Abstract
The neurosurgery division in University College Hospital (U.C.H.) admits approximately one traumatic spinal cord injured (SCI) patient per week, most of whom stay a minimum of 42 days on admission. A common complication in these patients is the development of pressure ulcers, which contributes to a longer hospital stay and increased hospital expenses. The purpose of this study was to investigate the pattern of presentation of pressure ulcers in patients on admission and to propose policies or protocols to reduce the incidence. It is a prospective study of traumatic SCI patients managed on the neurosurgery ward from January 2003 to June 2004. The data was analysed using descriptive statistics. Sixty‐seven patients were studied. The average hospital stay was 73 days. Thirteen (20%) of the patients were admitted with pressure ulcers, 32 (47·7%) developed it after admission. As much as 87·5% of pressure ulcers seen in the course of this study which occurred on admission in U.C.H. was in the first week of admission, 6·25% in the second week and the remaining 6·25% in the third week. Pressure ulcers were distributed as follows; 69% (42) in the sacral region, 18% (11) trochanteric, 5% (3) scalp, 1·5% (1) ankle, 1·5% (1) ischial tuberosity, the remaining 5% in other sites. Preventive measures for pressure ulcers consisted of basic skin care, pressure dispersion using fenestrated foams and alternating weight‐bearing sites by regular turning. Pressure ulcers are commonest in the sacral and gluteal regions and tend to occur within the first week of admission in the neurosurgical wards.
Keywords: Pressure ulcers, Traumatic spinal cord injury
INTRODUCTION
Pressure ulcers are caused by unrelieved pressure, resulting in damage to the underlying tissue. They usually occur over bony prominences and are classified as stages by the degree of tissue damage observed. Pressure ulcers is an ancient problem, observed at autopsy of Egyptian mummies by Thompson Rowling (1). The aetiology of pressure ulcers is multidimensional. Currently, the general accepted theory of pressure formation involves a direct effect by one extrinsic (primary) factor propitiated and modified by a number of intrinsic (secondary) factors (2). Extrinsic factors that exert mechanical force on soft tissue include pressure, shear and friction. Pressure is a perpendicular load exerted on a unit area; shear is a mechanical stress parallel to the plane and friction is the resistance of two surfaces moving across one another. Intrinsic factors include local ischaemia or fibrosis, diminished autonomic control, infection, small vessels occlusive disease, hypoproteinaemia, anaemia, sensory loss, impaired mobility, age, decreased mental status and faecal or urinary incontinence (2).
This pressure ischaemia theory holds that pressure ulcers form as a result of constant pressures on the soft tissues for a sufficiently long time. The exerted pressure must exceed the arterial capillary blood pressure of 32 mmHg (3) and must be sustained without interruption. The validity of this value should however be questioned, since the capillaries were cannulated when the study was done by Landis in 1930, this could have resulted in lower pressure readings as the bleeding vessels were not full or enclosed. Furthermore, capillary pressures can rise during mechanical loading due to autoregulation, in which case higher external pressures are needed to close the blood vessels 4, 5. Finally, capillary closure depends on local pressure gradients across the vessel wall and not just on interface pressures (6). Still, the capillary closing pressure found in Landis' study is frequently used in clinical practice as a threshold for tissue damage, where care is taken to avoid interface pressures higher than 32 mmHg. Techniques for the prevention of pressure ulcers consist of basic skin care, pressure dispersion and alternating weight‐bearing sites. Management options are non operative and operative. These include regular wound dressings, wound debridement and reconstructive options for wound cover of pressure sores.
Spinal cord injured (SCI) patients have a high risk of developing pressure ulcers because they are neurologically impaired and tend to have long hospital stay. The neurosurgery division in University College Hospital (U.C.H.) admits approximately one traumatic SCI patient per week, most of whom stay a minimum of 42 days on admission. A common complication in these patients is the development of pressure ulcers, which contributes to a longer hospital stay and increased hospital expenses.
The objectives of this study were as follows:
-
1
To identify the pattern of development of pressure ulcer in patients on admission.
-
2
To identify the factors that contribute to the development of pressure ulcers in these patients.
-
3
To advise on ways of reducing the incidence of pressure ulcers in these patients.
PATIENTS AND METHODS
The study was a prospective study of traumatic SCI patients managed on the neurosurgery ward from January 2003 to June 2004. The spinal cord injury management protocol consisted of initial maintenance of the cervical spine in a rigid cervical collar. Then appropriate radiological investigations were done to ascertain the spinal cord injury; these include cervical spine X‐rays, thoracolumbar spine X‐ray, computerised tomography scan or magnetic resonance imaging as required. Non operative care using Gardner‐Wells Tongs for postural reduction was then undertaken. The postural reduction was maintained with weights for at least 42 days. Subsequently a flexion–extension radiology study was done to confirm cervical spine stabilisation before discharge from the hospital. While on admission the patients were anticoagulated, had physiotherapy and were introduced intermittent urethral catheters. Pressure ulcer prevention measures consist of two hourly turning, use of fenestrated foam on pressure points especially hip–buttock region for pressure dispersion. None of the patients had operative spinal stabilisation done. Sixty‐seven consecutive patients who were managed on the wards were included in the study. The information was obtained into a questionnaire which had sections on demographic data, the ward, pressure ulcers, risk factors, preventive measures, investigations and management of pressure ulcers. This information was obtained from the patients and relatives, ward admission record and the patient's case‐notes. The grading of pressure ulcers was as follows (National Pressure Ulcer Advisory Panel):
Grade I – Non blanching erythema of intact skin
Grade II – Partial thickness skin loss
Grade III – Full thickness skin loss and subcutaneous tissue
Grade IV – Involvement of muscle, bone and/or joint structures
Data were analysed using descriptive statistics (cross tabulation using Pearson chi‐square and t‐test with statistical significance at P < 0·05).
RESULTS
Sixty‐seven patients were studied. Fifty‐three (79%) were tetraplegic and 14 (21%) were paraplegic. As much as 67·2% of patients had complete tetraplegia, 13·4% had incomplete tetraplegia, 19·4% had complete paraplegia (Figure 1). Fifty‐one were males and 16 females, with a ratio of 3:1. The age distribution ranged from 14 to 80 years, mean 38 years, standard deviation (SD) 16 years; the mean age for males was 39·9 years, SD 16·8, for females 32·8 years, SD 11·9 (t‐test = 0·126, not statistically significant). There was a peak in 20–29 age group (34·3% of patients) (Figure 2), 15 (65·2%) males and 8 (34·8%) females (P = 0·37, not statistically significant). As much as 74·6% of the patients were in the 20–49 age groups, 59·7% of patients were less than or 40 years of age, 15% of patients were greater or equal to 60 years. Hospital stay ranged from 1 to 258 days with an average of 73 days (median 66 days, SD 54 days).
Figure 1.
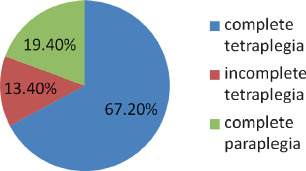
Spinal cord injury.
Figure 2.

Age distribution of traumatic spinal cord injured patients.
Forty‐five patients had pressure ulcers, 74·4% of these had complete tetraplegia, 7% incomplete tetraplegia and 18·6% complete paraplegia (P = 0·095, statistically significant) (Figure 3). Thirteen (20%) of the patients were admitted with pressure ulcers, 69·2% of those admitted with pressure ulcer had complete tetraplegia, 30·8% had complete paraplegia (P = 0·197, not statistically significant). Thirty‐two patients (47·7%) developed pressure ulcers after admission, 28 males, 8 were females (P = 0·175, not statistically significant). As much as 87·5% of pressure ulcers developed on admission in U.C.H. was in the first week of admission, 6·25% in the second week and the remaining 6·25% from the third week. The ratio of nurse to patient on the ward was one to seven.
Figure 3.
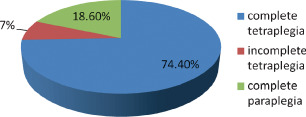
Pressure ulcers.
There were 61 pressure ulcer sites in this study which were located as follows: 69% (42) sacral region, 18% (11) trochanteric, 5% (3) scalp, 1·5% (1) ankle, 1·5% (1) ischial tuberosity, the remaining 5% in other sites (Figure 4). Four percent (2) had depth of Grade I, 38% (22) Grade II, 51% (30) Grade III and 7% (5) Grade IV (Figure 5). As much as 95·5% of pressure ulcers were in the hip–buttock region, 2·25% in the head and lower limbs, respectively. For patients with pressure ulcers in the hip–buttock region, 73·8% had complete tetraplegia, 7·1% had incomplete tetraplegia and 19% had complete paraplegia (Figure 6). Pressure ulcers in the head and lower limbs were in patients with complete tetraplegia (P = 0·952, not statistically significant). Ten patients had pressure ulcers in multiple sites, 34 patients had in one site. Of those with pressure ulcers in multiple sites, 70% had complete tetraplegia, 10% incomplete tetraplegia and 20% complete paraplegia. Patients with pressure ulcer on one site, 76·5% had complete tetraplegia, 5·9% incomplete tetraplegia and 17·6% complete paraplegia (P = 0·879, not statistically significant).
Figure 4.
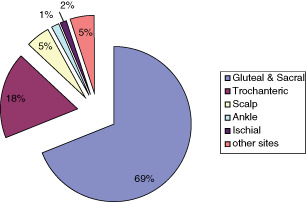
Pressure ulcer sites.
Figure 5.
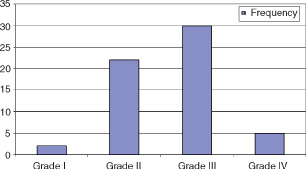
Depth of pressure sores.
Figure 6.
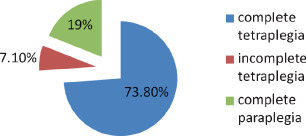
Pressure ulcers in hip‐buttock region.
Preventive measures for pressure ulcers consisted of basic skin care, pressure dispersion using fenestrated foams and alternating weight‐bearing sites by regular turning (2 hourly). The patients who had pressure ulcers had daily honey‐wound dressing; wound debridement was done when indicated. None of the patients had reconstructive surgery for pressure ulcers.
DISCUSSION
The annual incidence of pressure ulcers among individuals with SCI is between 23% and 30% (7), but up to 85% of SCI individuals develop a pressure ulcer at some point during their lifetimes 8, 9. More than 70% of SCI patients with a pressure ulcer have multiple ulcers. Barbenel and coworker (10) noted that 3–4·5% of all hospitalised patients will develop a pressure ulcer at some time in the hospital stay and more than two‐thirds of these patients are older than 70 years. Only 15% of our patients were 60 years and above, this is probably a reflection of our population demographics, the average life expectancy in Nigeria is 47 years (11). Dansereau and Conway (12) noted that the vast majority of pressure ulcers developed in the lower part of the body with two‐thirds in the hip–buttock areas and one‐fourth to one‐third in the lower limbs. This finding is similar to our study in which 95·5% had pressure ulcers in the hip–buttock region.
Pressure ulcers occur when external pressures that are high enough to close blood vessels will lead to ischaemia, this initiates a series of chemical and pH imbalances, accompanied by enhanced generation of injurious free radical species. The damage produced by short periods of ischaemia tends to be reversible if the circulation is restored, but cells subjected to long episodes of ischaemia become damaged irreversibly and die (13). In general, muscle appears to be tolerant of ischaemia for up to 4 hours, fat up to 13 hours and skin up to 24 hours at normothermia (14). For short exposures to pressure (less than 1 hour) and also for long exposures (over 2 hours), the magnitude of the pressure is the most important factor for causing cell death; the exposure time has little or no effect. However, for the intermediate exposures (between 1 and 2 hours), both pressure and time are important factors (15).
Although ischaemia will eventually lead to tissue death, it is probably not the only cause of pressure ulcers. One reason for this is that pressure ulcers can develop within 2 hours, whereas tissues can withstand ischaemia for longer times. Furthermore, ischaemia alone fails to explain why higher tissue pressures can create ulcers after a short period of ischaemia, whereas lower pressures, which still create an ischaemic state, need longer periods to cause the identical lesion (16). Tissue can also become damaged when the blood flow in the tissue is restored after an ischaemic period. Ischaemia and reperfusion of skeletal muscle can trigger a series of deleterious phenomena in tissue, such as cell oedema, increased permeability in the microcirculation, induction of the no‐reflow phenomenon, free oxygen radical production, electrolytic changes in mitochondria, cytosolic calcium overload and degradation of membrane phospholipids (17).
Ikebe et al. investigated the relationship between the duration of ischaemia and the subsequent reperfusion blood flow in rats (18). They found an increased reperfusion blood flow after 90 minutes and 3 hours of ischaemia, but after 6 hours of ischaemia there was no significant increase in postischaemic blood flow. This is called the ‘no‐reflow phenomenon’ and is probably caused by cellular swelling, thrombosis and white cell plugging in capillaries, which increases the resistance in the microcirculation (19). The incidence of no flow depends on the severity of both the ischaemic insult and the subsequent reperfusion injury (18). During reperfusion oxygen is provided to the tissues, which combines with the free radical species generated during ischaemia to form reactive oxygen species (ROS) (13). When the blood flow to the tissues is restored after ischaemia, oxygen combines. With free radical species generated during ischaemia to form ROS, this also causes cell damage. It is hypothesised that the reperfusion phase is more damaging than ischaemia alone. For example, when the total extent of ischaemia is constant, a larger number of reperfusion events during that period results in increased tissue damage 20, 21. Furthermore, gradual reperfusion leads to less tissue damage when compared to conventional reperfusion 22, 23.
Spinal cord injury impairs skin blood flow responses. Normally, skin responds to pressure, mechanical stimulation or inflammation with increased blood flow. Loss of this response not only adds to the vulnerability of the skin to pressure ulcers but reduces the ability of the skin to repair decubiti. Deitrick et al. (24) hypothesised that SCI patients get insufficient exercise due to the paralysed muscle mass in the lower extremities and therefore are prone to poor circulation in the legs, which represents an increased arteriosclerotic risk factor. The hypothesis was examined by measurement of the blood flow to the legs in SCI patients and an able‐bodied control group. It was shown that SCI patients in general have less blood flow in the legs, which might explain the poor healing rate observed in many SCI patients. Completeness of spinal cord injury is also a major risk factor 7, 24 for the development of pressure sores. Young et al. reported ‘the completeness of the injury appears to be a much more powerful determinant of pressure ulcer occurrence than level of the lesion’(7). This may be a function of the higher level of activity in paraplegics. Whether the risk of pressure ulcers is higher in tetraplegics or paraplegics remains unresolved. Many clinicians believe that higher spinal cord injuries (i.e. cervical) are associated with higher risk of pressure ulcers. The weight of evidence does not support this (25).
This belief was also not supported in our study as there was no statistical significance in the development of pressure ulcers in the patients with tetraplegia or paraplegia. The effect of completeness of the spinal cord injury in occurrence of pressure ulcer was also not demonstrated.
There are few local studies on pressure ulcers in SCI patients in Nigeria. Oluwatosin et al. (26) showed that 51% of patients with pressure ulcers in Ibadan had spinal injury; this suggests that patients with spinal cord injury form the majority of those with pressure ulcers in an hospital‐based study. Greatest distribution was in the hip–buttock, sacral 40·5%, trochanteric 29·7% and ischial 10·8%. Foam interposition was found to reduce pressure on bony prominence; fenestration or cutting a circular in the cushion at the site of bony prominence further reduced the pressure in such areas. This method of pressure reduction enhances healing in both operated patients and those managed conservatively.
In this study, we found that there is a male preponderance and a peak age group of 20–29 years. This is not surprising because the young men tend to travel more, most of the spinal cord injuries were sustained following vehicular road traffic accidents. In Solagberu's study in Ilorin (27), of patients admitted for spinal cord injury from 1995 to 1999 there were 39 patients, aged 19–60 years (mean 37·3), 36 male and 3 female patients. Igun et al. in Jos (28) noted that the mean age for their patients was 30 years with a male to female ratio of 10:1. The risk of pressure ulcers in SCI patients may increase after age 35 and again after age 65 (29). For the pressure ulcer risk in the SCI population, age may have a bimodal curve. A study relating the age of the population affected by pressure ulcer showed an increasing tendency in the number and seriousness of pressure ulcers with age. This increased incidence is particularly marked in patients over 40 years of age (30). This age relationship was not demonstrated in our study. The mean age in this study was 38 years, the above stated studies support the male preponderance and the economically productive age involved. The implication that approximately 75% of the patients in our study are in the 20–49 age group denotes that the financially productive group of the nation are worse hit.
The study by Pieper et al. (31) on risk factors, prevention methods and wound care for patients with pressure ulcers revealed that advanced practice nurses have a critical role in caring for patients with pressure ulcers and educating care providers. It is not surprising that 50% of the patients developed pressure ulcers after admission with a nurse to patient ratio of 1:7. However, the development of pressure ulcers means more work for the nurses since dressings will have to be done. As earlier stated this also means prolonged hospital stay and hospital expenses for the patient. So it is better to prevent pressure ulcers from developing than having to manage them.
In the first week of admission, 87·5% of pressure ulcers that developed on admission occurred; this denotes that preventive measures against the development of pressure ulcers are not effective earlier. Pieper et al. in their study ‘pressure ulcer prevention within 72 hours of admission in a rehabilitation setting’(32) stated that for at‐risk patients, it is necessary to implement pressure ulcer prevention strategies as early as possible after admission since pressure ulcers may quickly develop.
Preventive measures for pressure ulcers consisted of basic skin care, pressure dispersion using fenestrated foams and alternating weight‐bearing sites by regular turning (2 hourly).
The high incidence of pressure ulcers in the hip–buttocks sites is consistent with previous studies, and underscores the pressure theories; presumably, these are less in thoracic area partly because of weight and partly because of surface areas involved; the lesser incidence in occipital area may be due partly to retained sensation and movement, and partly to weight born in this region, the low incidence of pressure ulcers in the ischial tuberosity can be attributed to the fact that the patients are managed supine.
The patients who had pressure ulcers had daily honey‐wound dressings and wound debridement as indicated. None of the patients had reconstructive surgery for pressure ulcers. This is in contrast with Jiburum et al. (33) who reported early operative closure of pressure ulcers in the National Orthopaedic Hospital, Enugu, Nigeria. The reconstructive surgeries were in paraplegics, 21% of the patients in this study are paraplegics.
Reduction in the incidence of pressure ulcers in traumatic SCI patients and improved patient care can be enhanced by the establishment of a spinal cord unit in the hospital. The unit should have nurse to patient ratio of 3:1 and air‐fluidised beds or other pressure‐relieving mechanised beds. Inter‐disciplinary approach to the care of SCI patients, that is, spinal surgeons, urologists, specialised nurses etc. Adequately funding and equipping of these centres is essential.
Iwegbu (34) in his review of traumatic paraplegics in Zaria suggested that the establishment of a Centre for Injuries of the Spine, with the direct involvement of the Government through the Ministry of Social Welfare, in the management of these patients will definitely improve the overall results. Igun in Jos (28) concluded in their study that centres for spinal injuries should be established incorporating hospital wards, theatres, gymnasia, nursing units, occupational therapy units, activity centres and workshops. These centres will generate comprehensive data on morbidity and mortality needed for future planning. Most of these studies show that the preventive measures are less than optimal; this may be a reflection of the level of care of these categories of patients in a developing country. There is hence a need for increased advocacy to optimise care especially in this case prevention of pressure ulcers. Pressure ulcers account for one‐fourth of the cost of caring for SCI patients (35). Prevention of these ulcers would cost less than one‐tenth of the amount spent on treatment (36); clearly ‘more emphasis must be placed on their prevention’(37).
CONCLUSION
Pressure ulcers are commonest in the sacral region and tend to occur within the first week of admission in the neurosurgical wards. The low incidence of pressure ulcers in the ischial tuberosity can be attributed to the fact that the patients are managed supine. Pressure ulcer incidence can be reduced by prompt institution of all the three modalities of pressure sore prevention on admission. We recommend well‐equipped centres for SCI patients with appropriate nurse : patient ratio (3:1) should be established to facilitate improved patient care.
REFERENCES
- 1. Rowling JT. Pathological changes in mummies. Proc R Soc Med 1961;54:409. [PMC free article] [PubMed] [Google Scholar]
- 2. Enis J, Sarmiento A. The pathophysiology and management of pressure sores. Orthop Rev 1973;2:26. [Google Scholar]
- 3. Landis DM. Studies of capillary blood pressure in human skin. Heart 1930;15:209. [Google Scholar]
- 4. Bennett L, Lee BY. Pressure versus shear in pressure sore causation. In: Lee BL, editor. Chronic ulcers of the skin. McGraw‐Hill, New York, 1985:39–56. [Google Scholar]
- 5. Thompson D. A critical review of the literature on pressure ulcer aetiology. J Wound Care 2005;14:87–90. [DOI] [PubMed] [Google Scholar]
- 6. Bouten CV, Oomens CW, Baaijens FP, Bader DL. The etiology of pressure ulcers: skin deep or muscle bound? Arch Phys Med Rehabil 2003;84:616–9. [DOI] [PubMed] [Google Scholar]
- 7. Young JS, Burns PE. Pressure sores and the spinal cord injured: part II. Model systems. Sci Dig 1981;3:11–26,48. [Google Scholar]
- 8. De Lateur BJ, Berni R, Hangladarom T. Wheelchair cushions designed to prevent pressure sores: an evaluation. Arch Phys Med Rehabil 1976;57:129–34. [PubMed] [Google Scholar]
- 9. Fuhrer MJ, Garber SL, Rintala DH. Pressure ulcers in community‐resident persons with spinal cord injury: prevalence and risk factors. Arch Phys Med Rehabil 1993;74:1172–7. [PubMed] [Google Scholar]
- 10. Barbenel JC, Jordan MM, Nicol SM. Incidence of pressure sores in the greater Glasgow Health Board area. Lancet 1977;2:548. [DOI] [PubMed] [Google Scholar]
- 11. United Nations Children's Fund, UNICEF . 2006. At a glance:Nigeria‐statistics. URL: www.unicef. org/infobycountry/nigeria_statistics.html.
- 12. Dansereau JG, Conway H. Closure of decubiti in paraplegics. Plast Reconstr Surg 1964;33:474. [PubMed] [Google Scholar]
- 13. Rubin E, Gorstein F, Rubin R, Schwarting R, Strayer D. Rubin's pathology: clinicopathologic foundations of medicine, 4th edn. Lippincott Williams & Wilkins, Philadelphia, 2005. [Google Scholar]
- 14. Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 2002;10:620–30. [DOI] [PubMed] [Google Scholar]
- 15. Linder‐Ganz E, Engelberg S, Scheinowitz M, Gefen A. Pressure‐time cell death threshold for albino rat skeletal muscles as related to pressure sore biomechanics. J Biomech 2006;39:2725–32. [DOI] [PubMed] [Google Scholar]
- 16. Parish LC, Lowthian P, Witkowski JA. The decubitus ulcer: many questions but few definitive answers. Clin Dermatol 2007;25:101–8. [DOI] [PubMed] [Google Scholar]
- 17. Grisotto PC, dos Santos AC, Coutinho‐Netto J, Cherri J, Piccinato CE. Indicators of oxidative injury and alterations of the cell membrane in the skeletal muscle of rats submitted to ischemia and reperfusion. J Surg Res 2000;92:1–6. [DOI] [PubMed] [Google Scholar]
- 18. Ikebe K, Kato T, Yamaga M, Hirose J, Tsuchida T, Takagi K. Increased ischemia‐reperfusion blood flow impairs the skeletal muscle contractile function. J Surg Res 2001;99:1–6. [DOI] [PubMed] [Google Scholar]
- 19. Urbaniak JR, Seaber AV, Chen LE. Assessment of ischemia and reperfusion injury. Clin Orthop Relat Res 1997;334:30–6. [PubMed] [Google Scholar]
- 20. Peirce SM, Skalak TC, Rodeheaver GT. Ischemia‐reperfusion injury in chronic pressure ulcer formation: a skin model in the rat. Wound Repair Regen 2000;8:68–76. [DOI] [PubMed] [Google Scholar]
- 21. Tsuji S, Ichioka S, Sekiya N, Nakatsuka T. Analysis of ischemia‐reperfusion injury in a microcirculatory model of pressure ulcers. Wound Repair Regen 2005;13:209–15. [DOI] [PubMed] [Google Scholar]
- 22. Durrani NK, Yavuzer R, Mittal V, Bradford MM, Lobocki C, Silberberg B. The effect of gradually increased blood flow on ischemia‐reperfusion injury in rat kidney. Am J Surg 2006;191:334–7. [DOI] [PubMed] [Google Scholar]
- 23. Unal S, Ozmen S, Demir Y, Yavuzer R, Latifoglu O, Atabay K, Oguz M. The effect of gradually increased blood flow on ischemia‐reperfusion injury. Ann Plast Surg 2001;47:412–6. [DOI] [PubMed] [Google Scholar]
- 24. Deitrick G, Charalel J, Bauman W, Tuckman J. Reduced arterial circulation to the legs in spinal cord injury as a cause of skin breakdown lesions. Angiology 2007;58:175–84. [DOI] [PubMed] [Google Scholar]
- 25. Richardson RR, Meyer PR Jr. Prevalence and incidence of pressure sores in acute spinal cord injuries. Paraplegia 1981;19:235–47. [DOI] [PubMed] [Google Scholar]
- 26. Oluwatosin OM, Malomo OA, Oluwatosin OA, Shokunbi MT. Management of pressure ulceration using fenestrated foam and honey: preliminary report of 51 cases treated at Ibadan. Nig Q J Hosp Med 1998;8:264–66. [Google Scholar]
- 27. Solagberu BA. Spinal cord injuries in Ilorin, Nigeria. West Afr J Med 2002;21:230–2. [DOI] [PubMed] [Google Scholar]
- 28. Igun GO, Obekpa OP, Ugwu BT, Nwadiaro HC. Spinal injuries in the Plateau State, Nigeria. East Afr Med J 1999;76:75–9. [PubMed] [Google Scholar]
- 29. Salzberg CA, Byrne DW, Cayten CG, Niewerburgh P, Murphy JG, Viehbeck M. A new pressure ulcer risk assessment scale for individuals with spinal cord injury. Am J Phys Med Rehabil 1996;75:96–104. [DOI] [PubMed] [Google Scholar]
- 30. Vidal J, Sarrias M. An analysis of the diverse factors concerned with the development of pressure sores in spinal cord injured patients. Paraplegia 1991;29:261–7. [DOI] [PubMed] [Google Scholar]
- 31. Pieper B, Sugrue M, Weiland M. Risk factors, prevention methods and wound care for patients with pressure ulcers. Clin Nurse Spec 1998;12:7–12. [DOI] [PubMed] [Google Scholar]
- 32. Pieper B. Pressure ulcer prevention within 72 hours of admission in a rehabilitation setting. Ostomy Wound Manage 1997;43:14–8,20,22. [PubMed] [Google Scholar]
- 33. Jiburum BC, Achebe JU, Akpuaka FC. Early results of operative closure of pressure sores in traumaticparaplegics. Int Surg 1995;80:178–80. [PubMed] [Google Scholar]
- 34. Iwegbu CG. Traumatic paraplegia in Zaria, Nigeria: the case for a centre for injuries of the spine. Paraplegia 1983;21:81–5. [DOI] [PubMed] [Google Scholar]
- 35. Houle RJ. Evaluation of seat devices designed to prevent ischemic ulcers in paraplegic patients. Arch Phys Med Rehabil 1969;50:587–94. [PubMed] [Google Scholar]
- 36. Noble PC. The prevention of pressure sores in persons with spinal cord injuries. Monograph 11. New York: International exchange of information in rehabilitation. World Rehabilitation Fund, Inc, 1981. [Google Scholar]
- 37. Knutsdottir S. Spinal cord injuries in Iceland 1973‐1989 – a follow‐up study. Paraplegia 1993;31:68–72. [DOI] [PubMed] [Google Scholar]


