Abstract
This systematic review considers the evidence supporting the use of prophylactic dressings for the prevention of pressure ulcer. Electronic database searches were conducted on 25 July 2013. The searches found 3026 titles and after removal of duplicate records 2819 titles were scanned against the inclusion and exclusion criteria. Of these, 2777 were excluded based on their title and abstract primarily because they discussed pressure ulcer healing, the prevention and treatment of other chronic and acute wounds or where the intervention was not a prophylactic dressing (e.g. underpads, heel protectors and cushions). Finally, the full text of 42 papers were retrieved. When these 42 papers were reviewed, 21 were excluded and 21 were included in the review. The single high‐quality randomised controlled trial (RCT) and the growing number of cohort, weak RCT and case series all suggest that the introduction of a dressing as part of pressure ulcer prevention may help reduce pressure ulcer incidence associated with medical devices especially in immobile intensive care unit patients. There is no firm clinical evidence at this time to suggest that one dressing type is more effective than other dressings.
Keywords: Pressure ulcer prevention, Prophylactic dressings
Research questions
The questions that this systematic review considered were as follows:
What evidence was available to indicate that the use of dressings in pressure ulcer prevention leads to reduced pressure ulcer incidence?
What evidence was available to indicate that different types of prophylactic dressing may have a greater or lesser effect upon the prevention of superficial pressure ulcers?
Identification of studies and search strategy
This review was commissioned by a product manufacturer (Molnlycke Healthcare, Gothenburg, Sweden) and no review protocol was developed or made available for comment. The review process was conducted and the results were reported following the PRISMA statement 6.
The search strategy comprised the following main elements:
A search of four electronic bibliographic databases (Medline, Cochrane Library, CINAHL Plus and PubMed) was performed on 25 July 2013 for studies that met the inclusion criteria. The databases were searched from inception to the date of the search but limited to publications in English. The search strategy used in CINAHL Plus is described below.
S1 TX Pressure ulcer
S2 TX Pressure sore
S3 TX Decubitus ulcer
S4 TX Bedsore
S5 Pressure injury
S6 S1 OR S2 OR S3 OR S4 OR S5
S7 TX Prevention
S8 S6 AND S7
S9 TX Dressing
S10 S8 AND S9
Bibliographies of included studies were searched for further relevant studies. References were managed using EndNote version 17 (Thomson Reuters, New York, NY). Further Internet searches were performed on 26 July 2013 using Google and entering 'wound dressing and pressure ulcer prevention (along with common alternative terms – bedsore, decubitus ulcer, pressure sore and pressure injury)' as search terms. Additional publications were provided by the funder of the review work (Molnlycke Healthcare) and all of these had also been retrieved using the search strategy.
Inclusion criteria
The inclusion criteria for the systematic review are summarised in Table 1.
Table 1.
Inclusion criteria
| Questions | Criteria | Specification | Notes |
|---|---|---|---|
| 1 and 2 | Population | People at risk of developing pressure ulcers but with no signs of established pressure damage including category (1) pressure ulcers | |
| 1 and 2 | Intervention | Use of any type of wound dressing as part of pressure area care | Pressure area care considered to include load redistribution, risk assessment, skin care and nutritional support |
| 1 and 2 | Comparator | Pressure area care without the use of prophylactic dressings to augment prevention or pressure area care with an alternative prophylactic dressing used to augment prevention | |
| 1 | Outcome | Number and severity of new pressure ulcers | |
| 1 and 2 | Setting | Primary and secondary care | No restriction on geographical location |
| 1 and 2 | Study design and publication status | Systematic reviews, randomised, non‐randomised, cohort, case series and case studies, observational and qualitative studies. Excluded studies – those not using a prophylactic dressing to augment pressure ulcer prevention (e.g. underpads and padding), testimonials, non‐systematic reviews, editorials and in vitro studies | No restrictions on study type, review limited to published studies and in print manuscripts. Poster presentations excluded |
| 1 and 2 | Length of follow‐up | Until people receiving pressure ulcer prevention left the study or developed pressure ulcers | |
| 1 and 2 | Language | English language only | |
| 1 and 2 | Publication date | No limitation |
Exclusion criteria
Reports describing product news were excluded from the final review along with non‐systematic reviews containing no primary data, testimonials and laboratory‐based in vitro studies.
Study selection
Based on the above inclusion criteria, papers were selected for review from the titles and abstracts generated by the search strategy. This was done by a single reviewer (MC) with the other review authors independently checking the papers selected for review and the discrepancies were resolved through discussion. Retrieved papers were reviewed and selected against the inclusion criteria in the same manner.
Data extraction
Data were extracted from included studies by one reviewer (MC) using standardised data extraction forms made available by SIGN (Scottish Intercollegiate Guidelines Network) (http://www.sign.ac.uk/methodology/checklists.html) and checked by the other review authors. Data were gathered on the basis of design, participants, methods, outcomes, baseline characteristics and results of the studies.
Critical appraisal – assessing risk of bias
Studies were assessed for internal and external validity according to the criteria suggested by SIGN based on study type. The quality of case series was assessed using the checklist provided by Moga et al. 7 with this checklist validated for all published case series with multiple subjects.
Methods for analysis and synthesis
Analysis was carried out using Review Manager (RevMan) v5 (Cochrane Collaboration). The principal summary measure was relative risk (RR) with 95% confidence intervals (CIs) using random effects model.
Synthesis
All studies had a narrative synthesis.
Randomised controlled trials, controlled trials and cohort studies
Statistical heterogeneity was tested using the I 2 measure of inconsistency within RevMan 5 (Cochrane Collaboration) where an I 2 below 40% might indicate that statistical heterogeneity may not be important 8. Clinical and methodological heterogeneity were explored through assessment of the study populations, methods and interventions. Funnel plots were used to ascertain the potential of publication bias.
Case series
As with other study designs included in this review clinical heterogeneity was explored through assessment of the study populations, methods and interventions.
Results
Number and type of studies included
Electronic database searches were conducted on 25 July 2013. The searches found 3026 titles and after removal of duplicate records 2819 titles were scanned against the inclusion and exclusion criteria. Of these titles, 2777 were excluded on the basis of their title and abstract primarily because they discussed pressure ulcer healing, the prevention and treatment of other chronic and acute wounds or where the intervention was not a prophylactic dressing (e.g. underpads, heel protectors and cushions). Finally, the full text of 42 papers were retrieved. When these 42 papers were reviewed, 21 were excluded and 21 were included. The flow chart of the selection process is shown in Figure 1.
Figure 1.
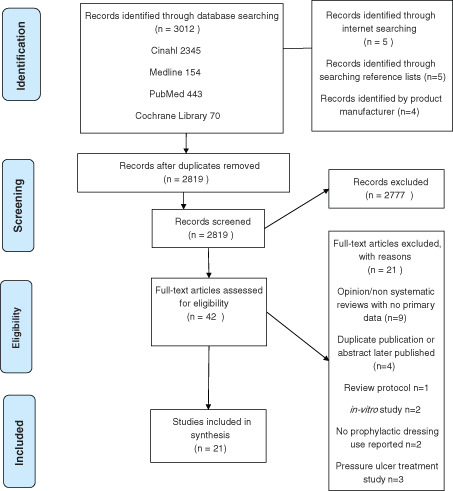
Flow chart showing records identified, screened and included in the synthesis.
Results
Of the 21 retrieved studies, 9 had a comparator arm 9, 10, 11, 12, 13, 14, 15, 16, 17 in which no prophylactic dressing was used to augment pressure ulcer prevention and these studies are summarised in Table 2. The study designs differed with two randomised controlled trials (RCTs) 9, 10, five cohort studies 11, 12, 13, 14, 15 and one within‐subject design where prophylactic dressings were applied to one trochanter with the other trochanter dressing free 16. The report of the final controlled study did not specify its design 17.
Table 2.
Summary characteristics of the ten studies where comparisons were made between the incidence of pressure ulcers where (a) the skin was protected with a prophylactic dressing and where no dressing was applied and (b) where the effect of different dressings upon pressure ulcer incidence was compared
| Study | Design | Population | Body sites reported | Prophylactic dressing | Pressure ulcer incidence with dressing use | Pressure ulcer incidence – no dressing use | Additional comments |
|---|---|---|---|---|---|---|---|
| Santamaria et al. 2013 9 | RCT | Trauma/critically ill | Sacrum/heel | Soft silicone foam | 5/161 (3·1%) | 20/152 (13·1%) | None |
| Callaghan and Trapp 1998 10 | RCT | Respiratory unit | Nose | Hydrocolloid | 2/8 (25%) | 8/10 (80%) | No report of randomisation procedure |
| Weng 2008 11 | Cohort | ICU | Nose | Transparent film and hydrocolloid | Transparent film 16/30 (53·3%) and hydrocolloid 12/30 (40%) | 29/30 (96·7%) | None |
| Huang et al. 2009 12 | Cohort | During surgery | Nose | Hydrocolloid | 6/10 (60%) | 8/8 (100%) | Lengthy surgical procedures (with nasal protection 10·4 ± 3·5 hours, no nasal protection 9·8 ± 1·7 hours) |
| Forni et al. 2011 13 | Cohort | Orthopaedic | Heel | Polyurethane foam | 2/56 (3·6%) | 21/49 (42·9%) | Incidence of pressure ulcers among subjects with category 1 heel ulcers at baseline. No reporting of entire study population |
| Brindle and Wegelin 2012 14 | Cohort | ICU | Sacrum | Soft silicone foam | 1/50 (2%) | 4/35 (11·4%) | None |
| Cubit et al. 15 | Cohort | Acute medical wards | Sacrum | Soft silicone foam | 1/51 (2%) | 6/58 (10·3%) | None |
| Nakagami et al. 2007 16 | Within‐subject | Elderly care | Trochanter | Layered dressing with hydrocolloid containing ceramide 2 in contact with skin | 2/37 (5·4%) | 11/37 (29·8%) | Persistent erythema as outcome |
| Imanishi et al. 2006 17 | Not specified | During surgery | Sacrum | Polyurethane film | 10/98 (10·2%) | 22/103 (21·4%) | Limited information upon study design |
| Torra i Bou et al. 2009 18 | RCT | Community care | Heel | Gauze dressing and bandage, hydrocellular dressing | 3% (number of patients and pressure ulcers unreported) | 44% (number of patients and pressure ulcers unreported). Comparison was gauze dressing and protective bandage applied to foot | Numbers in each arm unspecified, total population recruited 130. No report of randomisation procedure |
ICU, intensive care unit; RCT, randomised controlled trial.
The nine studies were clinically and methodologically heterogeneous given that they recruited subjects within different care settings, and reported on the four body sites where pressure ulcers might occur that were dressed with a variety of prophylactic dressings (Table 2). Statistical comparison of heterogeneity across the nine studies also indicated substantial heterogeneity (I 2 = 54%) between studies. Given this heterogeneity, no synthesis of all nine retrieved studies was undertaken.
Table 3 details the quality of the nine studies that ranged from low to moderately high. Common failings included no masked assessment of outcomes, while all except Santamaria et al. 9 and Cubit et al. 15 did not report the number of patients approached to participate in the study and with baseline characteristic of the control and intervention arms unreported in two studies 10, 17. A funnel plot (Figure 2) was constructed to assess the potential for publication bias with no apparent bias towards the less precise studies having the most positive effect on outcomes, although the variability detailed in Figure 2 may simply reflect the heterogeneity of methods used in the studies.
Table 3.
Quality of the randomised controlled, non‐randomised and cohort studies where comparisons were made between the incidence of pressure ulcers where (a) the skin was protected with a prophylactic dressing and where no dressing was applied and (b) where different dressings were applied to protect the skin
| Quality criterion | Santamaria et al. 9 | Callaghan and Trapp 10 | Weng 2008 11 | Huang et al. 2009 12 | Forni et al. 2011 13 | Brindle and Wegelin 2012 14 | Cubit et al. 15 | Nakagami et al. 2007 16 | Imanishi et al. 2006 17 | Torra i Bou et al. 2009 18 |
|---|---|---|---|---|---|---|---|---|---|---|
| The assignment of subjects to treatment groups is randomised | Y | Unclear* | N | N | N | N | N | N | N | Unclear* |
| Subjects and investigators are kept ‘blind’ about treatment allocation | N | N | N | N | N | N | N | N | N | N |
| The treatment and control groups are similar at the start of the trial | Y | Not reported | Y | Y | Y | Y | Y | Not applicable | Not reported | Y |
| The study indicates how many of the people asked to take part did so in each of the groups being studied | Y | N | N | N | N | N | Y | N | N | N |
| All relevant outcomes are measured in a standard, valid and reliable manner | Y | Y | Y | Y | Y | Y | Y | Y | Not reported | Y |
| Was the percentage of the individuals or clusters recruited into each treatment arm of the study who dropped out before the study was reported? | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Where the study is carried out at more than one site, results are comparable for all sites | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Not reported |
Subjects described as being randomly allocated to interventions but no details of the randomisation provided.
Figure 2.
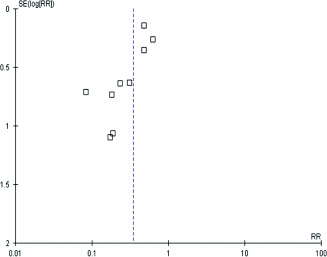
Funnel plot to ascertain risk of publication bias within the nine studies that compared pressure ulcer prevention with or without prophylactic dressings.
Pressure ulcers at the nose
Three studies investigated the role of prophylactic dressings to protect the nose from contact with medical devices (nasal masks and nasotracheal tubes) 10, 11, 12. The heterogeneity of these three studies was calculated (I 2 = 0%) and given the lack of statistical heterogeneity, the three studies were combined (Figure 3). Weng 11 compared the incidence of pressure ulcers among three cohorts of patients – with no dressing, a transparent film or a hydrocolloid dressing placed between nasal face masks and the skin. The results from the groups who received the film or the hydrocolloid dressing are combined in Figure 3 (pressure ulcer incidence category I film dressing 16/30 and hydrocolloid 12/30 combined incidence 28/60). In each study retrieved within this review there was no attempt to discriminate between blanching and non‐blanching erythema nor were other causes of skin changes, for example, incontinence‐associated dermatitis or allergic reaction to the dressing material considered. The three studies in nasal pressure ulcer prevention reported lower pressure ulcer incidence in the cohorts where dressings were used to protect the skin from medical devices (overall RR = 0·53, 95% CI 0·39–0·64). Use of a prophylactic dressing in the care of two patients would prevent one person developing a nasal pressure ulcer caused by medical devices.
Figure 3.
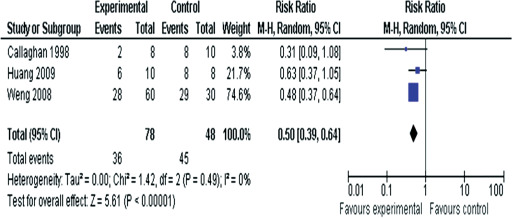
Forest plot synthesising the three studies that compared nasal pressure ulcer prevention with or without prophylactic dressings.
Pressure ulcers at the heels
Two studies 9, 13 reported the incidence of new heel ulcers irrespective of whether the heel was covered with a prophylactic dressing or not. Santamaria et al. 9 reported the results of 440 trauma and critically ill patients admitted to a single hospital through its emergency department. The subjects were randomly allocated pressure ulcer preventive care that included or lacked the application of soft silicone foam dressings to the sacrum and heels. Other elements of preventive care included all subjects being allocated a loss‐air‐loss bed, regular risk assessment and repositioning along with skin care. Three subjects developed heel ulcers (3/161; 1·9%) where a soft silicone dressing was applied to the heels with 12/152 (7·9%) having new heel ulcers where no dressing was applied. Forni et al. 13 recruited orthopaedic patients wearing a lower leg cast that incorporated the foot. In the first cohort (n = 86) no dressing was applied over the heel under the cast, while a later cohort (n = 72) received a polyurethane foam dressing to protect the heel. Only subsets of the study data were reported with the number of subjects with category I pressure ulcers at recruitment whose pressure ulcers later deteriorated being 21 of 49 (42·9%) where no dressing was applied, while 2 of 56 (3·6%) subjects exhibited similar deterioration where the dressing was applied between the heel and the cast. The studies by Santamaria et al. 9 and Forni et al. 13 were not combined given the differences in the reported outcome measures, such as incidence of new pressure damage and deterioration of existing pressure damage.
Pressure ulcers at the sacrum
Four studies 9, 12, 15, 17 described the incidence of sacral pressure ulcers where the skin was or was not protected with a prophylactic dressing. Figure 4 combines the four studies with a RR of 0·37 (95% CI 0·21–0·67). Three of the studies used a soft silicone foam dressing to protect the sacrum 9, 12, 15; the final study used a polyurethane film dressing, which was applied to the sacrum during the surgery 17. The heterogeneity of these four studies was calculated (I 2 = 0%) and given the lack of statistical heterogeneity, the studies were combined (Figure 4). Where a soft silicone foam dressing was applied to the sacrum in trauma and critically ill patients 9, 12, new pressure ulcers appeared in 3 of 161 9 and in 1 of 50 12 cases, whereas the incidence was higher if no dressing was applied – 8 of 152 9 and 4 of 35 12. Cubit et al. 15 compared the incidence of category I and II pressure ulcers among patients admitted to acute medical wards through a single‐centre emergency department – where soft silicone dressing was used, 1 of 50 developed a sacral pressure ulcer with 6 of 68 similar patients with no dressing in place to augment prevention, developed similar wounds. Studies 9, 12, 15 were clinically homogeneous (similar patient populations receiving appropriate pressure ulcer preventive care with or without dressing use) but methodologically different [RCT 9 and cohort studies 12, 15 with allocation to the group provided dressings to protect the skin based on which room the patient was allocated 9 or where the no dressing intervention group was dependent upon review of medical notes for outcome data]. Where polyurethane film dressings were applied to the skin of subjects undergoing gastrointestinal, urological and gynaecological surgery (position dorsosacral), 10 of 98 developed erythema at the end of surgery with 22 of 103 also showing erythema at the end of surgery if no dressing had been applied 16. Subjects were monitored for 24 hours with all bar 1 (film dressing group) and 4 (no dressing group) areas of erythema having resolved after 24 hours. Based on these four studies, three patients would need to have a dressing applied to prevent one sacral pressure ulcer.
Figure 4.
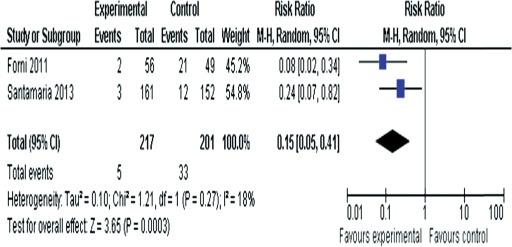
Forest plot synthesising the four studies that compared sacral pressure ulcer prevention with or without prophylactic dressings.
Comparison between different prophylactic dressings
There were only two comparisons between the effect of different prophylactic dressings upon pressure ulcer prevention 10, 18. Torra i Bou et al. 18 reported a comparison between the incidence of pressure ulcers at the heel where the heel was protected by a gauze dressing and bandage combination or a hydrocellular dressing. A total of 130 subjects were recruited from six nursing homes and primary health care centres in Spain; however, the number allocated to the intervention (hydrocellular dressing) or control (gauze dressing) arms was not reported. Subjects were followed up for 8 weeks and 44% (control) and 3·3% (intervention) of subjects developed pressure ulcers (severity unreported). The RR of developing pressure ulcers in the gauze and bandage arm was stated to be 13·42 (95% CI 3·31–54·3). In the second comparison between dressings, Weng 11 reported the incidence of nasal pressure damage under face masks where either a transparent film dressing or a hydrocolloid dressing was used to protect the skin. Sixty patients (30 per dressing type) were recruited with no pressure damage around the nose at baseline; the first 30 subjects were allocated the hydrocolloid dressing, of which 12 were reported to have developed category I pressure ulcers under the face mask with the length of follow‐up unreported with the skin checked for pressure damage every 30 minutes. The next 30 patients were provided with a film dressing to protect the skin and 16 developed category I pressure ulcers. The incidence of category I pressure ulcers in both dressing groups was similar (Χ 2 = 1·07, df = 1, P = 0·30). Both studies that compared the effect of different prophylactic dressings upon pressure ulcer incidence were generally of low quality (Table 4) with no random allocation of subjects to intervention and control arms, no masking of intervention to staff or data collectors and weak reporting of relevant data; for example, Torra i Bou et al. 18 did not report the number of subjects allocated to the intervention or to the control groups.
Table 4.
Summary characteristics of the 11 case series studies where comparisons were made between the incidence of pressure ulcers where the skin was protected with a wound dressing and either where no dressing was applied to prevent pressure damage or where alternative dressings had been used prior to the start of the study
| Study | Design | Population | Body sites reported | Prophylactic dressing | Comparator (if present) | Pressure ulcer incidence where dressings were used to augment prevention | Pressure ulcer incidence in comparator group (if present) | Other comments |
|---|---|---|---|---|---|---|---|---|
| Bots and Apotheker 2004 19 | Cohort, but one group had no reported data so treated in the review as a case series | General surgery, cardiopulmonary surgery, orthopaedic/trauma, vascular/gynaecology/urology in single centre | Heel | Hydropolymer adhesive dressing | Historical data | 10/117 (8·5%) | Prevalence 36·5% (number of patients and pressure ulcers unreported) | Dressing applied in at‐risk group anticipated to have surgery over 90 minutes in duration |
| Brindle 2009 20 | Case series | Single‐centre ICU | Sacrum | Soft silicone foam | None | 0/41 (0%) | None | Patients grouped as either high or low risk of pressure ulcer development, high‐risk patients received the dressing as part of their pressure ulcer preventive care |
| Cano et al. 2011 21 | Case series | Single‐centre ICU/CCU | Sacrum | Soft silicone foam | None | 1/166 (0·6%) | None | Conference abstract only |
| Chaiken 2012 22 | Case series | Single‐centre ICU | Sacrum | Soft silicone foam | Historical pressure ulcer prevalence data within ICU | 5/275 (1·8%) | Prevalence stated to be either 13·6% (abstract) and 12·3% (text) (stated to be data from 291 ICU patients) | Comparing prevalence and incidence data. Contemporary introduction of staff education with focus upon reduced head of bed elevation may also have reduced incidence? |
| Hsu et al. 2010 29 | Case series | Unspecified | Nose | Soft silicone foam | Historical incidence data where hydrocolloid dressing used to protect the skin under face masks | 0·9% (number of patients and pressure ulcers unreported) | 5·9% 47/797 (patients wearing face masks with the skin protected by hydrocolloid dressings in 2006) | None |
| Iwai et al. 2011 23 | Technical report | Single centre during Surgery | Nose | Hydrocolloid | None | 0% from 'over 500 patients' | None | Technical report upon placement of the dressing around a nasotracheal tube |
| Kiely 2012 24 | Case series | Single‐centre ICU | Sacrum | Soft silicone foam | Historical incidence data | 0·9% (number of patients and pressure ulcers unreported) | Stated to be five new sacral pressure ulcers per month in the ICU | Facility‐wide quality improvement programme with dressing use in ICU as one component |
| Koerner et al. 2011 25 | Case series | Single‐centre ICU | Sacrum | Soft silicone foam | Historical incidence data – medical/cardiac ICU and surgical ICU | 0% (number of patients followed up unreported) | 20% surgical ICU, 40% medical/cardiac ICU (number of patients and pressure ulcers unreported) | Conference abstract only |
| Lisco 2013 26 | Case series | Single‐centre ICU | Sacrum | Silicone adhesive hydrocellular foam | None | 0/22 (0%) | None | Conference abstract only |
| Sansom and Flynn 2007 27 | Case series | Single‐centre Emergency department | Heel | Foam | None | 0/20 (0%) | None | Data reported upon 20 of the 100 patients provided with the heel dressing |
| Walsh et al. 2012 28 | Case series | Single‐centre ICU | Sacrum | Soft silicone foam | None | 3/62 (4·8%) | None | Reduction in incidence of pressure ulcer in ICU 2008 to 2010 from 21·3% to 7%; reduced numbers of sacral pressure ulcers in ICU from 79 in 2008 to 13 in 2010 |
ICU, intensive care unit; CCU, critical care unit.
Case series reports of prophylactic dressing use
In addition to the ten studies detailed in Table 3 there were ten case series reports that compared the use of dressings to help prevent pressure ulcers with no dressing use 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 and one case series 29 that compared the incidence of nasal pressure ulcers under face masks where a soft silicone foam dressing or a hydrocolloid dressing was used to protect the skin. The characteristics of the case studies are listed in Table 4 and their methodological quality is reported in Table 5. The case series were typically undertaken in a single intensive care unit (ICU) (seven studies) and explored the prevention of sacral pressure ulcers (seven studies) where the sacrum was protected by a soft silicone foam dressing (six studies). All the case series reported low incidence of new pressure ulcers where dressings were used as part of pressure ulcer prevention. However, the case series studies were generally methodologically weak, often with non‐consecutive admission to the study and weak description of the main outcome in terms of failing to define pressure ulcers within the studies. In six case series, no comparison was made between the recorded pressure ulcer incidence and historical data 20, 21, 23, 26, 27, 28. In two case series, pressure ulcer incidence was compared with historical prevalence proportions 19, 22 and where pressure ulcer incidence was compared with older incidence data, the historical data were presented either as the average number of new ulcers per month 24 or as a percentage with no detail of the number of patients surveyed and the number who developed pressure ulcers 25. The final case series 29 compared nasal pressure ulcer incidence data gathered over 2006 within one facility, where 47 of 797 (5·9%) patients wearing face mask developed 86 pressure ulcers despite the skin being protected using hydrocolloid dressings below the face mask (51 category I, 33 category II pressure ulcers and 3 category III wounds) primarily on the cheeks (n = 51) and the bridge of the nose (n = 19). Hsu et al. 29 replaced the hydrocolloid dressings because skin peeling and pain on removal were observed on using this dressing (no data presented to support this observation). The skin under the face mask was then protected using a soft silicone foam dressing and the incidence of pressure ulcers due to face masks declined to 0·9% although the number of patients surveyed and the number and severity of encountered pressure ulcers were unreported. None of the case series performed any statistical comparison between the pressure ulcer incidence observed where a dressing was used as part of pressure ulcer prevention and historical pressure ulcer incidences captured in the same facility and unit.
Table 5.
Summary of key quality indicators of the case series
| Study | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Checklist | |||||||||||
| Study objective | |||||||||||
| 1. Is the hypothesis/aim/objective stated clearly in the abstract, introduction or methods section? | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Study population | |||||||||||
| 2. Are the characteristics of the participants included in the study described? | N | N | N | N | N | N | N | N | N | Y | N |
| 3. Were the cases collected in more than one centre? | N | N | N | N | N | N | N | N | N | N | N |
| 4. Are the eligibility criteria (inclusion and exclusion criteria) for entry into the study explicit and appropriate? | Y | Y | Y | N | N | N | N | N | N | Y | Y |
| 5. Were participants recruited consecutively? | N | Y | ? | ? | N | N | N | N | N | N | N |
| 6. Did participants enter the study at a similar point in the disease? | N | Y | Y | ? | N | N | Y | Y | ? | N | Y |
| Intervention and co‐intervention | |||||||||||
| 7. Was the intervention clearly described in the study? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 8. Were additional interventions (co‐interventions) clearly reported in the study? | Y | Y | N | N | N | N | N | N | N | Y | N |
| Outcome measure | |||||||||||
| 9. Are the outcome measures clearly defined in the introduction or methods section? | Y | Y | ? | N | Y | N | N | N | N | N | Y |
| 10. Were relevant outcomes appropriately measured with objective and/or subjective methods? | Y | Y | ? | Y | Y | N | N | N | N | N | Y |
| 11. Were outcomes measured before and after intervention? | Y | Y | ? | Y | Y | ? | ? | ? | ? | ? | Y |
| Statistical analysis | |||||||||||
| 12. Were the statistical tests used to assess the relevant outcomes appropriate? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Results and conclusions | |||||||||||
| 13. Was the length of follow‐up reported? | Y | N | N | N | N | N | N | N | N | N | Y |
| 14. Was the loss to follow‐up reported? | N | Y | N | N | N | N | N | N | N | N | Y |
| 15. Does the study provide estimates of the random variability in the data | N | N | N | N | N | N | N | N | N | N | N |
| Analysis of relevant outcomes? | |||||||||||
| 16. Are adverse events reported? | N | N | N | N | N | N | N | N | N | N | Y |
| 17. Are the conclusions of the study supported by results? | Y | Y | ? | Y | Y | Y | Y | N | N | Y | Y |
| Competing interests and sources of support | |||||||||||
| 18. Are both competing interests and sources of support for the study reported? | Y | Y | N | Y | N | N | Y | N | N | Y | Y |
Y, yes; N, no; N/A, not applicable; ?, unclear from study report.
Discussion
Statement of principal findings
Twenty‐one eligible publications were reviewed to establish whether the introduction of a prophylactic dressing applied upon intact skin could help reduce the incidence of pressure ulcers? And if so was there any evidence that different dressings would have a greater or lesser effect on improving pressure ulcer prevention? Three RCTs were identified; of these two 10, 18 were small and failed to describe how random allocation to treatment was achieved. The final RCT 9 was appropriately powered with adequate description of the elements of pressure ulcer prevention that were provided in addition to the placement of soft silicone foam dressing over the sacrum. In the study by Santamaria et al. 9, the use of the soft silicone foam dressing significantly reduced the incidence of pressure ulcers compared to similar patients who received preventive care but no dressing. While this study indicates that dressings may indeed augment pressure ulcer prevention, it does have limitations that may reduce its external validity to other facilities and patient populations; neither masking of staff to the intervention was possible nor was the reporting of outcomes undertaken with assessors masked to the intervention group. Santamaria et al. 9 recruited trauma patients and acutely ill patients admitted to the ICU through the emergency department of a single hospital among a specific patient population; it is possible that this group of generally immobile people may maintain their dressing in place whereas in a more mobile population, rolling of the edges of the dressing and perhaps dressing removal may occur. The 18 non‐RCT studies (7 cohort and 11 case series) reported low pressure ulcer incidence when the dressings were applied to the sacrum, heels, nose and trochanter. However, these studies were methodologically weak and Hawthorne effects cannot be discounted when considering their data.
There were few comparisons between the effects of different dressing materials upon reductions in pressure ulcer incidence. In one small RCT 18, a hydrocellular dressing was compared with a gauze and protective bandage when used to protect the heels of patients receiving care in community settings. The study reported that 3% and 44% of subjects developed heel pressure damage where the heel was protected by the hydrocellular or gauze dressing, respectively, with the numbers of patients in either arm unreported. Weng 11 reported the results of three cohorts of the ICU patients wearing face masks – when no dressing was applied under the mask, 29 of 30 developed pressure damage; with a transparent film between the skin and the mask, 16 of 30 developed damage; whereas a hydrocolloid dressing under the mask reduced the incidence of skin damage to 12 of 30. In the final comparison between different dressings, the incidence of nasal pressure damage among face mask wearers reduced from 5·9% to 0·9% where hydrocolloid or soft silicone foam dressings were positioned under the mask 29. However, there was no simultaneous comparison of the effect of the hydrocolloid or soft silicone foam dressings, and the changes in pressure ulcer incidence may be influenced by changes in practice or the face mask technology between the time subjects were recruited when hydrocolloid dressings were used and the later use of soft silicone foam.
Implications for health care
The introduction of dressings to increase the protection of vulnerable anatomical sites from pressure ulcers may offer another technique through which the incidence of superficial pressure ulcers may be reduced. Reductions in pressure ulcer incidence may improve both patient and staff satisfaction with health care while also improving the quality of health services through reducing patient harm. Widespread adoption of prophylactic dressings in pressure ulcer prevention may impact upon the costs of pressure area care. Santamaria et al. 30 reported the average marginal cost of using prophylactic dressings to be AUS $36·61, whereas the average cost of pressure ulcer treatment was lower in the intervention group allocated prophylactic dressings (AUS $70·82 for intervention and AUS $144·56 for control group with no prophylactic dressing use). This single economic study suggests that the cost of introducing appropriate use of prophylactic dressings may reduce overall treatment costs given the reductions in pressure ulcer incidence.
Research recommendations
There is limited high‐quality evidence linking dressings to improved pressure ulcer prevention. Large‐scale studies similar to that of Santamaria et al. 9 will be required to ascertain the most appropriate patient populations, care settings and even perhaps anatomical landmarks where the use of dressings along with other preventive care may reduce pressure ulcer occurrence. At the heel off‐loading is recommended to prevent pressure ulceration 1 and use of prophylactic dressings should be compared with heel off‐loading before the use of dressings at the heel becomes commonplace. There is also a need for comparative clinical studies between different dressing types to investigate whether in vitro performance differences between dressings, for example, Call et al. 31 translate into differential clinical effects upon pressure ulcer incidence.
Limitations of the review
It may appear strange to use the PRISMA statement to report upon mixed design studies that report the effect of prophylactic dressings upon pressure ulcer incidence with such methodological tools usually seen as appropriate for the synthesis of several RCTs. However, the PRISMA statement 6 comments that the process ‘can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions’. It may be helpful for future reviews on the effect of pressure ulcer prevention and healing also to follow the PRISMA statement in order to obtain consistently structured reviews across a range of interventions.
The main limitation of the review process was the exclusion of non‐English language publications – the search strategy was re‐run removing this limitation and two further clinical studies were identified – one in Spanish and the other in Farsi. The paucity of non‐English language clinical studies of prophylactic dressings suggests that the conclusions of the review may not have been altered if the two non‐English language publications had been included.
There were multiple weaknesses within the retrieved studies, for example, failure to define the length of dressing use, lack of clarity over observed skin changes and were these caused by pressure or could they have resulted from microclimate changes under the dressing or incontinence‐associated dermatitis. These failings are common within pressure ulcer prevention studies and not solely related to the topic of prophylactic dressing use.
Conclusions
The single high‐quality RCT and the growing number of cohort, weak RCT and case series all suggest that the introduction of a dressing as part of pressure ulcer prevention may help reduce pressure ulcer incidence associated with medical devices and in immobile ICU patients. There is no firm clinical evidence at this time to suggest that one dressing type is more effective than the other dressings.
Description of the health problem
Management of both the duration and magnitude of the mechanical loads applied to skin and soft tissues has long been seen as the essential element of pressure ulcer prevention and management 1. These mechanical loads, for example, direct pressure, shear or friction, have been typically considered to be best managed either through manual repositioning of patients or through the use of a variety of pressure‐redistributing support surfaces. Apart from reducing friction and at times shear, alternative approaches to achieving modification of applied mechanical loads were not considered within the 2009 International Pressure Ulcer guidelines 1.
Over the last 20 years there has been sporadic interest in the role that prophylactic dressings may play in both redistributing pressure and protecting the skin from the effects of shear and friction 2. There has also been recent discussion that microclimate control (defined as including management of temperature, humidity, moisture and skin surface pH) may play a key role in pressure ulcer prevention 3, 4; given the role of advanced wound dressings in exudate management it is most probable that wound dressings will alter the local wound or skin microclimate. The purpose of this systematic review was to consider whether the introduction of prophylactic dressings for pressure ulcer prevention can lead to reductions in the occurrence of superficial (category I and II) pressure ulcers 5.
Acknowledgements
This review was funded by Molnlycke Healthcare Ltd who had no input into the search parameters, the data extraction or its interpretation.
References
- 1. National Pressure Ulcer Advisory Panel and European Pressure Ulcer Advisory Panel . Prevention and treatment of pressure ulcers: clinical practice guideline. Washington, DC: National Pressure Ulcer Advisory Panel, 2009. [Google Scholar]
- 2. Clark M. The effect of a pressure‐relieving wound dressing on the interface pressures applied to the trochanter. Decubitus 1990;3:43–6. [PubMed] [Google Scholar]
- 3. Wounds International . Pressure ulcer prevention. Pressure, shear, friction and microclimate in context. London: Wounds International; URL http://www.woundsinternational.com/pdf/content_8925.pdf [accessed on 11 December 2012], 2010. [Google Scholar]
- 4. Gefen A. How do microclimate factors affect the risk for superficial pressure ulcers: a mathematical modeling study. J Tissue Viability 2011;20:81–8. [DOI] [PubMed] [Google Scholar]
- 5. Clark M, Cullum N. Matching patient need for pressure sore prevention with the supply of pressure redistributing mattresses. J Adv Nurs 1992;17:301–16. [DOI] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:b2535. DOI: 10.1136/bmj.b25357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moga C, Guo B, Schopflocher D, Harstall C. Development of a quality appraisal tool for case series studies using a modified Delphi technique. Edmonton: Institute of Health Economics, 2012. URL http://www.ihe.ca/documents/Case%20series%20studies%20using%20a%20modified%20Delphi%20technique.pdf [accessed on 7 June 2013]. [Google Scholar]
- 8. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. URL www.cochrane‐handbook.org. [Google Scholar]
- 9. Santamaria N, Gerdtz M, Sage S, McCann J, Freeman A, Vassiliou T, De Vincentis S, Ng AW, Manias E, Liu W, Knott J. A randomised controlled trial of the effectiveness of soft silicone multi‐layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J 2013. DOI: 10.1111/iwj.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Callaghan S, Trapp M. Evaluating two dressings for the prevention of nasal bridge pressure sores. Prof Nurse 1998;13:361–4. [PubMed] [Google Scholar]
- 11. Weng M. The effect of protective treatment in reducing pressure ulcers for non‐invasive ventilation patients. Intensive Crit Care Nurs 2008;24:295–9. [DOI] [PubMed] [Google Scholar]
- 12. Huang TT, Tseng CE, Lee TM, Yeh JY, Lai YY. Preventing pressure sores of the nasal ala after nasotracheal tube intubation: from animal model to clinical application. J Oral Maxillofac Surg 2009;67:543–51. [DOI] [PubMed] [Google Scholar]
- 13. Forni C, Loro L, Tremosini M, Mini S, Pignotti E, Bigoni O, Guzzo G, Bellini L, Trofa C, Di Cataldo AM, Guzzi M. Use of polyurethane foam inside plaster casts to prevent the onset of heel sores in the population at risk. A controlled clinical study. J Clin Nurs 2011;20:675–80. [DOI] [PubMed] [Google Scholar]
- 14. Brindle CT, Wegelin JA. Prophylactic dressing application to reduce pressure ulcer formation in cardiac surgery patients. J Wound Ostomy Continence Nurs 2012;39:133–42. [DOI] [PubMed] [Google Scholar]
- 15. Cubit K, McNally B, Lopez V. Taking the pressure off in the Emergency Department: evaluation of the prophylactic application of a low shear, soft silicon sacral dressing on high risk medical patients. Int Wound J 2012. DOI: 10.1111/j.1742-481X.2012.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakagami G, Sanada H, Konya C, Kitagawa A, Tadaka E, Matsuyama Y. Evaluation of a new pressure ulcer preventive dressing containing ceramide 2 with low frictional outer layer. J Adv Nurs 2007;59:520–9. [DOI] [PubMed] [Google Scholar]
- 17. Imanishi K, Morita K, Matsuoka M, Hayashi H, Furukawa S, Terashita F, Moriya E, Kanesaki U, Kinukawa N, Nose Y, Moroi Y, Urabe K, Furue M. Prevention of postoperative pressure ulcers by a polyurethane film patch. J Dermatol 2006;33:236–7. [DOI] [PubMed] [Google Scholar]
- 18. Torra i Bou J‐E, Rueda López J, Camañes G, Herrero Narváez E, Blanco Blanco J, Ballesté Torralba J, et al. Preventing pressure ulcers on the heel: a Canadian cost study. Dermatol Nurs 2009;21:268–72. [PubMed] [Google Scholar]
- 19. Bots TC, Apotheker BF. The prevention of heel pressure ulcers using a hydropolymer dressing in surgical patients. J Wound Care 2004;13:375–8. [DOI] [PubMed] [Google Scholar]
- 20. Brindle CT. Outliers to the Braden Scale: identifying high risk ICU patients and the results of prophylactic dressing use. World Council Enterostomal Ther 2009;30:11–8. [Google Scholar]
- 21. Cano A, Smits D, Corvino P. Efficacy of the prophylactic use of silicone foam dressing for the prevention of pressure ulcers in patients: an observational study in a 24 bed cardiovascular and cardiac intensive care unit. J Wound Ostomy Continence Nurs 2011;38(3S):S73. [Google Scholar]
- 22. Chaiken N. Reduction of sacral pressure ulcers in the intensive care unit using a silicone border foam dressing. J Wound Ostomy Continence Nurs 2012;39:143–5. [DOI] [PubMed] [Google Scholar]
- 23. Iwai T, Goto T, Maegawa J, Tohnai I. Use of a hydrocolloid dressing to prevent nasal pressure sores after nasotracheal intubation. Br J Oral Maxillofac Surg 2011;49:e65–6. [DOI] [PubMed] [Google Scholar]
- 24. Kiely C. Cultural transformation in pressure ulcer prevention and care. J Wound Ostomy Continence Nurs 2012;39:443–6. [DOI] [PubMed] [Google Scholar]
- 25. Koerner S, Adams D. Does the use of an absorbent soft silicone selfadherent bordered foam improve quality of care by decreasing incidence of hospital acquired pressure ulcers? J Wound Ostomy Continence Nurs 2011;38(3S):S70–S. [Google Scholar]
- 26. Lisco C. Evaluation of a new silicone gel‐adhesive hydrocellular foam dressing as part of a pressure ulcer‐prevention plan for ICU patients. J Wound Ostomy Continence Nurs 2013;40(3S):S61–2. [Google Scholar]
- 27. Sansom W, Flynn K. Risk assessment and anatomical foam heel dressings in emergency department contribute to reduced development of pressure ulcers. Prim Intent 2007;15:114. [Google Scholar]
- 28. Walsh NS, Blanck AW, Smith L, Cross M, Andersson L, Polito C. Use of a sacral silicone border foam dressing as one component of a pressure ulcer prevention program in an intensive care unit setting. J Wound Ostomy Continence Nurs 2012;39:146–9. [DOI] [PubMed] [Google Scholar]
- 29. Hsu M‐Y, Chung H‐C, Tang MT, Hsiu S‐R. Avoiding pressure damage when using ventilators. Wounds Int 2010;1 URL http://www.woundsinternational.com/practice‐development/avoiding‐pressure‐damage‐when‐using‐ventilators [accessed on 1 August 2013]. [Google Scholar]
- 30. Santamaria N, Liu W, Gerdtz M, Sage S, McCann J, Freeman A, Vassiliou T, De Vincentis S, Ng AW, Manias E, Knott J, Liew D. The cost‐benefit of using soft silicone multilayered foam dressings to prevent sacral and heel pressure ulcers in trauma and critically ill patients: a within‐trial analysis of the Border Trial. Int Wound J 2013. DOI: 10.1111/iwj.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Call E, Pedersen J, Bill B, Oberg C, Ferguson‐Pell M. Microclimate impact of prophylactic dressings using in vitro analog method. Wounds 2013;25:94–103. [PubMed] [Google Scholar]


