Abstract
Early detection and intervention of deep tissue injury are important to lead good outcome. Although the efficiency of ultrasonographic assessment of deep tissue injury has been reported previously, it requires a certain level of skill for accurate assessment. In this study, we present an investigation of the combination of thermographic and ultrasonographic assessments for early detection of deep tissue injury. We retrospectively reviewed 28 early‐stage pressure ulcers (21 patients) presenting at the University of Tokyo Hospital between April 2009 and February 2010, surveying the associated thermographic and ultrasonographic findings. The wound temperature patterns were divided into low, even and high compared with the surrounding skin. Ultrasonographic findings were classified into unclear layer structure, hypoechoic lesion, discontinuous fascia and heterogeneous hypoechoic area. All 13 ulcers that were associated with low temperature showed good outcome; three ulcers had even temperatures and 12 ulcers showed high temperature on thermographic assessment. The two deep tissue injuries were rated high on thermographic assessment and showed heterogeneous hypoechoic area findings on ultrasonographic assessment. No non‐deep tissue injury lesion was associated with these two findings simultaneously. The combination of thermographic and ultrasonographic assessments is expected to increase the accuracy of the early detection of deep tissue injuries.
Keywords: Deep tissue injury, Early detection, Pressure ulcer, Thermography, Ultrasonography
Introduction
Deep tissue injury, which is ‘a pressure‐related injury to subcutaneous tissues under intact skin 1, 2’, arises in the muscle layers adjacent to bony prominences following sustained loading and progresses outwards towards the skin 3, 4. Although these lesions are likely due to ischaemia of the muscle bed and occlusion of the vertical perforating blood vessels feeding the skin through the muscle 1, the exact underlying pathophysiology associated with the development of deep tissue injury remains poorly understood 5. Deep tissue injuries represent a serious type of pressure ulcer and are potentially dangerous lesions because they initiate in underlying tissues and are often not visible until they reach an advanced stage 4. As they are known to deteriorate quickly but do not resolve easily 3, treatment becomes problematic.
Early detection of deep tissue injury is critical because it leads to early intensive intervention. Because not all deep tissue injuries progress to full‐thickness defects 4, 5, early detection methods may permit the salvaging of ischaemic and injured tissues. Early detection and intervention can prevent the deterioration of lesions and promote good outcome 6. Although preventing the deterioration of deep tissue injury has become a key focus, it is still very difficult to predict the occurrence of injury at an early stage.
In this study, we present an investigation of the combination of thermographic and ultrasonographic assessments for early detection of deep tissue injury. We reviewed retrospectively the outcomes of early‐stage pressure ulcers to examine features that may suggest the presence of a deep tissue injury and to develop reliable cues for simple, accurate and early detection of deep tissue injury using thermography and ultrasonography.
Materials and methods
We performed a retrospective review of early‐stage pressure ulcers that received intervention from the pressure ulcer team at the University of Tokyo Hospital between April 2009 and February 2010. This study was conducted with approval from the appropriate Institutional Review Board. The patients were followed up for at least 2 weeks. Thermography was performed immediately after dressing removal followed by the routine care and ultrasonographic assessment. To prevent a secondary insult to the skin at the site of the pressure ulcer, patients were placed on a pressure‐relief bed and kept under intensive nursing care that included periodic position changes. Data regarding demographic distribution, patient's background, ulcer location, stage, thermographic findings and ultrasonographic findings were collected. DESIGN‐R, published as a clinical wound assessment tool by the Japanese Society of Pressure Ulcers in 2008 7, was used to assess the clinical stages of the pressure ulcers based on the depth of the observed tissue damage. DESIGN‐R stage d1 corresponds to National Pressure Ulcer Advisory Panel (NPUAP) stage I and DESIGN‐R stage d2 corresponds to NPUAP stage II (Table 1). DESIGN‐R stages d1 and d2 were defined as early‐stage pressure ulcers.
Table 1.
The DESIGN‐R scale
| Stage | Findings |
|---|---|
| Depth | |
| d0 | No injury and redness |
| d1 | Persistent redness |
| d2 | Lesion into dermis |
| D3 | Lesion into subcutaneous tissue |
| D4 | Lesion into muscle, tendon and bone |
| D5 | Lesion into body cavity |
| DU | Unstageable lesion |
Thermographic images were obtained using infrared thermography (Thermotracer TH5108ME; NEC Avio Infrared Technology Co. Ltd, Tokyo, Japan) with a measurable range of 0–70°C, an error range of 0·7°C and an accuracy of 0·1°C. It takes only a few minutes to capture thermographic images. In this study, we focused on graphical assessment, not detailed temperature readings. Thermographic findings were reviewed to assess the wound temperature compared with that of the surrounding skin; wound temperature patterns were divided into low, even and high versus the surrounding skin.
Ultrasonographic images were collected using a portable ultrasound system with a 10‐MHz probe (LOGIQ Book XP; GE Healthcare, Chalfont St Giles, UK), which provides reasonable resolution for an image 20–30 mm below the skin surface 8. Ultrasonographic findings were reviewed for the presence of four types of ultrasound features that signify deep tissue injury: unclear layered structure, hypoechoic lesion, discontinuous fascia and heterogeneous hypoechoic area 9. An unclear layered structure is a subcutaneous condition that does not show a clear layer, superficial fascia, deep fascia, muscular layer, bursa and bone (periosteum). In addition, this structure usually has a foggy‐appearing area with low‐contrast and rough resolution. A hypoechoic lesion is a small lesion with a relatively clear margin that has no or little echoic signal and may correspond to a non‐vascularised area such as haematoma, seroma or necrotic tissue. A discontinuous fascia is an interrupted high‐signal line corresponding to the superficial or deep fascia. A heterogeneous hypoechoic area is a round or oval area with a heterogeneous internal echo that disrupts the normally layered structure; this area sometimes has a diffuse border. The ulcers that progressed to DESIGN‐R stage D3 or worse after a week in spite of intensive care were diagnosed as deep tissue injuries.
Results
The survey included 28 early‐stage pressure ulcers in 21 patients [11 males (52·4%) and 10 females (47·6%); Table 2]. Patient's age at presentation ranged from 20 to 98 years, with an average age of 66·4 years. There were 4 pressure ulcers at DESIGN‐R stage d1 (14·3%) and 24 ulcers at stage d2 (85·7%) at the primary examination (Table 3). Fourteen pressure ulcers occurred on the sacral region (50%), nine on the greater trochanter region (32·1%), two on the coccyx region (7·1%), two on the iliac crest (7·1%) and one on the back (3·6%). There were two deep tissue injuries (case 14 and case 16).
Table 2.
Summarised data for 21 patients with 28 early‐stage pressure ulcers
| Patient | Age (years) | Sex | Location | Primary disease | Cause | Primary size (cm2) |
|---|---|---|---|---|---|---|
| 1 | 68 | F | Greater trochanter | Rheumatism | Immobilisation | 1·8 |
| 2 | 58 | F | Sacrum | Intravertebral canal haemorrhage | Immobilisation | 10·6 |
| 3 | 75 | M | Sacrum | Gastrointestinal haemorrhage | Immobilisation | 72·9 |
| Greater trochanter | 8·7 | |||||
| 4 | 70 | M | Sacrum | Parkinsonism, hepatocellular carcinoma | Immobilisation | 13·5 |
| 5 | 54 | F | Sacrum | Multiple sclerosis | Immobilisation | 1·5 |
| Greater trochanter, lt. | 2·8 | |||||
| 6 | 86 | M | Greater trochanter, lt. | Diabetes mellitus | Immobilisation | 0·6 |
| 7 | 98 | F | Sacrum | Pneumonia | Immobilisation | 8·8 |
| 8 | 82 | M | Sacrum | Heart failure | Immobilisation | 38·2 |
| 9 | 60 | F | Sacrum | Colon cancer | Immobilisation | 1·6 |
| 10 | 64 | M | Sacrum | Postresuscitation encephalopathy | Loss of consciousness | 1·4 |
| 11 | 74 | F | Sacrum | Sepsis | Immobilisation | 5·6 |
| 12 | 74 | M | Sacrum | Cervical spinal cord injury | Immobilisation | 72·1 |
| 13 | 64 | M | Sacrum | Heatstroke | Immobilisation | 50·8 |
| 14 | 50 | M | Sacrum | Septic shock | Immobilisation | 77·0 |
| 15 | 67 | F | Greater trochanter, rt. | Multiple sclerosis | Immobilisation | 17·1 |
| Ilium, rt. | 121·9 | |||||
| Back, rt. | 42·1 | |||||
| Greater trochanter, lt. | 30·9 | |||||
| Ilium, lt. | 9·8 | |||||
| 16 | 21 | F | Sacrum | Acute drug intoxication | Loss of consciousness | 33·3 |
| 17 | 63 | M | Sacrum | Oesophageal cancer | Prolonged surgery | 25·8 |
| 18 | 76 | M | Greater trochanter, rt. | Schizophrenia | Immobilisation | 1·6 |
| 19 | 85 | F | Greater trochanter, rt. | Hypothermia | Immobilisation | 1·6 |
| Greater trochanter, lt. | 3·7 | |||||
| 2·7 | ||||||
| 20 | 85 | F | Sacrum | Thoracic myelopathy | Immobilisation | 0·1 |
| 21 | 20 | M | Coccyx | Fracture of left lower extremity | Prolonged surgery | 9·6 |
Table 3.
Summarised data from thermography and ultrasonography for 28 early‐stage pressure ulcers in 21 patients
| Patient | Age (years) | Sex | Location | DESIGN‐R | Thermographic findings at primary examination | Ultrasonographic findings at primary examination | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primary stage | Stage after 1 week | Unclear layered structure | Hypoechoic lesion | Discontinuous fascia | Heterogeneous hypoechoic area | |||||
| 1 | 68 | F | Greater trochanter | d2 | d0 | Low | − | − | − | − |
| 2 | 58 | F | Sacrum | d2 | d2 | High | + | − | − | − |
| 3 | 75 | M | Sacrum | d2 | d2 | Low | − | − | − | − |
| Greater trochanter | d2 | d2 | Low | − | − | − | − | |||
| 4 | 70 | M | Sacrum | d2 | d2 | High | − | − | − | − |
| 5 | 54 | F | Sacrum | d2 | d2 | High | − | − | − | − |
| Greater trochanter, lt. | d2 | d2 | High | − | − | − | − | |||
| 6 | 86 | M | Greater trochanter, lt. | d2 | d2 | Low | − | − | − | − |
| 7 | 98 | F | Sacrum | d2 | d2 | Low | + | + | − | − |
| 8 | 82 | M | Sacrum | d2 | d2 | Low | − | − | − | − |
| 9 | 60 | F | Sacrum | d2 | d2 | Low | − | − | − | − |
| 10 | 64 | M | Sacrum | d2 | d2 | Low | − | − | − | − |
| 11 | 74 | F | Sacrum | d2 | d2 | Low | − | − | − | − |
| 12 | 74 | M | Sacrum | d1 | d1 | High | − | − | − | − |
| 13 | 64 | M | Sacrum | d1 | d2 | High | + | − | − | − |
| 14 | 50 | M | Sacrum | d2 | DU | High | + | − | − | + |
| 15 | 67 | F | Greater trochanter, rt. | d1 | d1 | Even | − | − | − | − |
| Ilium, rt. | d2 | d2 | High | + | − | − | − | |||
| Back, rt. | d2 | d2 | High | − | − | − | − | |||
| Greater trochanter, lt. | d2 | d2 | High | + | − | − | − | |||
| Ilium, lt. | d2 | d2 | High | − | − | − | − | |||
| 16 | 21 | F | Sacrum | d2 | DU | High | + | − | + | + |
| 17 | 63 | M | Sacrum | d2 | d2 | Low | + | − | − | − |
| 18 | 76 | M | Greater trochanter, rt. | d2 | d2 | Low | − | − | − | − |
| 19 | 85 | F | Greater trochanter, rt. | d2 | d2 | Low | − | + | − | − |
| Greater trochanter, lt. | d2 | d2 | Low | + | − | − | − | |||
| 20 | 85 | F | Sacrum | d2 | d0 | Even | − | − | − | − |
| 21 | 20 | M | Coccyx | d1 | d2 | Even | + | − | − | − |
Thirteen ulcers exhibited low temperature on thermographic assessment, all of which were associated with good outcome. Three ulcers were categorised as even temperature and 12 ulcers were categorised as high temperature (Table 4). No deep tissue injuries showed low or even temperature at the thermographic assessment; 2 of 12 ulcers with high temperature were associated with deep tissue injury.
Table 4.
Summary of findings at primary examination for 28 ulcers
| Ultrasonography | Deep tissue injury | ||||||
|---|---|---|---|---|---|---|---|
| Unclear layered structure | Hypoechoic lesion | Discontinuous fascia | Heterogeneous hypoechoic area | ||||
| Thermography | Low | 13 | 3 | 2 | 0 | 0 | 0 |
| Even | 3 | 1 | 0 | 0 | 0 | 0 | |
| High | 12 | 6 | 0 | 1 | 2 | 2 | |
| Total | 28 | 28 | 10 | 2 | 1 | 2 | 2 |
Ultrasonography showed unclear layered structures in ten ulcers and hypoechoic lesions in two ulcers. One ulcer exhibited discontinuous fascia and two ulcers contained heterogeneous hypoechoic areas. These two ulcers simultaneously showed high temperature at thermographic assessment and were associated with deep tissue injury.
Case reports
Case 1
A 68‐year‐old female had a d2 pressure ulcer on her greater trochanter region due to immobilisation resulting from rheumatism (Figure 1). The wound temperature was low and the ultrasonographic findings were intact at the primary examination. The lesion healed completely with in 1 week.
Figure 1.
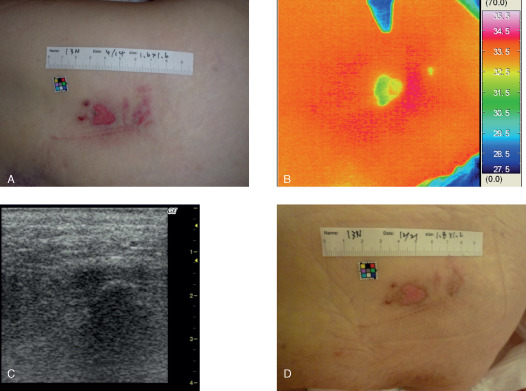
Case 1. (A) A 68‐year‐old female had a d2 pressure ulcer on her greater trochanter region. (B) Thermography showed that the wound temperature was low and (C) the ultrasonographic findings were intact at the primary examination. (D) The lesion healed completely in 1 week.
Case 19
An 85‐year‐old female had a d2 pressure ulcer on her left greater trochanter region due to immobilisation after hypothermia (Figure 2). The wound temperatures were low, and ultrasonography showed an unclear layered structure at the primary examination. The lesions healed completely within a week.
Figure 2.
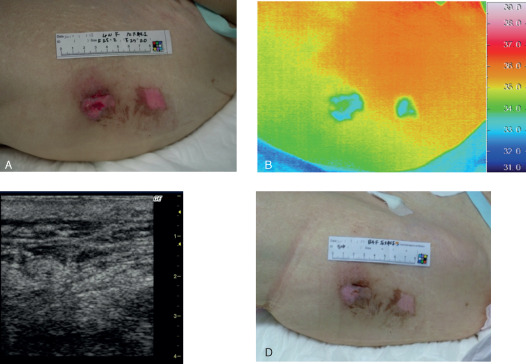
Case 19. (A) An 85‐year‐old female had a d2 pressure ulcer on her left greater trochanter region. (B) Thermographic findings indicated that the wound temperatures were low and (C) the ultrasonographic findings reflected an unclear layered structure at the primary examination. (D) The lesion healed completely in 1 week.
Case 14
A 50‐year‐old male had a d2 pressure ulcer on his sacral region due to immobilisation after sepsis (Figure 3) with a relatively high wound temperature. An unclear layered structure and a heterogeneous hypoechoic area were detected by ultrasonography at the primary examination. The lesion worsened and an ischaemic part of the skin broke out in the centre of the lesion within 1 week, and the lesion was estimated as DESIGN‐R stage DU (stage D3 or worse), indicating the presence of a deep tissue injury.
Figure 3.
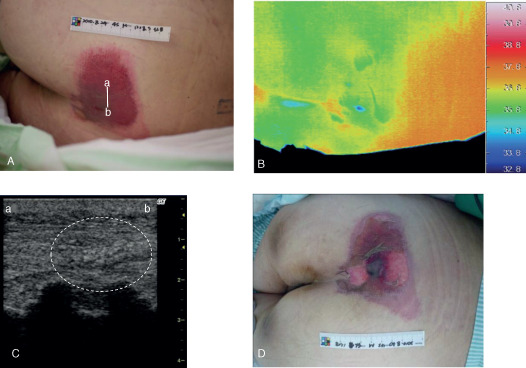
Case 14. (A) A 50‐year‐old male had a d2 pressure ulcer on his sacral region. The bar indicates the location of the ultrasonography probe. (B) The thermographic findings showed that the wound temperature was relatively high and (C) ultrasonography showed an unclear layered structure and a heterogeneous hypoechoic area (white dashed circle) at the primary examination. (D) The lesion worsened and was estimated as stage DU (stage D3 or worse) within 1 week, indicating a deep tissue injury. Line a–b indicates the direction of the applied probe in panels (A) and (C).
Case 16
A 21‐year‐old female had a d2 pressure ulcer on her sacral region due to immobilisation after acute intoxication from taking a large amount of antipsychotic medicines (Figure 4). Thermography indicated that the wound temperature was high in the greater part of the lesion, whereas ultrasonography showed an unclear layered structure, discontinuous fascia and a heterogeneous hypoechoic area at the primary examination. The wound worsened and skin necrosis broke out in the lesion within 1 week, and the lesion was estimated as DESIGN‐R stage DU (stage D3 or worse), reflecting deep tissue injury.
Figure 4.
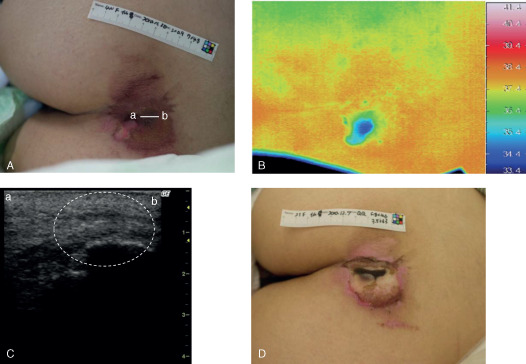
Case 16. (A) A 21‐year‐old female had a d2 pressure ulcer on her sacral region. The bar indicates the location of the ultrasonography probe. (B) Thermography indicated that the wound temperature was high in the greater part of the lesion and (C) ultrasonography showed an unclear layered structure, discontinuous fascia and heterogeneous hypoechoic area (white dashed circle) at the primary examination. (D) The wound worsened and was estimated as stage DU (stage D3 or worse) within 1 week, suggesting a deep tissue injury. Line a–b indicates the direction of the applied probe in panels (A) and (C).
Discussion
The management of pressure ulcers remains a great challenge, with failure resulting in the deterioration in the patient's clinical condition 10, 11. Early detection and intervention of deep tissue injury are therefore an essential step in preventing injury deterioration. Current tools for early detection of deep tissue injury include ultrasonography, computed tomography and magnetic resonance imaging, which are also appropriate for the evaluation of pressure ulcers 6, 12, 13, 14, 15, 16.
Ultrasonography, a familiar tool, is expected to become common in daily practice to detect deep tissue injury because it is minimally invasive and can be used easily and repeatedly at the bedside. Several authors have reported the usefulness of ultrasonography to examine pressure ulcers including deep tissue injury 8, 17, 18, 19. Aoi et al. reported four types of ultrasound features that reflect deep tissue injury 9: unclear layered structure, hypoechoic lesion, discontinuous fascia and heterogeneous hypoechoic area. An ultrasound system with a 10‐MHz probe showed all four of these findings. Ten ulcers showed unclear layered structures and two ulcers had hypoechoic lesions. One ulcer was associated with discontinuous fascia. Two ulcers showed heterogeneous hypoechoic areas, and both of these ulcers had deep tissue injuries. These two deep tissue injuries, the only such injuries in our sample, both showed heterogeneous hypoechoic areas on ultrasonographic assessment, suggesting that the heterogeneous hypoechoic areas are closely related to deep tissue injury. Although ultrasonography may be a useful tool to detect deep tissue injury, a disadvantage of ultrasonographic assessment is the requirement for a certain level of skill for accurate assessment. Some supplementary devices are required to make it easier to use.
Thermography has been used in the examination of pressure ulcers since the early 1970s 20, 21, 22, 23. Using thermography, it is very easy to assess the wound temperature and compare it with the surrounding skin. As it takes only 1–2 minutes to capture images, thermographic assessment can be performed while the patients are receiving their routine care and it would not be a heavy load. We used a portable infrared thermographic system at the bedside, where it was possible to repeatedly use the device. We detected 13 ulcers with low temperature, all of which showed good prognosis and were not associated with deep tissue injuries. Twelve ulcers had high temperatures on thermographic assessment; two of these ulcers were associated with deep tissue injuries, the only such injuries in our sample. If lesions with low temperature consistently show good prognosis and lesions with high temperature consistently include deep tissue injury, thermographic assessment may provide an efficient method for detecting deep tissue injuries.
Ultrasonography and thermography are both minimally invasive procedures that can be performed at the bedside. A disadvantage of ultrasonographic assessment is the requirement for a certain level of skill for accurate assessment, although even beginners can perform thermography and assess its findings accurately after some practice. One of the disadvantages of thermographic assessment is that not all of the high‐temperature ulcers were associated with deep tissue injury (2 of 12 ulcers). However, combination of thermographic and ultrasonographic assessments showed that the two deep tissue injuries simultaneously harboured high temperatures and heterogeneous hypoechoic areas.
In contrast, no non‐deep tissue injury case was simultaneously associated with these two findings. We expect that combining thermographic and ultrasonographic assessments minimises the disadvantages of each procedure, raising the accuracy of early detection of deep tissue injury.
We usually perform thermographic assessment immediately after dressing removal followed by the routine care and ultrasonographic assessment. If thermography indicates that the wound temperature is high, a completely watchful investigation is needed using ultrasonography. If, on the other hand, thermography indicates that the wound temperature is low, the situation may be very promising (Figure 5). Only 2 of the 12 high‐temperature ulcers indicate deep tissue injuries, which is a low percentage. However, using such algorithm, it may be possible to determine uneventful cases in an instant and to focus full attention on proper cases with more potential risk of deeper injury, which is regarded as one of the most important actual clinical benefits of combination of thermographic and ultrasonographic assessments. The determination of ulcer depth based on clinical examination has not been perfect so far. Combining thermographic and ultrasonographic assessments will help to improve accurate determination of ulcer depth and provide patients with timely and apt pressure ulcer care. The ability to quickly and accurately diagnose ulcer depth will likely represent the next paradigm shift in improving pressure ulcer care.
Figure 5.
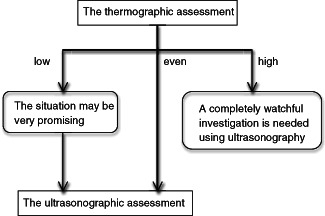
Algorithm for thermographic and ultrasonographic assessments of early‐stage pressure ulcers. If thermography indicates that the wound temperature is high, a completely watchful investigation is needed using ultrasonography. If thermography indicates that the wound temperature is low, the situation may be very promising.
It is interesting that the low‐temperature wounds, which would usually indicate ischaemia, do not progress to further damage, whereas the high temperature might progress to deeper damage. There may be several possible causes of changing of the wound temperature, for example, infection, critical colonisation, hyperaemia, insufficient relief of mechanical loading and ischaemia 24. If the wound has scabbed over, thermography would show low temperature because of ischaemia or insufficient blood flow on the surface of the wound. In this study, however, all ulcers were in early stage at their primary examination and were not covered by necrotic tissues like scabs. As showed in Table 3, all low‐temperature ulcers were at stage d2. Although it is currently difficult to elucidate the actual cause of changing of the wound temperature, it is assumed that most d2 ulcers show low temperature compared with the surrounding skin under normal circumstances because they loss the surface of the skin and hold exudate on it, and the normal wound healing process does not produce excessive inflammation enough to increase the skin surface temperature. When the healing processes are uneventful, the wound may keep low temperature and heal smoothly. In contrast, if deep tissue injury or inflammation occurs, the wound temperature may increase and the wound healing may delay.
In conclusion, this report is the first to note the thermographic findings of deep tissue injury as well as the efficiency of the combination of thermographic and ultrasonographic assessments for early detection of deep tissue injury. The investigation of this study therefore suggests new criteria for early detection of deep tissue injury based on thermographic and ultrasonographic assessments.
References
- 1. Black JM. National Pressure Ulcer Advisory Panel. Moving toward consensus on deep tissue injury and pressure ulcer staging. Adv Skin Wound Care 2005;18:415–6, 418, 420–1. [DOI] [PubMed] [Google Scholar]
- 2. Fleck CA. Suspected deep tissue injury. Adv Skin Wound Care 2007;20:413–5. [DOI] [PubMed] [Google Scholar]
- 3. Agam L, Gefen A. Pressure ulcers and deep tissue injury: a bioengineering perspective. J Wound Care 2007;16:336–42. [DOI] [PubMed] [Google Scholar]
- 4. Stekelenburg A, Gawlitta D, Bader DL, Oomens CW. Deep tissue injury: how deep is our understanding? Arch Phys Med Rehabil 2008;89:1410–3. [DOI] [PubMed] [Google Scholar]
- 5. Ankrom MA, Bennett RG, Sprigle S, Langemo D, Black JM, Berlowitz DR, Lyder CH. National Pressure Ulcer Advisory Panel. Pressure‐related deep tissue injury under intact skin and the current pressure ulcer staging systems. Adv Skin Wound Care 2005;18:35–42. [DOI] [PubMed] [Google Scholar]
- 6. Mao CL, Rivet AJ, Sidora T, Pasko MT. Update on pressure ulcer management and deep tissue injury. Ann Pharmacother 2010;44: 325–32. [DOI] [PubMed] [Google Scholar]
- 7. Matsui Y, Furue M, Sanada H, Tachibana T, Nakayama T, Sugama J, Furuta K, Tachi M, Tokunaga K, Miyachi. Development of the DESIGN‐R with an observational study: an absolute evaluation tool for monitoring pressure ulcer wound healing. Wound Repair Regen 2011;19:309–15. [DOI] [PubMed] [Google Scholar]
- 8. Nagase T, Koshima I, Maekawa T, Kaneko J, Sugawara Y, Makuuchi M, Koyanagi H, Nakagami G, Sanada H. Ultrasonographic evaluation of an unusual peri‐anal induration: a possible case of deep tissue injury. J Wound Care 2007;16:365–7. [DOI] [PubMed] [Google Scholar]
- 9. Aoi N, Yoshimura K, Kadono T, Nakagami G, Iizuka S, Higashino T, Araki J, Koshima I, Sanada H. Ultrasound assessment of deep tissue injury in pressure ulcers: possible prediction of pressure ulcer progression. Plast Reconstr Surg 2009;124:540–50. [DOI] [PubMed] [Google Scholar]
- 10. Redelings MD, Lee NE, Sorvillo F. Pressure ulcers: more lethal than we thought? Adv Skin Wound Care 2005;18:367–72. [DOI] [PubMed] [Google Scholar]
- 11. VanGilder C, MacFarlane GD, Harrison P, Lachenbruch C, Meyer S. The demographics of suspected deep tissue injury in the United States: an analysis of the International Pressure Ulcer Prevalence Survey 2006–2009. Adv Skin Wound Care 2010;23:254–61. [DOI] [PubMed] [Google Scholar]
- 12. Firooznia H, Rafii M, Golimbu C, Sokolow J. Computed tomography of pressure sores. J Comput Tomogr 1983;7:367–73. [DOI] [PubMed] [Google Scholar]
- 13. Esposito G, Ziccardi P, Meoli S, Rengo C, Scioli M, di Caprio G, Fucci G, Scuderi N. Multiple CT imaging in pressure sores. Plast Reconstr Surg 1994;94:333–42. [DOI] [PubMed] [Google Scholar]
- 14. Hencey JY, Vermess M, van Geertruyden HH, Binard JE, Manchepalli S. Magnetic resonance imaging examinations of gluteal decubitus ulcers in spinal cord injury patients. J Spinal Cord Med 1996;19:5–8. [DOI] [PubMed] [Google Scholar]
- 15. Ruan CM, Escobedo E, Harrison S, Goldstein B. Magnetic resonance imaging of nonhealing pressure ulcers and myocutaneous flaps. Arch Phys Med Rehabil 1998;79:1080–8. [DOI] [PubMed] [Google Scholar]
- 16. Larson DL, Gilstrap J, Simonelic K, Carrera GF. Is there a simple, definitive, and cost‐effective way to diagnose osteomyelitis in the pressure ulcer patient? Plast Reconstr Surg 2011;127:670–6. [DOI] [PubMed] [Google Scholar]
- 17. Quintavalle PR, Lyder CH, Mertz PJ, Phillips‐Jones C, Dyson M. Use of high‐resolution, high‐frequency diagnostic ultrasound to investigate the pathogenesis of pressure ulcer development. Adv Skin Wound Care 2006;19:498–505. [DOI] [PubMed] [Google Scholar]
- 18. Lyder C. The use of technology for improved pressure ulcer prevention. Ostomy Wound Manage 2007;53:14–6. [PubMed] [Google Scholar]
- 19. Kanno N, Nakamura T, Yamanaka M, Kouda K, Tajima F. Low‐echoic lesions underneath the skin in subjects with spinal‐cord injury. Spinal Cord 2009;47:225–9. [DOI] [PubMed] [Google Scholar]
- 20. Verhonick PJ, Lewis DW, Goller HO. Thermography in the study of decubitus ulcers: preliminary report. Nurs Res 1972;21:233–7. [DOI] [PubMed] [Google Scholar]
- 21. Barton AA, Barton M. The clinical and thermographical evaluation of pressure sores. Age Ageing 1973;2:55–9. [DOI] [PubMed] [Google Scholar]
- 22. Trandel RS, Lewis DW, Verhonick PJ. Thermographical investigation of decubitus ulcers. Bull Prosthet Res 1975; Fall: 137–55. [PubMed] [Google Scholar]
- 23. Newman P, Davis NH. Thermography as a predictor of sacral pressure sores. Age Ageing 1981;10:14–8. [DOI] [PubMed] [Google Scholar]
- 24. Nakagami G, Sanada H, Iizaka S, Kadono T, Higashino T, Koyanagi H, Haga N. Predicting delayed pressure ulcer healing using thermography: a prospective cohort study. J Wound Care 2010;19:465–6, 468, 470,472. [DOI] [PubMed] [Google Scholar]


