectin and fibrinogen 14. Bacterial adhesins from a number of common bacteria found in infected wounds, and their tissue targets, are summarised in Table 1. Given the potential for macromolecular targets of these bacterial adhesins to be present in wound slough, it is worthwhile to further investigate the protein composition of slough from chronic wounds for their presence.
Table 1.
Bacterial adhesins identified in common wound colonizing bacteria
| Wound infection | Bacterial adhesin | Tissue target | References |
|---|---|---|---|
| Pseudomonas aeruginosa | Agglutinin I (PA‐IL) | Galactose and mannose in fibronectin, fibrinogen, laminin, collagens; mannose in ABO(H) and P blood group glycosphingolipids | 33, 34 |
| Agglutinin II (PA‐IIL) | Mannose in ABO(H) and P blood group glycosphingolipid. Potentially to mannose in the carbohydrate side chains of fibrinogen, fibronectin and laminin | 35, 36 | |
| Sialic acid (N‐acetyl neuraminic acid) binding protein | Potentially binds to N‐acetyl neuraminic acid on fibrinogen and fibronectin | 37 | |
| Staphylococcus aureus | Fibronectin binding proteins A and B | Fibronectin, fibrinogen, elastin | 14, 38 |
| Collagen binding protein | Binds to four repeats of Gly‐Pro‐Hyp or Gly‐Pro‐Pro present in the collagen sequence | 39 | |
| Clumping factors A and B | Fibrinogen. Clumping factor B may also bind to keratin | 40, 41 | |
| Elastin binding protein S (EbpS) | Elastin | 42 | |
| Protein A | Binds the A1 domain of vW factor and the Fc region of IgG | 43 | |
| Staphylococcus epidermidis | SdrG | Binds to the Bβ chain of fibrinogen at the thrombin cleavage site | 44 |
| Autolysin/adhesin (Aae) | Binds vitronectin and the β chain of fibrinogen | 45 | |
| AtI E | Vitronectin | 46 | |
| Elastin binding protein (epb) | Elastin | 47 | |
| Fibronectin binding protein | Fibronectin | 48 | |
| Escherichia coli | Type I fimbriae | Mannose residues on laminin, fibronectin and collagen types 1 and IV | 49 |
| P‐fimbriae | Potentially binds to galactose residues on collagen and fibronectin | 50 | |
| S‐fimbriae | Potentially binds to galactose residues on collagen and fibronectin and N‐acetyl glucosamine residues on laminin, fibronectin, elastin and fibrinogen | 51 | |
| Long polar fimbriae | Binds to mannose residues on laminin, fibronectin and collagen types 1 and IV | 52 | |
| Proteus mirabilis | Mannose‐resistant fimbriae (MRF) | Potentially binds mannose residues on laminin, fibronectin and collagens types I and IV | 53 |
| Uroepithelial cell adhesion (UCA) also known as non‐agglutinating fimbriae (NAF) |
GalNAcβ1‐4Gal present in asialo‐GM1 and asialo‐GM2 glycosphingolipids present in epithelial cells Has the potential to bind to galactose and N‐acetylgalactose residues on fibronectin, laminin, collagen and fibrinogen |
54, 55 | |
| Ambient‐temperature fimbriae (ATF) | Homology to E. coli type I fimbriae (mannose binding), has potential to bind laminin, fibronectin, types 1 and IV collagen | 56 | |
| Enterococcus faecalis | Aggregation substance | Aggregation substance enhances the binding of Enterococcus faecalis to fibronectin, thrombospondin, vitronectin and type I collagen | 57 |
| Klebsiella oxytoca | Mannose‐resistant fimbriae (MRF) homologous to Proteus mirabilis | Has the potential to bind mannose residues on laminin, fibronectin and collagens types I and IV | 58 |
Tissues in bold are the bacterial adhesin tissue target molecules detected in VLU slough which are degraded by Lucilia sericata chymotrypsin 1 (ISP).
As a result, and building on previous observations 9, the present manuscript describes a more comprehensive proteinaceous profile of wound slough from VLUs, accomplished by SDS‐PAGE analysis and Western blotting, using antibodies against a number of wound components considered important for bacterial colonisation.
This study also confirmed the effectiveness of L. sericata chymotrypsin (ISP) in degrading wound slough, and describes the resistance of the maggot debridement enzyme to wound gelatinase activity. These could be pertinent observations, given the clinical need for effective debridement agents and the fact that a recent trial has demonstrated that L. sericata maggots are efficient at debriding necrotic tissue 8.
Materials and methods
Collection and extraction of patient samples
Ethical and R&D approval for the collection of wound slough was obtained from the University of Nottingham ethics committee (REC05/Q2404/194; QDE100502). Wound slough was collected with consent from outpatients attending the Dermatology Clinic at the Treatment Centre located at the Queens Medical Centre, Nottingham. All slough samples were taken from non‐healing VLUs prior to maggot therapy, using the sterile spoon of a Sarstedt faeces sample collection tube (Cat No: 80.734.311). Microbiological analysis of individual slough samples was carried out by the Microbiology Department, Queens Medical Centre, Nottingham. Details of subjects who participated in the study and their microbiological status are shown in Table 2. Wound slough was extracted into sterile phosphate buffered saline (PBS) using a sterile plastic tissue homogeniser suitable for use in 1·5 ml microcentrifuge tubes, then centrifuged at 13 000 g for 15 minutes to remove insoluble material and the supernatant was stored at –20 °C. The protein concentration of the extracted slough was determined using the Biorad protein assay (Bio‐Rad Laboratories; Hemel Hempstead, UK) 15.
Table 2.
Clinical data from patients used in the study
| Patient sample no | Age | Microbiology | Clinical outcome* |
|---|---|---|---|
| 1 | 72 | S. aureus, P. aeruginosa, mixed colonizing bacteria | Healed |
| 2 | 81 | S. aureus, Methicillin‐resistant S. aureus, P. aeruginosa, mixed colonizing bacteria | Healed |
| 3 | 83 | S. aureus | Healed |
| 4 | 77 | S. aureus, β‐haemolytic group C Streptococcus, mixed colonizing bacteria | Healed |
| 5 | 85 | S. aureus, mixed colonizing bacteria | Healed |
| 6 | N/A | N/A | N/A |
| 7 | 80 | Methicillin‐resistant S. aureus, P. mirabilis, mixed colonizing bacteria | Healed |
Microbiological analysis of individual slough samples was carried out by the Microbiology Department, Queens Medical Centre, Nottingham.
N/A indicates data not available.
Post‐treatment with L. sericata.
Wound slough protein degradation by recombinant L. sericata chymotrypsin (ISP)
Recombinant L. sericata chymotrypsin was expressed in insect Sf9 cells and activated and assayed as described previously, using the fluorogenic substrate Suc‐Ala‐Ala‐Pro‐Phe‐AMC 9. ISP used in this study had a specific activity of 7·7 pmol of AMC released/min/mg.
To assess the ability of ISP to degrade wound slough proteins, 7 µg of wound slough was incubated overnight at 37°C alone, or with 1 µg of ISP in a final volume of 50 µl of PBS. Degradation products were precipitated by adding 200 µl of ice cold acetone and incubating at –20°C for 15 minutes followed by centrifugation at 13 000 g for 10 minutes. The precipitated products were re‐suspended in non‐reducing tricine‐SDS‐PAGE sample buffer (200 mM Tris‐HCl, pH 6·8, 2% SDS, 10% glycerol, 0·04% Coomassie Blue G‐250) and incubated for 30 minutes at 37°C. Wound slough proteins and their degradation products were analysed by non‐reducing 5% tricine‐SDS‐PAGE 16. Electrophoresis was carried out at a constant 100 V. Following electrophoresis, gels were either stained with silver 17 or transferred onto nitrocellulose overnight at a constant 26 V 18. Wound slough proteins were resolved under non‐reducing conditions in an attempt to reflect the natural configuration of the proteins in the wound environment. Limited data obtained from a prior analysis of a single sample of VLU tissue 9 aided the selection of a panel of antibodies in order to identify and confirm common protein patterns from VLU slough using Western blotting, giving a holistic view of slough from the wound environment.
Western blot analysis of wound slough proteins
Western blots of the wound slough proteins were blocked for 1 hour in Tris buffered saline (TBS) containing 5% skimmed milk powder. Blocked Western blots were incubated overnight at 4°C in primary antibody diluted in TBS/skimmed milk powder. Rabbit anti‐human fibrinogen, fibronectin, human serum albumin and MMP 9 were diluted at 1:10 000. Rabbit anti‐human MMP 8, was diluted at 1:5000. Goat anti‐collagen types I, III, IV and V were diluted at 1:1000. Primary antibody was discarded and the blots washed (3 × 20 minutes) with TBS containing 0·05% Tween 20 (TBS/Tween). Antibody binding was detected by incubating the blots for 2 hours at room temperature with alkaline phosphatase conjugated goat anti‐rabbit IgG diluted 1:10 000 in TBS/skimmed milk powder or donkey anti‐goat IgG diluted at 1:5000 in TBS/skimmed milk powder. Following incubation in secondary antibody, the blots were washed (4 × 20 minutes) with TBS/Tween. Blots were developed with 200 μM 5‐bromo‐4‐chloro‐3‐indolyl phosphate disodium salt (BCIP) and 20 μM nitro blue tetrazolium (NBT) in 0·75 M Tris pH 9·6. Western blots probed with protein G were blocked as above and incubated for 2 hours at room temperature with alkaline phosphatase conjugated protein G diluted at 1:10 000. Following incubation, blots were washed and developed as described above.
Western blots of wound slough protein pre‐ and post‐ISP degradation were also analysed for the presence of residual ISP. In this case wound slough proteins were resolved by 10% non‐reducing tricine‐SDS‐PAGE prior to transfer onto nitrocellulose. Western blots were probed as described above with rabbit anti‐ISP 19 diluted at 1:1000 in 5% skimmed milk powder followed by alkaline phosphatase conjugated goat anti‐rabbit IgG diluted at 1:10 000 in TBS/skimmed milk powder.
Detection of gelatinolytic activity in wound slough
A total of 2·5 µg of slough protein was incubated overnight at 37°C with 0·5 µg of ISP in a final volume of 10 µl of PBS. Control incubations of wound slough and ISP alone were also included. Gelatinase activity was determined using gelatin substrate SDS‐PAGE where 0·1% gelatin was incorporated into a 5% tricine resolving gel. Following digestion, 10 µl of non‐reducing sample buffer was added to the reaction mix, which was incubated for 30 minutes at 37°C prior to gel loading. Post‐electrophoresis (100 V constant) the gels were washed for 30 minutes with 2·5% Triton X100 followed by 30 minutes with water. To promote gelatinolytic activity, gels were incubated overnight at 37°C in PBS containing 2 mM CaCl2. Gels were stained with Coomassie Brilliant Blue R250 then destained with 25% methanol and 10% acetic acid until gelatinolytic activity was observed as clear banding against a blue background.
Results
Macromolecular profiling of VLU wound slough
VLU slough samples from seven patients (Table 2) were profiled under non‐reducing conditions by tricine‐SDS‐PAGE and Western blotting in an attempt to reflect the natural configuration and interactions of the proteins in the wound environment. Protein profiles were generally similar, with a prominent 60 kDa protein and larger molecular mass proteins resolving from approximately 70 kDa to >250 kDa.
ISP resulted in protein degradation within slough samples, as demonstrated by the disappearance of the predominant 60 kDa and high molecular mass proteins, and the appearance of degradation products below 60 kDa (Figure 1A). The prominent 60 kDa protein resolved with a relative mobility identical to human serum albumin (HSA—Figure 1B); the reference protein appeared as a monomer and dimer.
Figure 1.
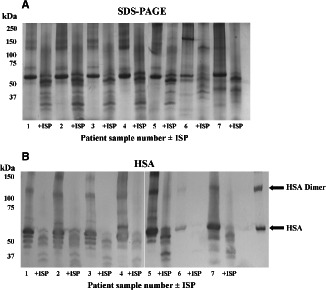
Macromolecular profiles of VLU slough samples and the effects of Lucilia sericata chymotrypsin 1 (ISP). Wound slough from seven patients, pre‐ and post‐treatment with ISP, was separated by 5% tricine‐SDS‐PAGE under non‐reducing conditions. Gels were silver stained (A). Probing for human serum albumin (HSA) resulted in recognition patterns which suggest that the predominant 60 kDa protein is indeed HSA (B). The HSA reference protein would appear to exist in monomeric and dimeric forms. +ISP, maggot chymotrypsin‐treated.
Probing VLU slough for MSCRAMM targets
Fibrinogen (Figure 2A) and fibronectin (Figure 2B) were detected following Western blot with specific polyclonal antibodies. These proteins resolved poorly, and appeared to be degraded when compared to their native molecular masses 20, 21, 22, and internal reference proteins, possibly as a result of autolysis. ISP degraded fibrinogen and fibronectin further. IgG, detected using protein G, was also prominent in five samples (Figure 2C), and was also degraded by ISP. Each of these molecules presents docking sites for MSCRAMMS, therefore their degradation by the maggot enzyme could be significant in terms of the clinical action of L. sericata (Table 1).
Figure 2.
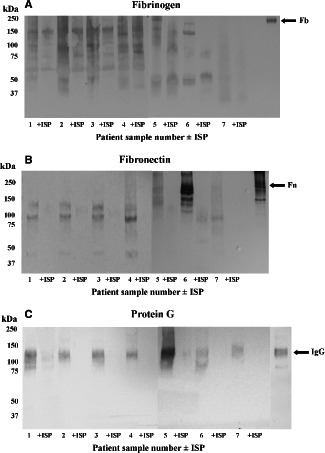
(A–C) Detection of fibrinogen (Fb), fibronectin (Fn) and IgG in VLU samples by Western blotting and the degradative effects of ISP. Wound slough separated by 5% tricine‐SDS‐PAGE under non‐reducing conditions was transferred onto nitrocellulose and probed with polyclonal antibodies specific for Fb and Fn, or with protein G to detect IgG. The relative mobilities of intact reference proteins are indicated by arrows. +ISP, maggot chymotrypsin‐treated.
Collagens, a target for debridement agents like Santyl and for bacterial adhesins (Table 1) were also detected in VLU slough. Collagen types I, III, IV and V were detected in a majority of samples (Figure 3), and were poorly resolved, exhibiting molecular masses >100 kDa. Treatment of wound slough with ISP generally resulted in the degradation of collagens.
Figure 3.
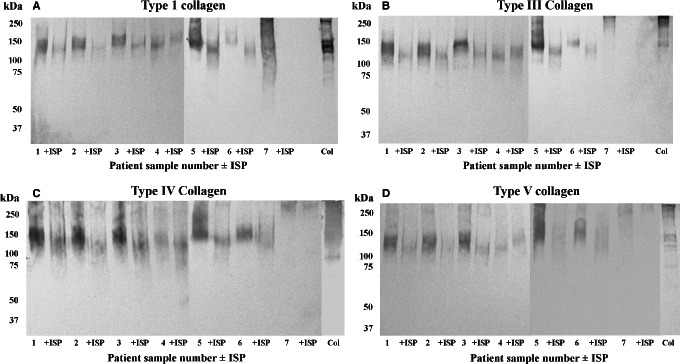
Detection of collagen in VLU slough. Wound slough from seven patients pre‐ and post‐treatment with ISP was separated by 5% tricine‐SDS‐PAGE under non‐reducing conditions, transferred onto nitrocellulose and probed with antibodies to collagen type I (A), type III (B), type IV (C) and type V (D). +ISP, maggot chymotrypsin‐treated.
To summarise at this stage, the predominant macromolecules identified in VLU slough were fibrinogen, fibronectin, human serum albumin, IgG and collagens I, III, IV and V. The majority of these proteins possess docking sites for MSCRAMMs (Table 1), and ISP had a degradative effect in the majority of the analyses conducted. Given that these proteins often resolved at masses lower than their native states before ISP treatment, possibly as a result of autolysis in the wound, slough was subsequently probed for the presence of matrix metalloproteinases (MMPs).
MMP 8 was not detected in any sample following Western blot (not shown). In contrast, MMP 9 was detected in all VLU samples, albeit again in a poorly resolved manner (Figure 4A) and at higher molecular mass than its native form [82 kDa 23], raising the possibility of the presence of MMP 9/protein complexes. Treatment of wound slough with ISP resulted in the downward shift of the MMP 9 profile in some samples. This suggests that the maggot chymotrypsin degrades a tissue gelatinase.
Figure 4.
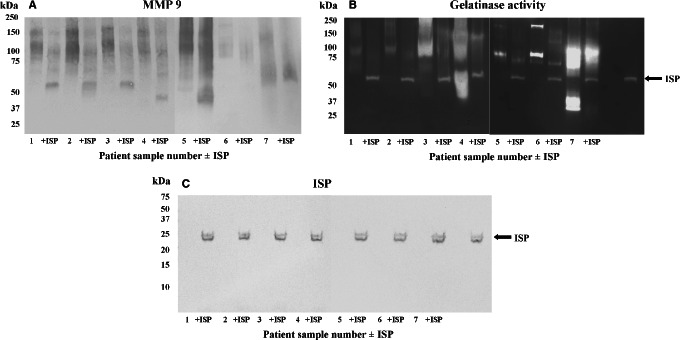
Detection of MMP 9 and gelatinase activity in wound slough. Wound slough was probed for the presence of MMP 9 (A) and analysed by gelatin substrate SDS‐PAGE (B). The presence of residual and intact ISP in VLU samples rich in gelatinolytic activity was detected by Western blot (C). +ISP, maggot chymotrypsin‐treated.
ISP survives gelatinolytic activity in VLU samples
Following the detection of MMP 9 by Western blot, VLU samples were analysed by gelatin substrate SDS‐PAGE pre‐ and post‐incubation with ISP (Figure 4B). Gelatinolytic activity was particularly evident in samples 3, 4 and 7. Treatment with ISP altered the gelatinolytic profile and ISP remained active in the presence of wound gelatinase activity. The intact active ISP reference is indicated by arrows, and a proteinase with identical mobility can be seen in VLU samples where ISP has been added. The molecular integrity of ISP was confirmed by Western blot of VLU samples with an antibody specific to ISP 19 following the addition of ISP to the samples (Figure 4C).
Discussion
This study was designed to increase knowledge of the protein composition of VLU slough, particularly in relation to the presence of macromolecular targets for MSCRAMMs. The effect of maggot chymotrypsin 1 (insect) serine proteinase (ISP) on the macromolecular profile was then confirmed in an increased number of patient samples to that previously reported. The study provided evidence that ISP remained active against macromolecules presenting MSCRAMM targets in what is essentially a gelatinase rich environment.
Clearly, many of the tissue targets for MSCRAMMS, fibrinogen, fibronectin, collagens and IgG, were detected and then degraded by ISP. Further experiments would be required to assess whether the degradation was sufficient to destroy the molecular structures of the exact docking sites on these molecules for MSCRAMMs. In addition, as some of the docking sites involve recognition of sugars on glycosylated proteins, it is also possible that proteolytic digestion supports further activity by glycosidases known to be present in the secretions of L. sericata 24.
This combination of enzymes could facilitate the radical debridement of VLU slough, combining a perturbation of the native state of slough macromolecules with the direct effect of ISP on some MSCRAMMs themselves 25, resulting in an improved VLU wound environment in terms of the removal of necrotic tissue and its associated bacterial burden 5, 26.
Each VLU slough extract was shown to contain gelatinolytic proteinases and the presence of MMP 9 was confirmed by Western blot. MMP 9 (gelatinase B) is a zinc‐dependent enzyme involved in the breakdown of the extra cellular matrix (ECM), tissue remodelling and wound healing 27. It is secreted as an inactive 92 kDa zymogen. The activated 82 kDa enzyme degrades collagens IV, V, XI and laminin 23, 28 following interaction with three fibronectin type 3 repeats 29. In this study, MMP 9 was detected at >100 kDa, suggesting it may have been complexed. Alternatively MMP 9 dimers may have been present 27. However, it is very difficult to obtain accurate molecular masses for many of the constituents of VLU slough following gel analysis, given the nature of the biological material, which is also known to contain DNA.
Despite the clear presence of MMP 9, and intrinsic gelatinase activity, ISP persisted in VLU slough, supporting the use of L. sericata and its chymotrypsin as wound management agents. An analysis of the sequence of L. sericata ISP revealed an absence of MMP 9 cleavage sites 9, 29, possibly explaining the resistance of ISP to the proteinase activity present in the wound environment and the survival of L. sericata serine proteinases in wounds 30.
MMP 9 activity in VLU slough samples could also be responsible for autolysis and the appearance of the macromolecules detected at masses less than their predicted native molecular size. However, some macromolecules resolved at masses higher than their native masses, suggesting as yet unexplained protein–protein interactions in VLUs.
Finally, the relative abundance of HSA in slough needs to be discussed. As the most abundant plasma protein, its presence could be relegated to irrelevant bystander capacity. However, given that HSA has extraordinary ligand‐binding properties 31, for fatty acids and pharmaceuticals, its presence should not perhaps be ignored in the context of developing a full understanding of the wound ecosystem.
In conclusion, we have demonstrated that VLU slough contains the plasma‐derived proteins such as fibrinogen, fibronectin, human serum albumin and IgG, as seemingly intact macromolecules and in varying states of (autolytic?) degradation. In addition, collagens I, III, IV and V were present. Many of these proteins carry motifs receptive for MSCRAMMs (Table 1), therefore their removal by ISP may impede bacterial colonisation and subsequent biofilm formation. Furthermore, ISP appears to be suited to be active in the wound environment, given its resistance to MMP 9 and endogenous wound proteinase inhibitors 32.
This increase in understanding of the macromolecular composition of slough, and its potential to harbour, interact with and possibly protect bacteria could also be of assistance to those in the dressings industry striving to design more effective wound management products.
References
- 1. Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44–5. [PubMed] [Google Scholar]
- 2. Lewis R, Whiting P, ter Riet G, O'Meara S, Glanville A. A rapid and systematic review of the clinical effectiveness and cost effectiveness of debriding agents in treating surgical wounds healing by secondary intention. Health Technol Assess 2008;5:1–131. [DOI] [PubMed] [Google Scholar]
- 3. Ramundo J, Gray M. Enzymatic wound debridement. J Wound Ostomy Continence Nurs 2008;35:273–80. [DOI] [PubMed] [Google Scholar]
- 4. Steed DL. Debridement. Am J Surg 2004;187:71S–4. [DOI] [PubMed] [Google Scholar]
- 5. Vowden K, Vowden P. Debridement made easy. Wounds UK 2011;7:1–4. [Google Scholar]
- 6. Milne CT, Ciccarelli AO, Lassy M. A comparison of collagenase to hydrogel dressings in wound debridement. Wounds 2010;22:270–4. [PubMed] [Google Scholar]
- 7. Singer AJ, Taira BR, Anderson R, Ryon BA, McClain SA, Rosenberg L. Re‐epithelialization of mid‐dermal porcine burns after rapid enzymatic debridement with Debrase® . J Burn Care Res 2011;32:647–53. [DOI] [PubMed] [Google Scholar]
- 8. Dumville JC, Worthy G, Bland JM, Cullum N, Dowson C, Iglesias C, Mitchell JL, Nelson EA, Soares MO, Torgerson DJ. Larval therapy for leg ulcers (VenUS II): randomised controlled trial. BMJ 2009;338:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Telford G, Brown AP, Seabra RAM, Horobin AJ, Rich A, English JSC, Pritchard DI. Degradation of eschar from venous leg ulcers using a recombinant chymotrypsin from Lucilia sericata . Br J Dermatol 2010;163:523–31. [DOI] [PubMed] [Google Scholar]
- 10. Harrison‐Balestra C, Cazzaniga AL, Davis SC, Mertz PM. Wound isolated Pseudomonas aeruginosa grows a biofilm in vitro within 10 hours and is visualized by light microscopy. Dermatol Surg 2003;29:631–5. [DOI] [PubMed] [Google Scholar]
- 11. van der Plas MJA, Jukema GN, Wai S‐W, Dogterom‐Ballering HCM, Lagendijk EL, van Gulpen C, van Dissel JT, Bloemberg GV, Nibbering PH. Maggot excretions/secretions are differentially effective against biofilms of Staphylococcus aureus and Pseudomonas aeruginosa . J Antimicrob Chemother 2008;61:117–22. [DOI] [PubMed] [Google Scholar]
- 12. Hall‐Stoodly L, Stoodly P. Evolving concepts in biofilm infections. Cell Microbiol 2009;11:1034–43. [DOI] [PubMed] [Google Scholar]
- 13. Rebiere‐Huët J, Di Martino P, Hulen C. Inhibition of Pseudomonas aeruginosa adhesion to fibronectin by PA‐IL and monosaccharides: involvement of a lectin‐like process. Can J Microbiol 2004;50:303–12. [DOI] [PubMed] [Google Scholar]
- 14. Henderson B, Nair S, Pallas J, Williams MA. Fibronectin: a multidomain host adhesin targeted by bacterial fibronectin‐binding proteins. FEMS Microbiol Rev 2011;35:147–200. [DOI] [PubMed] [Google Scholar]
- 15. Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 16. Schagger H, Von Jagow G. Tricine sodium dodecyl sulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 kDa to 100 kDa. Anal Biochem 1987;166:368–79. [DOI] [PubMed] [Google Scholar]
- 17. Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987;8:93–9. [Google Scholar]
- 18. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocelluose sheets: procedure and some applications. Proc Nat Acad Sci USA 1979;76:4350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pritchard DI, Telford G, Diab M, Low W. Expression of a cGMP compatible Lucilia sericata insect serine proteinase debridement enzyme. Biotechnol Prog 2012;28:567–72. [DOI] [PubMed] [Google Scholar]
- 20. Roy S, Sun A, Redman C. In vitro assembly of the component chains of fibrinogen requires endoplasmic reticulum factors. J Biol Chem 1996;271:24544–50. [DOI] [PubMed] [Google Scholar]
- 21. Ruoslahti E, Pierschbacher M, Engvall E, Oldberg Å, Hayman EG. Molecular and biological interactions of fibronectin. J Invest Dermatol 1982;79(S1):658–88. [DOI] [PubMed] [Google Scholar]
- 22. Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology 2005;41:1211–9. [DOI] [PubMed] [Google Scholar]
- 23. Toth M, Chvyrkova I, Bernardo MM, Hernandez‐Barrantes S, Fridman R. Pro‐MMP‐9 activation by the MT1‐MMP/MMP‐2 axis and MMP‐3: role of TIMP‐2 and plasma membranes. Biochem Biophys Res Commun 2003;308:386–95. [DOI] [PubMed] [Google Scholar]
- 24. Telford G, Brown AP, Rich A, English JS, Pritchard DI. Wound debridement potential of glycosidases of the wound healing maggot, Lucilia sericata . Med Vet Entomol 2012;26:291–9. [DOI] [PubMed] [Google Scholar]
- 25. Harris LG, Nigam Y, Sawyer J, Mack D, Pritchard DI. Lucilia sericata chymotrypsin disrupts protein mediated Staphylococcal biofilm formation. App Environ Microbiol 2013;79:1393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolcott RD, Kennedy JP, Dowd SE. Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care 2011;18:54–6. [DOI] [PubMed] [Google Scholar]
- 27. Olson MW, Bernardo MM, Pietila M, Gervasi DC, Toth M, Kotra LP, Massova I, Mobashery S, Fridman R. Characterization of the monomeric and dimeric forms of latent and active matrix metalloproteinase‐9.Differential rates for activation by stromelysin 1. J Biol Chem 1999;275:2661–8. [DOI] [PubMed] [Google Scholar]
- 28. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardivasc Res 2006;69:562–73. [DOI] [PubMed] [Google Scholar]
- 29. Kridel SJ, Chen E, Kotra LP. Substrate hydrolysis by matrix metalloproteinase 9. J Biol Chem 2001;276:20572–8. [DOI] [PubMed] [Google Scholar]
- 30. Schmidtchen A, Wolff H, Rydengard V, Hansson C. Detection of serine proteases secreted by Lucilia sericata in vitro and during treatment of a chronic leg ulcer. Acta Derm Venereol 2003;83:310–1. [DOI] [PubMed] [Google Scholar]
- 31. Fasano F, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005;57:787–96. [DOI] [PubMed] [Google Scholar]
- 32. Telford G, Brown AP, Rich A, English JSC, Pritchard DI. Maggot chymotrypsin I from Lucilia sericata is resistant to endogenous wound protease inhibitors. Br J Dermatol 2011;164:192–6. [DOI] [PubMed] [Google Scholar]
- 33. Townsend RR, Hilliker E, Li Y‐T, Laine RA, Bell WR, Lee YC. Carbohydrate structure of human fibrinogen. J Biol Chem 1982;257:9704–10. [PubMed] [Google Scholar]
- 34. Chen C‐P, Song S‐C, Gilboa‐Garber N, Chang KSS, Wu AM. Studies on the binding site of the galactose‐specific agglutinin PA‐IL from Pseudomonas aeruginosa . Glycobiology 1998;8:7–16. [DOI] [PubMed] [Google Scholar]
- 35. Winzer K, Falconer C, Garber NC, Diggle SP, Camara M, Williams P. The Pseudomonas aeruginosa lectins PA‐IL and PA‐IIL are controlled by quorum sensing and by RpoS. J Bacteriol 2000;182:6401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu AM, Wu JH, Singh T, Liu J‐H, Tsai A‐S, Gilboa‐Garber N. Interactions of the fucose‐specific Pseudomonas aeruginosa lectin, PA‐IIL, with mammalian glycoconjugates bearing polyvalent Lewis and ABH blood group glycotopes. Biochimie 2006;88:1479–92. [DOI] [PubMed] [Google Scholar]
- 37. Ramphal R, Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa in the lower respiratory tract. Infect Immun 1983;41:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burke FM, McCormack Rindi S, Speziale P, Foster TJ. Fibronectin‐binding protein B variation in Staphylococcus aureus . BMC Microbiol 2010;10:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Symersky J, Patti JM, Carson M, House‐Pompeo K, Teale M, Moore D, Jin L, Schnieder A, Delucas LJ, Höök M, Narayana SVL. Structure of the collagen‐binding domain from a Staphylococcus aureus adhesin. Nat Struct Biol 1997;4:833–8. [DOI] [PubMed] [Google Scholar]
- 40. McDevitt D, Francois P, Vaudaux P, Foster TJ. Identification of the ligand‐binding domain of the surface‐located fibrinogen receptor (clumping factor) of Staphylococcus aureus . Mol Microbiol 1995;16:895–907. [DOI] [PubMed] [Google Scholar]
- 41. Eidhin DN, Perkins S, Francois P, Vaudaux P, Höök M, Foster TJ. Clumping factor B (ClfB), a new surface‐located fibrinogen‐binding adhesin of Staphylococcus aureus . Mol Microbiol 1998;30:245–57. [DOI] [PubMed] [Google Scholar]
- 42. Downer R, Roche F, Park PW, Mecham RP, Foster TJ. The elastin‐binding protein of Staphylococcus aureus (EbpS) is expressed at the cell surface as an integral membrane protein and not as a cell wall‐associated protein. J Biol Chem 2002;277:243–50. [DOI] [PubMed] [Google Scholar]
- 43. O'Seaghdha M, van Schooten CJ, Kerrigen SW, Emsley J, Silverman GJ, Cox D, Lenting PJ, Foster TJ. Staphylococcus aureus protein A binding to von Willebrand factor A1 domain is mediated by conserved IgG binding regions. FEBS J 2006;273:4831–41. [DOI] [PubMed] [Google Scholar]
- 44. Davis SL, Gurusiddappa S, McCrea KW, Perkins S, Höök M. SdrG, a fibrinogen‐binding bacterial adhesin of the microbial surface components recognizing adhesive matrix molecules subfamily from Staphylococcus epidermidis, targets the thrombin cleavage site in the Bβ chain. J Biol Chem 2001;276:27799–805. [DOI] [PubMed] [Google Scholar]
- 45. Heilmann C, Thumm G, Chaatwal S, Hartlieb J, Uekötter A, Peters G. Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis . Microbiology 2003;149:2769–78. [DOI] [PubMed] [Google Scholar]
- 46. Heilmann C, Hussain M, Peters G, Gtz F. Evidence for autolysin‐mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol 1997;24:1013–24. [DOI] [PubMed] [Google Scholar]
- 47. Park PW, Rosenbloom J, Abrams WR, Rosenbloom J, Mecham RP. Molecular cloning and expression of the gene for elastin‐binding protein (ebpS) in Staphylococcus aureus . J Biol Chem 1996;271:15803–9. [DOI] [PubMed] [Google Scholar]
- 48. Williams RJ, Henderson B, Sharp LJ, Nair SP. Identification of a fibronectin‐binding protein from Staphylococcus epidermidis . Infect Immun 2002;70:6805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krogfelt KA, Bergmans H, Klemm P. Direct evidence that the FimH protein is the mannose‐specific adhesin of Escherichia coli Type 1 fimbriae. Infect Immun 1990;58:1995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Korea C‐G, Ghigo J‐M, Beloin C. The sweet connection: solving the riddle of multiple sugar‐binding fimbrial adhesins in Escherichia coli . Bioessays 2011;33:300–11. [DOI] [PubMed] [Google Scholar]
- 51. Moch T, Hoschützky H, Hacker J, Kröncke K‐D, Jann K. Isolation and characterization of the a‐sialyl‐, 8‐2,3‐galactosylspecific adhesin from fimbriated Escherichia coli . Proc Natl Acad Sci USA 1987;84:3462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Farfan MJ, Cantero L, Vidal R, Botkin DJ, Torres AG. Long polar fimbriae of enterohemorrhagic Escherichia coli O157:H7 bind to extracellular matrix proteins. Infect Immun 2011;79:3744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rocha SPD, Pelayo JS, Elias WP. Fimbriae of uropathogenic Proteus mirabilis . FEMS Immunol Med Microbiol 2007;51:1–7. [DOI] [PubMed] [Google Scholar]
- 54. Lee KK, Harrison BA, Latta R, Altman E. The binding of Proteus mirabilis nonagglutinating fimbriae to ganglio‐series asialoglycolipids and lactosyl ceramide. Can J Microbiol 2001;46:961–6. [PubMed] [Google Scholar]
- 55. Altman E, Harrison BA, Latta RK, Lee KK, Kelly JF, Thibault P. Galectin‐3‐mediated adherence of Proteus mirabilis to Madin‐Darby canine kidney cells. Biochem Cell Biol 2001;79:783–8. [PubMed] [Google Scholar]
- 56. Massad G, Bahrani FK, Mobley HL. Proteus mirabilis fimbriae: identification, isolation, and characterization of a new ambient‐temperature fimbria. Infect Immun 1994;62:1989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rozdzinski E, Marre R, Susa M, Worth R, Muscholl‐Silberhorn A. Aggregation substance‐mediated adherence of Enterococcus faecalis to immobilized extracellular matrix proteins. Microb Pathog 2001;30:211–20. [DOI] [PubMed] [Google Scholar]
- 58. Old DC, Adegbola RA. A new mannose‐resistant haemagglutinin in Klebsiella . J Appl Bacteriol 1983;55:165–72. [DOI] [PubMed] [Google Scholar]


