Abstract
Infiltration of surgical wounds with long‐acting local anaesthetics (LA) is used to reduce postoperative incisional pain. We hypothesised that infiltration with LA interferes with wound healing in rats. Seventy‐two rats were allocated into nine groups. After intraperitoneal anaesthesia, the interscapular dorsal region was infiltrated with equivolumes of saline, 0·5% bupivacaine or ropivacaine, in a randomised double‐blind fashion. A standardised incision was performed in the infiltrated area and sutured closed. The rats were euthanised on the 3rd or 14th day after the operation and tissue from the incision site was subjected to histochemical analyses and mechanical testing (MT). Compared with the control group, bupivacaine displayed a significant increase in the macrophage number on day 3 (+63% versus +27% for ropivacaine). The transforming growth factor β‐1 expression had a significant increase in the LA (versus saline) groups, +63% in ropivacaine group and +115% in bupivacaine group on day 3 (P < 0·05). The collagen fibres as measured by dyed area were significantly higher in the bupivacaine group on day 3 (+56%, P < 0·01 versus +15% for ropivacaine). CD34 was reduced in bupivacaine group (−51%, P < 0·05 versus +3% for ropivacaine). On day 14, no statistical differences were observed in either LA group (versus saline) with respect to histopathologic or inflammatory mediators. MT on day 14 showed no differences between the LA and saline groups. The LA‐induced increases in histological markers did not extend beyond the third day, suggesting that wound infiltration with long‐acting LA does not impair the wound healing process in rats.
Keywords: Anaesthetics, Immunohistochemistry, Local, Mechanical test, Wound Healing
Introduction
Infiltration of the surgical incision site with long‐acting local anaesthetics (LA) at the end of an operation is a common clinical practice to reduce incisional pain. Despite their widespread use, concerns have been raised regarding the potential adverse effects of long‐acting LA on wound healing (1). When skin is injured by a surgical incision, platelets release numerous chemotactic factors, including the transforming growth factor β‐1 (TGF‐β1). This growth factor is responsible for accumulation of leucocytes at the site of injury, thereby initiating the inflammation phase. TGF‐β1 is involved in all stages of wound healing (2). The recruitment of inflammatory cells into the wound site appears to play an important role in the quality of the wound‐healing response (3).
Tissue injury can also induce a cascade of inflammatory cytokines and the resultant peripheral and central nervous system (CNS) sensitisation can lead to postoperative hyperalgesia (4). When long‐acting LA are injected at incision sites for postoperative pain management, they have been reported to reduce the stimulation produced by cytokines and minimise hyperalgesia (5). Although some studies suggest that LA have anti‐inflammatory effects (6), other reports suggest the opposite effect 1, 7). Wound healing is a biological process and a logical sequence of phases has been demonstrated in animal models (8, 9).
The pharmacokinetic and dynamic profiles of bupivacaine and ropivacaine are similar in both animals and humans 8, 9. Despite they possessing comparable analgesic profiles (10), animal studies have suggested that bupivacaine and ropivacaine may differ with respect to both CNS and cardiotoxicity (11). Although opioid analgesics have been reported to increase collagen synthesis and wound tensile strength (12), little is known regarding the influence of these two long‐acting LA on the mechanical properties of wound healing.
Using a rodent model, we designed a randomised, double‐blind placebo‐controlled study to evaluate the inflammatory and wound healing processes when the incision site was infiltrated with long‐acting LA. We hypothesised that subcutaneous administration of these long‐acting LA (versus saline) would interfere with the wound healing process, resulting in reduced tensile strength at the incision site at 2 weeks after surgery.
Materials and methods
Study animals
Seventy‐two male Wistar rats, weighing approximately 200 gm each, were housed in separate cages in a temperature‐controlled room (24°C ± 1°C) and maintained on a 12h:12h light–dark cycle with a relative humidity of 60–70% at the Ribeirão Preto School of Medicine. The rats were provided with the same diet, a regular rodent chow (57·3% carbohydrate, 41·2% as starch, Labina; Purina®;, São Paulo, Brazil) (13) and tap water on an ad libitum basis. The animals were maintained in agreement with the guidelines of the Committee on Care and Uses of Laboratory Animals of the National Research Council of the N.I.H. (USA) and the study was approved by the local Commission of Ethics in Animal Research (Protocol #039/2010).
Experimental design
A total of nine groups of rats (each with an n = 8) received equivolumes of 0·9% saline (S), plain 0·5% bupivacaine (B) or plain 0·5% ropivacaine (R) at a standardised 1·5 cm long incision. Three groups were euthanised on the third day (S3, B3 and R3), and three groups on the 14th day (S14, B14 and R14) after the operation for the histological and inflammatory mediator assays (Figure 1). In addition, mechanical testing (MT) was performed on three additional groups of rats (Smt, Bmt and Rmt) on the 14th day after the incision.
Figure 1.
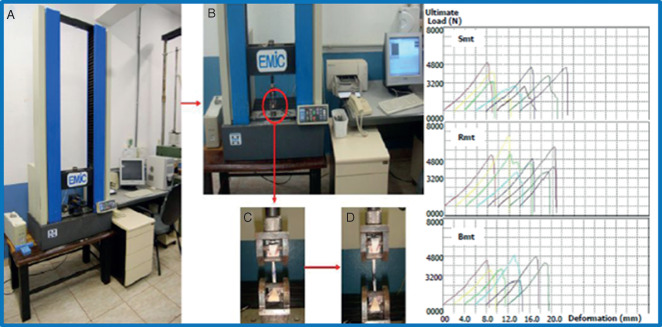
Mechanical testing machine with the graphs obtained from each animal (A); with the skin attached (B); skin before the rupture (C) and breakpoint of skin at the end of the mechanical test (D). mt, mechanical testing; S, saline; B, bupivacaine; R, ropivacaine.
All animals were anaesthetised with an intraperitoneal injection of ketamine (40 mg/kg) and xylazine (10 mg/kg). After shaving the skin and applying povidone‐iodine (PVP‐iodine), the subcutaneous interscapular dorsal region of the animals was infiltrated with 1 ml of the ‘blinded’ study medication containing saline (S), bupivacaine (B) or ropivacaine (R) according to a computer‐generated randomisation table. In order to ensure proper binding, tissue samples were identified by a number and lettering system which avoided any reference to the specific treatment group. All study groups were blinded until after the statistical analysis had been completed.
A transversal cutaneous incision interscapular (1‐cm length) was performed using a surgical blade #15, deep enough to reach the dorsal muscle aponeurosis (2 mm) in the area that the study medication was injected. Sterile black non‐absorbable surgical sutures composed of inorganic protein, 3–0 polyester/cotton (Ethicon Inc., San Angelo, TX) was used to close the incision. The whole surgical procedure required approximately 10 minutes. Supplemental oxygen was administered by a mask until the rats were awake.
The animals were euthanised (using Carbon dioxide asphyxiation) on the 3rd or 14th day after the operation. For the histological essaying, the skin containing the incision site was removed (1 cm2) and fixed in 10% buffered paraformaldehyde.
Mechanical testing
For the mechanical tests, the animals were euthanised on the 14th day in the biomechanical laboratory and the fresh skin flap containing the original incision was immediately placed in the device used for tensile strength measurements (Figure 1). The specific treatment group (e.g. control [saline], bupivacaine, or ropivacaine) was blinded to the investigators at all times during the evaluation periods. The mechanical traction testing was performed using a Universal Testing Machine (EMIC®;, DL10000, São José dos Pinhais, PR, Brazil) working with a load cell capacity of either 500 or 10 000 N. The tissue strip was placed on a support and a traction load was vertically applied to the tip of the tissue at a rate of 2 mm/min. Data were processed by software that computed the ultimate load, deformation, stiffness and maximum tension.
Histological analysis
Tissue paraffin‐embedded 5‐µm‐thick sections were stained with haematoxylin–eosin (HE) for general analysis, Verhoeff for elastin fibres and Picrosirius red (PSR) for collagen fibres. Images for the tissue sections were examined using a microscopic system (Leica, Microsystems GmbH, Wetzlar Germany), recorded by a video camera (Leica Microsystems Ltd, Heebrugg, Switzerland) and registered in a computer. For the quantification of the area occupied by collagen and elastic fibres (i.e. the morphometric analyses), the images were assessed by a blinded investigator using Leica Qwin software (Leica Microsystems Image Solutions, Cambridge, UK).
Areas of elastin and collagen in the skin were determined by optical density in 20 randomly chosen non‐coincident fields at a magnification of 400×, giving a total area of 0·5 mm2. The elastic and collagen fibres had particular coloration, and their occupied areas could be measured by the microscope software system and expressed as percentages. Inflammatory cells and neovessels were scored by immunohistochemistry technique and counted in 20 randomly chosen non‐coincident microscope fields. The magnification used was 400×, in a total area of 0·5 mm2 and the mean values were calculated as cells or vessels/area (mm2).
The antibodies used for immunohistochemical study were CD68 (clone ED‐1, 1/100, Serotec®; Ltd, Oxford, UK) for the macrophage study; COX‐2 (clone 4H12, 1:200, Novocastra®;, Newcastle upon Tyne, UK) for the inflammatory response; CD34 (clone QBEnd/10, 1:100, Novocastra®;) and the VEGF (clone SC‐152, 1:100, Santa Cruz Biotechnology®;, Inc, Santa Cruz, CA) for the neovascularisation and the TGF‐β1 (clone TGFB17, 1:100, Novocastra®;) was used to evaluate the fibroblast proliferation (14).
Statistical analyses
Considering earlier wound healing studies in the rat model 15, 16, 17) and assuming a statistical power of 0·90 and a significance criterion of 0·05, for a two‐tailed statistical analysis, the minimum group sizes were >5 (18). Eight animals were analysed in each group. For each lamina chosen for histological study, 20 randomly selected, non‐coincident fields were chosen, for a minimum total of 100. All the data were expressed as mean values ± SD. The comparisons between the groups were made by one‐way analysis of variance and post hoc Newman–Keuls' test for multiple comparisons. The level of significance was set at 5% (P < 0·05). The software used was the GraphPad Prism software version 4·0 (GraphPad Software Inc., San Diego, CA).
Results
On the 3rd and 14th days after the operations, neither ropivacaine nor bupivacaine was associated with significant changes in elastic fibres concentration, VEGF or COX‐2 expression (Table 1). Similarly, the tissue MT properties (i.e. ultimate load, deformation, stiffness and maximum tension) were not different between the local anaesthetic and saline groups on the 14th postoperative day (POD; Table 2). One of the rats in the bupivacaine group (Bmt) was excluded from the final analysis because the surgical area had been scratched.
Table 1.
Histological and immunohistochemical analysis on the 3rd and 14th postoperative days *
| Postoperative day #3 | Postoperative day #14 | |||||
|---|---|---|---|---|---|---|
| Elastic fibres/area % | COX‐2 Li | VEGF‐Li | Elastic fibres/area % | COX‐2 Li | VEGF‐Li | |
| Saline | 11·0 ± 3·0 | 2·2 ± 0·8 | 0·43 ± 0·24 | 10·0 ± 4·7 | 2·0 ± 0·59 | 0·64 ± 0·27 |
| Ropivacaine | 12·1 ± 2·5 | 2·4 ± 0·6 | 0·4 ± 0·22 | 11·7 ± 0·72 | 2·0 ± 0·54 | 0·88 ± 0·22 |
| Bupivacaine | 9·5 ± 2·0 | 1·9 ± 0·4 | 0·65 ± 0·25 | 11·4 ± 1·3 | 1·2 ± 0·3 | 0·59 ± 0·28 |
*There were no statistical differences among the three different treatment groups (mean values ± SD).
Table 2.
Mechanical testing for the three treatment groups on the 14th postoperative day *
| Ultimate load (N) | Deformation (mm) | Stiffness (N/mm) | Maximum tension (PA) | |
|---|---|---|---|---|
| Saline | 4·93 ± 1·06 | 8·25 ± 1·39 | 0·49 ± 0·061 | 637 265 ± 142 957 |
| Ropivacaine | 3·89 ± 0·77 | 6·96 ± 1·33 | 0·44 ± 0·060 | 562 308 ± 146 010 |
| Bupivacaine | 4·21 ± 0·51 | 6·94 ± 0·59 | 0·47 ± 0·65 | 524 019 ± 105 406 |
*There were no statistically significant differences among the three treatment groups (mean values ±SD).
On third POD, the collagen concentration was increased in the B3 group compared with the S3 (+56%; P < 0·01) and the R3 (+36%; P < 0·05) groups (Figure 2). TGF‐β1‐positive cell infiltration in the wound site was significantly increased in R3 (+63%) and in B3 (+115%) groups compared with the S3 group (Figure 3). Macrophage number (CD68) was increased +63% (P < 0·05) in the B3 group and +27% (P > 0·05) in the R3 group compared with the S3 group (Figure 4). A significant reduction of CD34+ cells infiltrating in the wound site in the B3 group (−51%) was observed compared with the S3 and the R3 (−53%) groups (P < 0·05 and <0·01, respectively; Figure 5).
Figure 2.
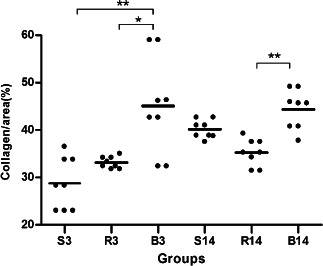
Collagen concentration expressed on the 3rd day (S3, R3 and B3) and 14th day (S14, R14 and B14) in the S, saline; B, bupivacaine; R, ropivacaine groups. *P < 0·05, ∗∗ P < 0·01 and ∗∗∗ P < 0·001. The lines represent the mean values ± SEM for the three treatment groups and the individual data points are represented by dots above and below the mean value.
Figure 3.
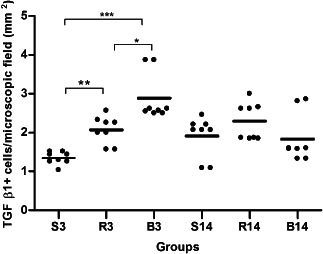
TGF‐β1 expression on 3rd day (S3, R3 and B3) and 14th day (S14, R14 and B14) with S, saline; B, bupivacaine and R, ropivacaine. ∗ P < 0·05, ∗∗ P < 0·01 and ∗∗∗ P < 0·001. The lines represent the mean values ± SEM for the three treatment groups and the individual data points are represented by dots above and below the mean value.
Figure 4.
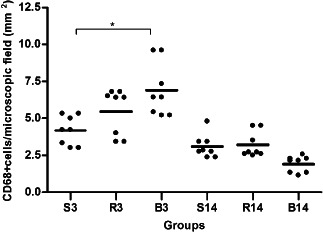
CD68 expression on day 3 (S3, R3 and B3) and day 14 (S14, R14 and B14); (S,R,B) saline, bupivacaine and ropivacaine. ∗ P < 0·05. The lines represent the mean values ± SEM for the three treatment groups and the individual data points are represented by dots above and below the mean value.
Figure 5.
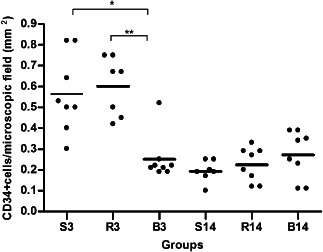
CD34 expression on day 3 (S3, R3 and B3) and day 14 (S14, R14 and B14); (S,R,B) saline, bupivacaine and ropivacaine. ∗ P < 0·05, ∗∗ P < 0·01 and ∗∗∗ P < 0·001. The lines represent the mean values ± SEM for the three treatment groups and the individual data points are represented by dots above and below the mean value.
On the 14th POD, the collagen concentration in both groups (R14 and B14) was not significantly different from saline group, although there was an increase in B14 group, when compared with R14 group (+23%, P < 0·01). All the other parameters analyzed on the 14th POD were unaltered by either bupivacaine or ropivacaine compared to saline (Figure 3).
Discussion
Using a rat model of surgical wound healing, we were unable to find any deleterious effects at the incision site with respect to changes in histochemical markers and mechanical (strength) testing of the tissue associated with infiltration of the surgical incision using a long‐acting LA. Fibroblasts have an important role in the wound healing process (19). The myofibroblast differentiation process is regulated by a complex repertoire of profibrotic and antifibrotic cytokines, including the transforming growth factor isoform TGF‐β1 (20). We observed that the increase in the collagen concentration was directly proportional to TGF‐β1‐positive cell infiltrating in the wound site. Collagen type I is the most abundant structural protein in skin, and it directly influences tissue mechanical behaviour (21). During the first 14 days of the repair process, the wound tensile strength is directly proportional to the amount of collagen deposited (22). On the basis of this finding, we performed MT of wound strength on the 14th POD to evaluate the influence of bupivacaine and ropivacaine on the wound healing process.
The traction resistance at the surgical incision site is reported as the maximum tensile strength. The wound tensile strength is related to the collagen deposition and the maximum extension is related to the elastic fibre content (23). In our study, we found that neither of the two long‐acting LA altered the amount of elastic fibre at the incision site as a result elasticity (deformation) compared with saline (control) group. The greater initial increase in collagen triggered by the increase in TGF‐β1 in the bupivacaine group on POD 3 did not alter any of the mechanical parameters evaluated on POD 14 (e.g. ultimate load, deformation, stiffness and maximum tension). These data suggest that wound infiltration with these long‐acting LA should not adversely affect the tissue mechanical properties of the surgical wound during the healing process. Of interest, Chang et al. (12) demonstrated that high‐doses of systemic morphine enhanced TGF‐β1 expression, and the opioid analgesic increased the collagen deposition in the subcutaneous tissue, increasing wound tensile strength.
Wound inflammation also plays an important role in the development of new tissue (24). Inflammation causes the induction of cyclooxygenase‐2 (COX‐2), which sensitise peripheral nociceptor terminals and can lead to localised hypersensitivity to pain. Although LA have been reported to produce anti‐inflammatory actions (6), pro‐inflammatory cytokines secreted by macrophages can exert inflammatory effects on surrounding cells and tissues (7). At the incision site, monocytes are recruited by the local tissue trauma and differentiate into macrophages. The macrophages play a central role in all stages of wound healing process. The specific laboratory marker used to quantify the macrophage number is CD68. The presence of growth factors, like TGF‐β, is important for the production of fibroblasts (and keratinocytes). Fibroblasts are responsible for collagen fibres production, another essential factor in the wound repair process. Finally, new vessel formation (angiogenesis) is essential to bring nutrients and oxygen for new tissue formation and the overall success of the entire wound reparative process. The CD34+ cell count is an early marker of vasculogenesis, and VGEF is the growth factor that is used to evaluate the overall process of vasculogenesis (25, 26). The activation response of macrophages following phagocytosis is varied depending on the extracellular environment (8, 9). In the current study, the extracellular environment was apparently stimulated by both bupivacaine and ropivacaine. However, the increase in the macrophages number on POD #3 in the bupivacaine group (and to a lesser extent in the ropivacaine group) may have counteracted any anti‐inflammatory action of these two LA.
The inflammatory process has direct effects on normal and abnormal wound healing and in the balance of cytokines, like transforming growth factor‐β (TGF‐β) and prostaglandin E2 (PGE2) (27). The administration of IL‐1 receptor antagonist (100 mg/kg ANAK) within the perioperative period could decrease postsurgical wound pain in a murine incisional wound model (28). Deegan et al. (29), studied 11 cytokines and 3 matrix metalloproteinase (MMP) in patients who have undergone breast surgery with and without thoracic paravertebral block and found no difference between the two groups with respect to 10 of the 14 inflammatory molecules they evaluated. Patients who received paravertebral analgesia exhibited decreases in IL‐1β, MMP‐3 and MMP‐9, and a significant increase in IL‐10, an important inhibitor of the pro‐inflammatory cytokines IL‐1, IL‐6, IL‐8, TNF and MMPs (29).
While LA block the conduction of nociceptive input, the induction of cytokines is not necessarily inhibited. Bupivacaine has a pro‐inflammatory effect, and has been associated with stimulation of COX‐2 gene expression and higher PGE2 production, and pain after the local anaesthetic effect dissipates (30). Bupivacaine and tetrodotoxin did not diminish cytokine induction during carrageenan hind paw inflammation in rats (31), and sciatic nerve blockade in animals with hind paw inflammation did not prevent increases in central COX‐2 or PGE2 levels as effectively as administration of a cytokine inhibitor (32). In our study, the LA were not associated with an anti‐inflammatory response due to the relative short duration of their blocking action following a single injection at the incision site.
For effective wound healing an adequate supply of oxygen and nutrients is essential, consequently, the vascularisation has an important role. As the circulating CD34+ cells function as endothelial cell progenitors, they represent the vasculogenesis state (33). To measure the quantity of these cells, the CD34+ cell incorporation test was used. It has been reported that participation of CD34+ cell is a key process in the initial phase of wound healing process (34). In our study, bupivacaine reduced the CD34 expression on POD #3, but the effect did not persist until POD #14. Vasculogenesis is an essential component of normal wound healing and repair by removal of debris and assists the development of a granulation tissue framework for wound closure (35). VEGF may contribute to the angiogenic stimuli in wounds by direct effects on proliferation and migration of endothelial cells or indirectly by affecting persistent vascular permeability at the level of existing microvessels, which occurs during the early phases of wound repair (36). In our study, we did not find statistically significant differences in VEGF expression in the ropivacaine and bupivacaine groups compared with the saline groups, suggesting that these LA did not impaired the vasculogenesis process. Nevertheless, it must be emphasised that these preliminary findings in normal animals may not be the same in animals or humans with impaired wound‐healing conditions (e.g. diabetes). The fall in the number of CD34+ cells in the bupivacaine‐treated group (B3) may have deleterious effects on wound healing in diabetic patients.
The deficiencies in this study design relate the use of a single application of the LA. Consequently there was a short exposure to potential deleterious effects of the drugs on the local tissue. It would be interesting to compare different concentrations of these LA, as well as localised infusions of bupivacaine and ropivacaine in future studies. Although experimental rat models are commonly used for wound healing studies (9), these finding may not be extrapolated directly to human beings. Studies involving a longer duration of exposure to the LA would be of interest to determine if there are any long‐term effects on the wound healing process. The small group sizes (n = 8 each) could have increased the possibility of a type II statistical error. However, given the genetic homogeneity of the rodents used in this study, the small group sizes were justified.
The short incision was performed to minimise the possibility of contamination of the healing process due to scratching of the area by the rat itself (one animal had to be eliminated and replaced due to local tissue infection secondary to scratching of the incision site). Importantly, the length of the incision (1 cm) was adequate for both the histochemical and mechanical strength testing procedures. In this preliminary study, the two time points chosen to assess wound healing (namely, 3 and 14 days after the incision) were based on the typical time required for wound healing in this rat model. However, it is important to point out that wound healing in rats occurs more rapidly than in humans. Therefore, further investigative studies in other animal models and humans are necessary to insure that the negative findings we reported in this preliminary rat model can be translated into the clinical practice of wound healing in post‐surgical populations. These further studies are also warranted to confirm the safety of the common clinical practice of infiltrating LA both prior to the skin incision, as well as at end of the surgical procedure.
Compared with the saline (control) group, there appeared to be a small positive benefit of bupivacaine (and to a lesser extent ropivacaine) at the third POD with respect to the biochemical measures. However, there were no significant differences among the three study groups with respect to tensile strength on the 14th POD. Therefore, we conclude that preincisional infiltration of a surgical wound with the long‐acting LA bupivacaine and ropivacaine did not adversely influence histological wound healing parameters or reduce the tensile strength of the locally infiltrated (skin) tissue in this rodent model.
Acknowledgement
All the coauthors made substantial contributions to this study and have read and approved the content of this manuscript. None of the authors have any conflicts of interest to report with respect to the study medications or the content of this manuscript. Financial support was provided by Faculty of Medicine of Ribeirão Preto, University of São Paulo, Ribeirão Preto, São Paulo, Brazil, and White Mountain Institute, 144 Ashby Lane, Los Altos, CA 94022.
References
- 1. Grishko V , Xu M , Wilson G , Pearsall AW. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg 2010. ; 92 : 609 – 18. [DOI] [PubMed] [Google Scholar]
- 2. O'Kane S , Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol 1997. ; 29 : 63 – 78. [DOI] [PubMed] [Google Scholar]
- 3. Cowin AJ , Brosnan MP , Holmes TM , Ferguson MW. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev Dyn 1998. ; 212 : 385 – 93. [DOI] [PubMed] [Google Scholar]
- 4. Buvanendran A , Kroin JS. Does manipulating local surgical wound cytokines improve surgical outcomes?. Anesth Analg 2010. ; 111 : 1335 – 6. [DOI] [PubMed] [Google Scholar]
- 5. Gordon SM , Brahim JS , Dubner R , McCullagh LM , Sang C , Dionne RA. Attenuation of pain in a randomized trial by suppression of peripheral nociceptive activity in the immediate postoperative period. Anesth Analg 2002. ; 95 : 1351 – 7. table of contents [DOI] [PubMed] [Google Scholar]
- 6. Cassuto J , Sinclair R , Bonderovic M. Anti‐inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand 2006. ; 50 : 265 – 82. [DOI] [PubMed] [Google Scholar]
- 7. Brand S. Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut 2009. ; 58 : 1152 – 67. [DOI] [PubMed] [Google Scholar]
- 8. Arthur GR , Feldman HS , Covino BG. Comparative pharmacokinetics of bupivacaine and ropivacaine, a new amide local‐anesthetic. Anesth Analg 1988. ; 67 : 1053 – 8. [PubMed] [Google Scholar]
- 9. Yi JW , Lee BJ , Kim DO , Park SW , Choi YK , Chang HK , Kim CJ , Park JH. Effects of bupivacaine and ropivacaine on field potential in rat hippocampal slices. Br J Anaesth 2009. ; 102 : 673 – 9. [DOI] [PubMed] [Google Scholar]
- 10. Zink W , Graf BM. The toxicity of local anesthetics: the place of ropivacaine and levobupivacaine. Curr Opin Anaesthesiol 2008. ; 21 : 645 – 50. [DOI] [PubMed] [Google Scholar]
- 11. Heavner JE. Local anesthetics. Curr Opin Anaesthesiol 2007. ; 20 : 336 – 42. [DOI] [PubMed] [Google Scholar]
- 12. Chang PJ , Chen MY , Huang YS , Lee CH , Huang CC , Lam CF , Tsai YC. Morphine enhances tissue content of collagen and increases wound tensile strength. J Anesth 2010. ; 24 : 240 – 6. [DOI] [PubMed] [Google Scholar]
- 13. de Moura RF , Ribeiro C , de Oliveira JA , Stevanato E , de Mello MA. Metabolic syndrome signs in Wistar rats submitted to different high‐fructose ingestion protocols. Br J Nutr 2009. ; 101 : 1178 – 84. [DOI] [PubMed] [Google Scholar]
- 14. Andrade TA , Iyer A , Das PK , Foss NT , Garcia SB , Coutinho‐Netto J , Jordao‐Jr AA , Frade MA. The inflammatory stimulus of a natural latex biomembrane improves healing in mice. Braz J Med Biol Res 2011. ; 44 : 1036 – 47. [DOI] [PubMed] [Google Scholar]
- 15. Li H , Fu X , Zhang L , Huang Q , Wu Z , Sun T. Research of PDGF‐BB gel on the wound healing of diabetic rats and its pharmacodynamics. J Surg Res 2008. ; 145 : 41 – 8. [DOI] [PubMed] [Google Scholar]
- 16. Seveljevic‐Jaran D , Cuzic S , Dominis‐Kramaric M , Glojnaric I , Ivetic V , Radosevic S , Parnham MJ. Accelerated healing of excisional skin wounds by PL 14736 in alloxan‐hyperglycemic rats. Skin Pharmacol Physiol 2006. ; 19 : 266 – 74. [DOI] [PubMed] [Google Scholar]
- 17. Sun TZ , Fu XB , Zhao ZL , Chen W , Sun XQ , Zhou G , Shen ZY. Experimental study on recombinant human platelet‐derived growth factor gel in a diabetic rat model of cutaneous incisal wound healing. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2003. ; 15 : 596 – 9. [PubMed] [Google Scholar]
- 18. Eng J. Sample size estimation: how many individuals should be studied?. Radiology 2003. ; 227 : 309 – 13. [DOI] [PubMed] [Google Scholar]
- 19. Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 2003. ; 200 : 500 – 3. [DOI] [PubMed] [Google Scholar]
- 20. Lagares D , Garcia‐Fernandez RA , Jimenez CL , Magan‐Marchal N , Busnadiego O , Lamas S , Rodriguez‐Pascual F. Endothelin 1 contributes to the effect of transforming growth factor beta1 on wound repair and skin fibrosis. Arthritis Rheum 2010. ; 62 : 878 – 89. [DOI] [PubMed] [Google Scholar]
- 21. Puxkandl R , Zizak I , Paris O , Keckes J , Tesch W , Bernstorff S , Purslow P , Fratzl P. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Phil Trans R Soc Lond Ser B-Biol Sci 2002. ; 357 : 191 – 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ehrlich HP , Keefer KA , Maish GO , Myers RL , Mackay DR. Vanadate ingestion increases the gain in wound breaking strength and leads to better organized collagen fibers in rats during healing. Plast Reconstr Surg 2001. ; 107 : 471 – 7. [DOI] [PubMed] [Google Scholar]
- 23. Nunes JA Jr , Ribas‐Filho JM , Malafaia O , Czeczko NG , Inacio CM , Negrao AW , Lucena PL , Moreira H , Wagenfuhr J Jr , Cruz Jde J. Evaluation of the hydro‐alcoholic Schinus terebinthifolius Raddi (Aroeira) extract in the healing process of the alba linea in rats. Acta Cir Bras/Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia 2006. ; 21 ( Suppl 3 ): 8 – 15. [DOI] [PubMed] [Google Scholar]
- 24. Eming SA , Krieg T , Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007. ; 127 : 514 – 25. [DOI] [PubMed] [Google Scholar]
- 25. Scull CM , Hays WD , Fischer TH. Macrophage pro‐inflammatory cytokine secretion is enhanced following interaction with autologous platelets. J Inflamm (Lond) 2010. ; 7 : 1 – 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Behm B , Babilas P , Landthaler M , Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol 2012. ; 26 : 812 – 20. [DOI] [PubMed] [Google Scholar]
- 27. Su WH , Cheng MH , Lee WL , Tsou TS , Chang WH , Chen CS , Wang PH. Nonsteroidal anti‐inflammatory drugs for wounds: pain relief or excessive scar formation? Mediat Inflamm 2010. ; 1 – 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu Y , Liang D , Li X , Liu HH , Zhang X , Zheng M , Dill D , Shi X , Qiao Y , Yeomans D , Carvalho B , Angst MS , Clark JD , Peltz G. The role of interleukin‐1 in wound biology. Part II: in vivo and human translational studies. Anesth Analg 2010. ; 111 : 1534 – 42. [DOI] [PubMed] [Google Scholar]
- 29. Deegan CA , Murray D , Doran P , Moriarty DC , Sessler DI , Mascha E , Kavanagh BP , Buggy DJ. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Region Anesth Pain Med 2010. ; 35 : 490 – 5. [DOI] [PubMed] [Google Scholar]
- 30. Gordon SM , Chuang BP , Wang XM , Hamza MA , Rowan JS , Brahim JS , Dionne RA. The differential effects of bupivacaine and lidocaine on prostaglandin E2 release, cyclooxygenase gene expression and pain in a clinical pain model. Anesth Analg 2008. ; 106 : 321 – 7. table of contents. [DOI] [PubMed] [Google Scholar]
- 31. Beloeil H , Ababneh Z , Chung R , Zurakowski D , Mulkern RV , Berde CB. Effects of bupivacaine and tetrodotoxin on carrageenan‐induced hind paw inflammation in rats (Part 1): hyperalgesia, edema, and systemic cytokines. Anesthesiology 2006. ; 105 : 128 – 38. [DOI] [PubMed] [Google Scholar]
- 32. Samad TA , Moore KA , Sapirstein A , Billet S , Allchorne A , Poole S , Bonventre JV , Woolf CJ. Interleukin‐1[beta]‐mediated induction of Cox‐2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001. ; 410 : 471 – 5. [DOI] [PubMed] [Google Scholar]
- 33. Sivan‐Loukianova E , Awad OA , Stepanovic V , Bickenbach J , Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res 2003. ; 40 : 368 – 77. [DOI] [PubMed] [Google Scholar]
- 34. Kiernan TJ , Boilson BA , Witt TA , Dietz AB , Lerman A , Simari RD. Vasoprotective effects of human CD34+ cells: towards clinical applications. J Transl Med 2009. ; 7 : 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nissen NN , Polverini PJ , Koch AE , Volin MV , Gamelli RL , DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 1998. ; 152 : 1445 – 52. [PMC free article] [PubMed] [Google Scholar]
- 36. Battegay EJ. Angiogenesis – Mechanistic Insights, neovascular diseases, and therapeutic prospects. J Mol Med 1995. ; 73 : 333 – 46. [DOI] [PubMed] [Google Scholar]


