Abstract
Carica papaya L. (Linn) (Caricaceae) is traditionally used to treat various skin disorders, including wounds. It is widely used in developing countries as an effective and readily available treatment for various wounds, particularly burns. This study evaluated the wound‐healing and antimicrobial activity of C. papaya seed extract. Ethanol extract of C. papaya seed (50 mg/kg/day) was evaluated for its wound‐healing activity in Sprague‐Dawley rats using excision wound model. Animals were randomly divided into four groups of six each (group 1 served as control, group 2 treated with papaya seed extract, group 3 treated with a standard drug mupirocin and papaya seed extract (1:1 ratio) and group 4 treated with a mupirocin ointment. Rate of wound contraction and hydroxyproline content were determined to assess the wound‐healing activity of the seed extract. The group 2 animals showed a significant decrease in wound area of 89% over 13 days when compared with groups 1 (82%), 3 (86%) and 4 (84%) respectively. The hydroxyproline content was significantly higher with the granulation tissue obtained from group 2 animals which were treated with C. papaya seed extract. Histological analysis of granulation tissue of the group 2 animals showed the deposition of well‐organized collagen. The extract exhibited antimicrobial activity against Salmonella choleraesuis and Staphylococcus aureus. Our results suggest that C. papaya promotes significant wound healing in rats and further evaluation for this activity in humans is suggested.
Keywords: Carica papaya, Excision wound, Hydroxyproline, Wound area
INTRODUCTION
The dynamic process of wound healing involves a series of events including inflammation, granulation tissue formation, epithelisation, collagen synthesis and tissue remodelling (1). Normal wound‐healing response begins at the moment the tissue is injured. The healing cascade begins immediately following injury when the platelets come into contact with exposed collagen. As platelet aggregation proceeds, clotting factors are released resulting in the deposition of a fibrin clot at the site of injury. The fibrin clot serves as a provisional matrix and sets the stage for the subsequent events of healing (2).
The inflammatory cells also arrive along with the platelets at the site of injury and they provide key signals that are known as cytokines or growth factors (3). The fibroblast is the connective‐tissue cell responsible for collagen deposition that is needed to repair the tissue injury. Collagen is the most abundant protein in the animal kingdom, accounting for 30% of the total protein in the human body (4). In normal tissues collagen provides strength and structural integrity. When tissues are disrupted following injury, collagen is needed to repair the defect and restore anatomic structure and function. A treatment could influence the healing of wounds by intervening in one or more phases of wound healing. Wound care and maintenance involve a number of measures including dressing and administration of analgesics, use of anti‐inflammatory agents, topical and systemic antimicrobial agents and healing promoting drugs.
The use of Carica papaya L. (Caricaceae) in traditional medicine relies on papain, a proteolytic enzyme, is the active principle which exerts an ulcer protective effect (5). The C. papaya possesses antimicrobial (6) antioxidant and anti‐inflammatory activities (7). C. papaya has antibacterial effects that could be useful in treating chronic skin ulcers to promote healing (6). C. papaya is traditionally used to treat various skin disorders, including wounds. It is widely used in developing countries as an effective and readily available treatment of various wounds, particularly burns. A decoction made from the seeds of C. papaya has been used to treat skin ulcers and inflammation.
All the above reported medicinal uses either from fruit, seed or leaves of C. papaya prompted us to investigate the antimicrobial and the wound‐healing activity of the seed extract of C. papaya.
MATERIALS AND METHODS
Preparation of seed extract
The seeds (400 g) were collected from ripened fruits of locally available C. papaya and shade dried. Later it was blended using a blender to obtain a fine powder. The fine powder of seed extract was mixed with 1000 ml of ethanol and kept for 24 hours and filtered. Then the filtrate was kept in a fume hood for complete evaporation of ethanol to get the final yield 58 g of extract for use.
An acute toxicity study was conducted using the crude seed extract by the stair‐case method (8). The experimental animals used for this toxicity study with increasing doses (50, 100, 150, 200 and 250 mg/kg body weight) of the extract. The toxicity was assessed by mortality and behavioural changes of rats over the period of 13 days.
Animals
The study was approved by the Ethics Committee for animal experimentation (AHC06/07/1) of the Faculty of Medical Sciences, The University of the West Indies, St. Augustine, Trinidad. Healthy inbred Sprague‐Dawley male rats weighing 200–250 g were individually housed and maintained on normal food and water ad libitum. Animals were periodically weighed before and after the experiment. Animals were closely observed for any infection and if those showing signs of infection were separated from the study and replaced. The excision wound model was used to evaluate the wound‐healing activity of C. papaya extract. The animals were randomly distributed into four groups of six each.
Excision wound model
Rats were inflicted with excision wounds according to the method of Morton and Malone (9). Animals were anesthetised with 1 ml of intraperitoneal ketamine hydrochloride (120 mg/kg body weight) and shaved on both sides of the back with an electric clipper. The area of the wound to be created was outlined on the back of the animals with methylene blue using a circular stainless steel stencil. A full thickness excision wound of circular area 200 mm2 and 2 mm in depth was created along the markings. The entire wound was left open. Animals were closely observed for any infection and those which showed signs of infection were separated, excluded from the study and replaced. Animals were divided into four groups of six each. The group 1 animals were applied with vaseline only, group 2 was topically applied with C. papaya extract (50 mg/kg body weight), group 3 animals were applied with a combination of mupirocin and extract (1:1 ratio) and the group 4 was applied with mupirocin ointment. The topical application was carried out in all the cases for 13 days. Wound areas were measured on alternate days (1, 3, 5, 7, 9, 11 and 13) using a transparency sheet and a permanent marker. Recordings of the wound areas were measured on graph paper. All the animals were sacrificed on day 13 using intraperitoneal ketamine (400 mg/kg body weight) and pieces of granulation tissue were excised from the healed area for hyrdroxyproline estimation and histological studies.
Phytochemical screening methods
Saponins
One gram of extract was boiled with 10 ml water for 4 minutes; the mixture was cooled and mixed vigorously and left for few minutes. The formation of frothing indicates the presence of saponins (10).
Tannins
Two millilitres of extract was added with 2 ml of ferric chloride (1%). Colour development from red‐brown to blue‐black indicates the presence of tannins (10).
Triterpenes
The extract (1·0 g) was mixed with 10 ml of chloroform and warmed at 55°C for 30 minutes. To this 1·0 ml of concentrated sulphuric acid was added and mixed well. The appearance of a reddish brown colour indicates the presence of triterpenes (11).
Sterols
The extract (1·0 g) was mixed with 10 ml of chloroform and warmed at 55°C for 30 minutes. This was added with few drops (1–2 ml) of concentrated sulphuric acid and mixed well. The appearance of reddish brown colour indicates the presence of sterols (11).
Alkaloids
The extract (1 g) was boiled with 50 ml of methanol (90%) for 20 minutes in a water bath and the cooled filtrate was tested separately with Mayer's, Wagner's Hager's and ammonium reineckate reagents. Cloudy white precipitate of the alcoholic layer indicates the presence of alkaloids (11).
Flavonoids
One gram of extract was boiled with 10 ml of ethyl acetate over a steam bath for 3 minutes. The 4·0‐ml filtrate was mixed with 1 ml of dilute ammonia solution and a yellow precipitate indicates the presence of flavonoids (10).
Antimicrobial activity
Salmonella choleraesuis (ATCC 14028), Staphylococcus aureus (ATCC 4827), Escherichia coli (ATCC 25922) and Klebsiella pneumoniae (ATCC 700603) were the organisms tested. The bacterial strains were obtained from fresh colonies grown on Mac Conkey blood agar plates. Sensitivity testing was performed using Muller Hinton Agar plates. Known volume of bacterial suspension was transferred to each microplate well. Ten microlitres (5 mg/ml) of ethanol extract of C. papaya was added to the microplate wells and incubated at 35–37°C for 18–20 hours. Results were determined by visual inspection of zones of growth inhibition.
Statistical analysis
The means of wound area measurements between groups at different time intervals were compared using one‐way analysis of variance (ANOVA), followed by Tukey's post hoc tests. One‐way ANOVA was used to examine the mean differences in the rate of wound healing between the groups in excision wound models. Data were analysed using the SPSS (Version 15.0, SPSS Inc., Chicago, IL) and P value was set <0·05 for all analyses.
RESULTS
In acute toxicity studies, the extract in doses up to 250 mg/kg body weight did not produce any signs of toxicity and mortality over the period of 13 days. The animals were physically active and were consuming food and water in a regular way. No abnormal behaviour was noticed.
A significant increase in wound‐healing activity was observed in rats treated with C. papaya extract. In the excision wound model, animals of group 2 showed an increased percentage of wound contraction when compared with the animals of groups 1, 3 and 4 (Table 1). On day 13, group 2 showed wound contraction of 89% (P < 0·001) and it was similar to group 4 (84·28%) (1, 2). The animals of groups 1 and 2 showed 82% and 86% of wound contraction, respectively.
Table 1.
Rate of wound closure over the course of 13 days
| Groups | Wound area on day 1 area (mm2) | Wound area on day 13 (mm2) | Rate of Wound closure | Wound closure (%) |
|---|---|---|---|---|
| (Mean ± SE) | (Mean ± SE) | (mm2/day) | ||
| Group 1 (control) | 196·67 ± 2·66 | 34·50 ± 6·19 | 12·47 | 82.46 |
| Group 2 (papaya only) | 199·33 ± 14·08 | 22·00 ± 11·24 * | 13·64 | 88.96 * |
| Group 3 (mupirocin + C. papaya) | 194·16 ± 10·82 | 27·67 ± 4·08 | 12·80 | 85.75 |
| Group 4 (mupirocin) | 193·00 ± 7·21 | 30·33 ± 3·44 | 12·57 | 84.28 |
N = 6, * P < 0·001.
Figure 1.

Comparative rates of wound contraction at different courses of treatment over a period of 13‐days treatment. Each vertical bar represents mean ± SE (P > 0·001) when compared with the (control) group 1.
Figure 2.

Percentage of wound closure over a 13‐day period for different treatment regimes.
Animals treated with C. papaya (group 2) showed the presence of more hydroxyproline content (126 mg) as compared with 63, 87 and 81 mg in groups 1, 3 and 4 respectively (Figure 3). Histological analysis of granulation tissue obtained from group 2 animals showed significant collagen deposition and fibroblast activity (Figure 4) when compared with groups 1, 3 and 4 (5, 6, 7. These results suggest that C. papaya has significant wound‐healing activity. Phytochemical analysis showed the presence of alkaloids, flavonoids, triterpenes and sterols.
Figure 3.
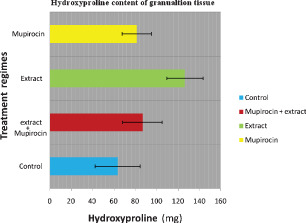
Hydroxyproline content in (mg/g tissue) of each group after 13 days of treatment. Each bar represents the mean ± SE (P < 0·05).
Figure 4.

Haematoxylin and eosin preparation of granulation tissue from the group 2 animals (C. papaya only) on day 13. The histological analysis shows significant, dense collagen–fibre deposition, as compared with the group 2 animals; numerous fibroblasts are visible, most actively synthesising ground substance and collagen. Some monocytes are visible. Also significant vascularisation was observed. M, monocyte; F, fibroblast; B, blood vessel (with erythrocytes) and C, collagen fibres.
Figure 5.
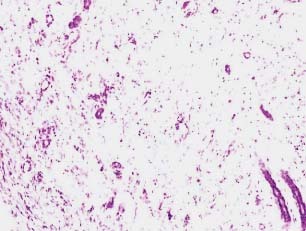
Histology of the granulation tissue obtained from the animals of control group stained with haematoxylin and eosin (day 13). No collagen deposition or fibroblast is visible. A significantly large extent inflammatory infiltrates were observed.
Figure 6.
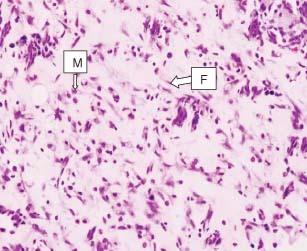
Haematoxylin and eosin preparation of granulation tissue from a specimen of group 3 (mupirocin + C. papaya) on day 13. The histological analysis shows significant collagen–fibre deposition, as compared with group 1, a few fibroblasts are visible. Some monocytes are visible, but no significant vascularisation was observed. M, macrophages and F, fibroblast.
Figure 7.
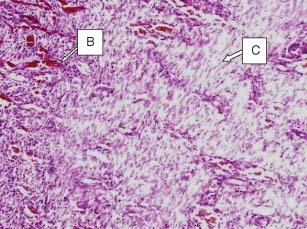
Haematoxylin and eosin preparation of granulation tissue obtained from the group 4 animals treated with mupirocin (day 13). The histological analysis shows that sparse collagen–fibre deposition is greater than in groups 1 and 2, but not in group 3. Numerous fibroblasts are visible. Some monocytes are visible. Also significant vascularisation was observed. B, blood vessel (with erythrocytes) and C, collagen fibres.
The extract showed antimicrobial activity against selected organisms, viz. S. choleraesuis and S. aureus. No activity was found for E. coli and K. pneumoniae.
DISCUSSION
C. papaya is widely known as a therapeutic plant, extracts of its waxy exudates and fruit have proven to be beneficial for wound healing. There is limited research data available on medicinal benefits of the seeds and its effect on wound healing.
Our experiment seeks to establish possible scientific evidence to support the claims made by practioners of traditional medicine. Our results showed that wounds treated with an ethanolic extract of C. papaya exhibited a higher rate of wound contraction when compared with untreated wounds, wounds treated with the mupirocin only and wounds treated with a preparation of mupirocin and C. papaya). The phytochemical components of the C. papaya seed might have been aided in the regeneration of lost tissue as evidenced by changes in histology and hydroxyproline content of the granulation tissue obtained from the group 2 animals treated with C. papaya. Similar type of observation was reported for C. papaya latex used in mice burn model (12).
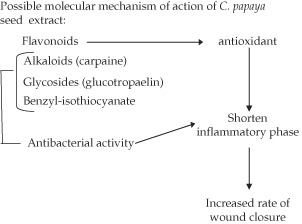
Phytochemical analysis of C. papaya seeds showed the presence of, triperpenes, sterols, alkaloids and flavonoid. Ayotunde and Ofem (13) showed that the seed of C. papaya contained a significant quantity of a glycoside described as glucotropaelin, this glycoside was suggested to have a very potent bactericidal action; an aglycone known as benzyl‐isothiocyanate. This chemical may be attributed to the antimicrobial effect observed with ethanolic extract of C. papaya. Another phytochemical of substantial value found in the seed of C. papaya is myrosin; this protein serves as the enzyme that hydrolyses the glucotropaelin glycoside into rhamnose, sulphate‐ions and benzyl‐isothiocyanate. Another possible mechanism which can be used to explain the efficacy of C. papaya is the presence of significant quantities of the alkaloid, which is known to be a very effective antimicrobial agent.
The possible synergistic effect of mupirocin and C. papaya evaluated in this study showed that there was no significant synergism between C. papaya and mupirocin. This can be because of the possible activity of enzymes such as myrosinase or carpasemine both found in large quantities in the seed of C. papaya, which may have contributed to some limited deactivation or degradation of the mupirocin structural moiety; therefore, the possible synergistic effect may not be present. The rate at which cutaneous wounds heal is often dependent on the existence of the inflammatory phase. Therefore, it is often beneficial to use antimicrobial therapy which reduces the proliferation of a pathogenic load; and ultimately reduced the extent of inflammatory responses. C. Papaya seed extract was found to have significant amounts of sterols and triterpene derivatives; this was consistent with the research carried out by Rios (14) who suggested potential anti‐inflammatory properties of these phytochemicals. It can be concluded from our study that C. papaya has significant would healing activity. This might have been because of the phytochemical constituents of C. papaya seed extract which possess antimicrobial (6), anti‐inflammatory and antioxidant (15) activities observed.
CONCLUSIONS
These results suggest that C. papaya promotes significant wound healing in rats. The results of this experiment warrant further investigation to validate it's suitability for humans.
REFERENCES
- 1. Reddy G. Laser photo stimulation accelerates wound healing in diabetic rats. Wound Repair Regen 2009;9:248–55. [DOI] [PubMed] [Google Scholar]
- 2. Clark RA. Fibrin and wound healing. Ann N Y Acad Sci 2001;936:355–67. [DOI] [PubMed] [Google Scholar]
- 3. Lawrence WT, Diegelmann R‐F. Growth factors in wound healing. Clin Dermatol 1994;12:157–69. [DOI] [PubMed] [Google Scholar]
- 4. Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases and potentials for therapy. Ann Rev Biochem 1995;64:403–34. [DOI] [PubMed] [Google Scholar]
- 5. Emeruwa A. Antibacterial substance from Carica Papaya fruit extract. J Nat Prod 1982;45:132–7. [DOI] [PubMed] [Google Scholar]
- 6. Dawkins G, Hewitt H, Wint Y, Obiefuna PC, Wint B. Antibacterial effects of Carica papaya fruit on common wound organisms. West Indian Med J 2003;52:290–2. [PubMed] [Google Scholar]
- 7. Gupta OP, Sing S, Bani S, Sharma N, Malhotra S, Gupta BD, Banerjee SK, Handa SS. Anti‐inflammatory and anti‐arthritic activities of silymarinacting through inhibition of 5‐lipoxygenease. Phytomedicine 2000;7:21–4. [DOI] [PubMed] [Google Scholar]
- 8. Jalalpure SS, Patil MB, Prakash NS, Hemalatha K, Manvi FV. Hepatoprotective activity of fruits of Piper longum L. Indian J Pharm Sci 2003;65:360–6. [Google Scholar]
- 9. Morton JJP, Malone MH. Evaluation of vulnerary activity by an open wound procedure in rats. Arch Int Pharmacodyn 1972;196:117–26. [PubMed] [Google Scholar]
- 10. Kapoor LD, Singh A, Kapoort SL, Strivastava SN. Survey of Indian Medicinal Plants for Saponins. Alkaloids and Flavonoids. Lloydia 1969;32:297–302. [PubMed] [Google Scholar]
- 11. Harborne JB. Photochemical methods. A guide to modern techniques of plant analysis. London: Chapman A & Hall, 1973:279. [Google Scholar]
- 12. Gurung S, Basnet NS. Wound healing properties of Carica papaya latex: In vivo evaluation in mice burn model. J Ethnopharmacol 2009;121:338–41. [DOI] [PubMed] [Google Scholar]
- 13. Ayotunde EO, Ofem BO. Acute and chronic toxicity of pawpaw (Carica papaya) seed powder to Nile Tilapia Oreochromis niloticus (Linne 1757), fingerlings. Adv Environ Biol 2008;2:101–7. [Google Scholar]
- 14. José‐Luis R. Effects of triterpenes on the immune system. J Ethnopharmacol 2010;128:1–14. 20079412 [Google Scholar]
- 15. Srikanth G, Manohar Babu S, Kavitha CHN, Bhanoji Rao ME, Vijaykumar N, Pradeep CH. Studies on in‐vitro antioxidant activities of Carica papaya aqueous leaf extract. RJPBCS 2010;1:59–65. [Google Scholar]


