Abstract
Pyoderma gangrenosum (PG) is an uncommon ulcerative, non‐infective chronic inflammatory skin disorder of unknown aetiology. Systemic therapies are necessary to control the associated medical diseases, and, due to the inflammatory nature of PG, topical or systemic immunosuppressant agents are effective, but wound healing is usually slow. Negative wound pressure therapy (NPWT) has become an important tool for the management of complex skin ulcers, and usage in PG has been recently described in the literature: we present four cases of classic PG in which NPWT in association with systemic therapy achieved wound healing and a drastic pain reduction.
Keywords: Inflammatory ulcer; Negative pressure wound therapy; Pain; Pyoderma gangrenosum; Vacuum therapy
Introduction
Pyoderma gangrenosum (PG) is an uncommon ulcerative, non‐infective chronic inflammatory skin disorder of unknown aetiology characterised by a destructive, painful and progressive necrotic process. PG is nowadays classified within the neutrophilic dermatosis. The pathogenesis is unknown; in about 50% of cases, PG is associated with systemic diseases such as inflammatory bowel disease, systemic arthritis, haematological diseases, HIV infection, hereditary hypogammaglobulinemia and malignancies. Pain in these patients is out of proportion to clinical scenario, and the level of pain in patients can be used as a judgement of disease activity. Infection can potentially act as a pathergy‐triggering factor in PG ulcers.
On one hand, systemic therapies are necessary to control the associated medical diseases; on the other hand, due to the inflammatory nature of PG, topical or systemic immunosuppressant agents are effective, but wound healing is usually slow. Because of the pathergic nature of PG, surgical intervention and wound debridements can lead to worsening of lesions. Surgical options include aggressive ulcer excision, recipient site preparation, grafting of autologous cultured keratinocytes with or without topical treatment with hyaluronic acid derivatives and skin graft or muscle flap coverage.
Recently, advanced wound care and devices such as negative wound pressure therapy (NPWT) have become important tools for the management of complex skin ulcers from a wide variety of causes. The usage of NWPT in PG has been recently described in the literature, and we present four cases of classic PG in which NWPT in association with systemic therapy achieved reepithelisation and pain reduction.
Methods
Informed consent was obtained from each patient. The study protocol is conformed to ethical guidelines of the 1975 Declaration of Helsinki and approved by local ethics committee.
Case reports
Case 1
A 53‐year‐old male was referred to our Clinic in December 2010 for the onset of an inflammatory nodular skin lesion on a leg, rapidly evolving in a painful wound of 12 × 13 cm. Margins were well demarcated, with undermining necrotic tissue, subcutaneous fistulae with Achilles tendon exposure. Beyond the violaceous borders, an important inflammatory reaction was present. Screening test results for autoimmune diseases were negative, whereas medical history was positive for inflammatory bowel disease. The clinical diagnosis of PG was supported by the clinical history and by a non‐specific histopathologic pattern at a perilesional skin biopsy (chronic acroangiodematosis). An immunosuppressive treatment with oral corticosteroids was started (1 mg/kg daily); dapsone and cyclosporine were added as steroid‐sparing agents. The severe concomitant pain needed a treatment with transdermic fentanyl. Moreover, a topical treatment with gauze‐based NPWT was started. The synergistic approach of systemic therapy and NPWT led to a fast reduction of the pain, allowing opioid suspension. A pain reduction from 8 (before the application of the gauze‐based negative pressure therapy) to 1 (during all the time of treatment) in the visual analog scale (VAS) was noticed. In January 2012, the patient presented a reduction of the wound dimensions (3 × 2 cm) with complete coverage of the Achilles tendon.
Case 2
A 59‐year‐old female was admitted to our Clinic in November 2010 for a painful large wound (20 × 10 cm) evolved from an inflammatory plaque on the left breast. The patient had a personal history of an infiltrating ductal left breast cancer successfully treated with radiotherapy in early 2003. Restaging radiological examinations failed to show a local cancer relapse or secondary lesions. Skin lesion biopsy showed an unspecific pattern, thus a PG diagnosis was made. A systemic steroid (1 mg/kg daily) treatment was started; because of an unsatisfactory response, after 10 weeks of treatment, high‐dose intravenous immunoglobulin therapy at immunosuppressive dosage (400 mg/kg daily for 5 days monthly) was added, for three cycles totally. At last, cyclosporine (3 mg/kg daily) was started, together with a gauze‐based NPWT. Pain relief rapidly improved, but hardly managed with opioids. Corticosteroid was tapered. Cyclosporine was interrupted after 6 months of treatment. A complete clinic healing of the wound was obtained 12 months after initiation of NPWT. The introduction of the gauze‐based NPWT allowed to reduce pain perception (VAS 10 before treatment with NPWT to 0 during and at the end of the treatment) with complete suspension of opioids (1, 2, 3, 4).
Figure 1.
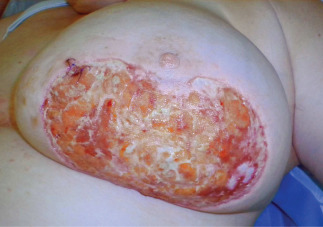
Patient described in Case 2. Picture before the application of gauze‐based NPWT (August 2010).
Figure 2.
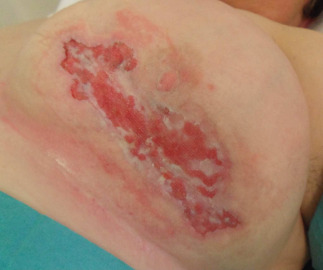
Patient described in Case 2. Picture taken during NPWT (January 2011).
Figure 3.
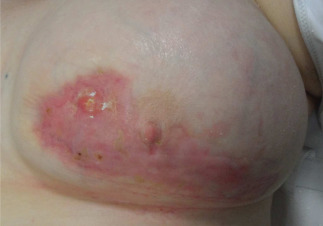
Patient described in Case 2. Picture taken at the end of NPWT, note the complete wound closure for secondary intention (September 2011).
Figure 4.
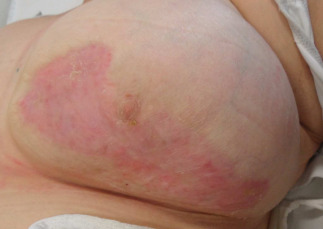
Patient described in Case 2. Result stable after 7 months (April 2012).
Case 3
A 72‐year‐old woman was referred to our Institution in March 2012 for the relapse of a large (8 × 6 cm) ulcerative lesion on a lower limb. The patient had a first diagnosis of classic PG in 1995, no other concomitant diseases – except for a type II diabetes mellitus – were present at clinical history; after a long‐lasting (<10 years) complete response to systemic steroids, PG lesions relapsed as an enlarging slough painful wound on the left leg in late 2011. Cutaneous culture was positive to Pseudomonas aeruginosa and Staph aureus; perilesional skin biopsy showed an unspecific inflammatory pattern. Autoimmune antibody screening test results were still negative.
Cyclosporine (3 mg/kg daily) was started, together with systemic antibiotic and a gauze‐based NPWT. Pain relief rapidly improved. At the time of writing, cyclosporine treatment is ongoing as well as the NPWT, with a clear reduction of wound depth and pain; granulation tissue is present. Bacterial infection is completely resolved. The reduction observed in the VAS score for pain was from 10 (before treatment with NPWT) to 3 during the treatment, and in May 2012, the bed of the wound was debrided with granulation tissue.
Case 4
A 45‐year‐old female had a previous diagnosis in 2007 of ulcerous rectocolitis, policondritis, vasculitis, polinevritis and scleritis; since then, she was treated with immunosuppressants, initially consisting in systemic corticosteroids and low‐dose methotrexate, changed with corticosteroids (1 mg/kg daily) and cyclosporine (3 mg/kg daily) because of hepatic toxicity. In 2008, the diagnosis of PG was carried out for a chronic ulcer at the anterior region of the left leg. A medical treatment with biologic drugs (anti‐tumor necrosis factor (TNF)) was performed to manage the active phase of the disease; wound closure by secondary intention was reached in >1 year. In February 2012, the patient referred to our Institution for the relapse of a large PG ulcer (30 × 18 cm) in the left leg, with tendons exposure (tendons of tibialis anterior, peroneus longus and brevis). An immunosuppression was again managed with systemic corticosteroids, cyclosporine and ev. cyclophosphamide; pain due to polyneuritis was managed with oral opioids until the gauze‐based NPWT was applied. Thereafter, VAS changed from 9 to 3, opioids were interrupted and an optimal pain control was achieved with paracetamol alone. At the time of writing, the tendons are almost covered by granulation tissue, and the dimension of the ulcer is 25 × 15 cm.
Discussion
PG is an uncommon ulcerative cutaneous disease of unknown aetiology characterised by a destructive, painful and progressive necrotic process.
The pathogenesis is unknown (1), but autoimmune mechanisms including immune complex‐mediated neutrophilic vascular reactions have been suggested (2).
Histological examination of the advancing inflamed border shows dense perivascular lymphocytic inflammation associated with vascular destruction. Haemorrhage and necrosis of the epidermis are usually present 3, 4. However, none of the histological features is pathognomonic for PG (5).
Clinically, the ulcers present circumscribed, violaceous, raised edges with a central fibrinopurulent necrotic area (6). Ulcers may occur at any anatomical site, but lower extremities are the most frequently affected (1).
Diagnosis is primarily based on history and clinical presentation. Many patients are first misdiagnosed with soft tissue infections and then subsequently diagnosed PG only after treatment with antibiotics has failed. Its presentation may be isolated or, more frequently, associated with systemic diseases in 50 to 70% of PG (7). The treatment of the associated systemic disease is a key point for the healing of the wound: in Table 1, some of the pharmacological and other adjuvant non‐surgical treatments for both systemic and local conditions are shown. However, the management of PG remains a challenge and randomised, double‐blinded, prospective, multicentre trials are not available in the literature because of the low prevalence and incidence of this disease.
Table 1.
Systemic treatment of disseminated or localised disease and other non‐surgical therapies
| Systemic treatment of disseminated or localised disease | Other |
|---|---|
| Corticosteroids | Hyperbaric oxygen |
| Cyclosporine | Radiotherapy and electron beam irradiation |
| Mycophenolate mofetil | Topical pharmacological treatment of localised disease |
| Azathioprine | NWPT |
| Methotrexate | |
| Tacrolimus | |
| Infliximab | |
| Thalidomide | |
| Alkylating agents | |
| Dapsone | |
| Interferon alfa | |
| Phendimetrazine | |
| Various associations | |
| Intravenous immunoglobulin therapy | |
| Plasmapheresis | |
| Granulocyte and monocyte adsorption apheresis |
Unfortunately, some PG ulcers may take months or years to completely heal with systemic treatment alone, and even in patients with adequate medical therapy, this may not be sufficient. Even if local debridement and compression dressings treatment alone (where systemic therapy was impracticable) have been described in a few cases (8), surgery should be avoided (9), especially during the active phase of PG, because trauma (10) and surgical trauma 11, 12, 13, 14 have been accounted to be a potential trigger of PG [pathergy, a phenomenon by which minor trauma can result in progressive destruction of healthy‐appearing skin (5)]. The rationale for surgical treatment is limited to sepsis prevention in immunosuppressed patients with extensive open surgical wounds. The currently available adjuvant surgery (7) includes aggressive ulcer excision, recipient site preparation, grafting of autologous cultured keratinocytes with or without topical treatment with hyaluronic acid derivatives and skin graft or muscle flap coverage.
Recently, some reports 6, 11, 15, 16, 17 described the usage of the VAC system (KCI International, San Antonio, TX) with polyurethane foam in association with debridements (6), skin grafts or in association with hyperbaric oxygen and skin grafts (17). It was found to be a well‐tolerated treatment that can be used alone until complete wound closure (16). The rationale is the control of the advancing ischaemic process and the rapid production of a healthy granular wound bed, the reduction of localised tissue oedema, along with augmentation of perfusion. The mechanical stress applied on the wound surface enhances cellular proliferation (18).
In this article, we describe the use of gauze‐based RENASYS (V1STA Blue Sky Medical/Smith & Nephew, London, UK) NWPT in PG which, in our opinion, presents several advantages 19, 20.
All patients were hospitalised during the first phase of the disease, in order to perform diagnostic exams, to start a systemic drug therapy and the NPWT. Subsequently, the negative pressure therapy was managed in a home care setting, with weekly ambulatory follow‐up.
Our choice of using gauze NPWT system is based on its main role in pain control 19, 20, 21 probably due to the different type of granulation: (21) with foam‐based systems, granulation tissue grows deeply in foam micropores making the foam removal painful. Moreover, gauze‐based NPWT requires a lower dressing change rate. We noticed a surprising antalgic effect of the gauze‐based NPWT, allowing to reduce pain medication in these patients; as the NPWT was suspended for a short period, a local pain relapse was observed. Moreover, pain in PG is generally considered a judgement of disease activity (22).
Some authors questioned the long‐term usage of NWPT (23): histological examinations show that the best wound bed quality is achieved in 30–45 days, then the onset of a stable tissue limits the effectiveness of negative pressure therapy; therefore, the cost‐effectiveness ratio of this device must be carefully evaluated.
In this study, we prolonged the NPWT for several months; our rationale was not only to stimulate granulation tissue but also to provide a local management of the moisture, exudates and oedema thus achieving an important pain relief.
In conclusion, even if more data are needed, we believe that gauze‐based NPWT is a useful tool added to a good systemic drug therapy, not only to obtain healing of PG lesions but also to reduce and manage pain in these patients.
References
- 1. Callen JP. Pyoderma gangrenosum. Lancet 1998. ; 351 : 581 – 5. [DOI] [PubMed] [Google Scholar]
- 2. Jorizzo JL , Solomon AR , Zanolli MD , Leshin B. Neutrophilic vascular reactions. J Am Acad Dermatol 1988. ; 19 : 983 – 1005. [DOI] [PubMed] [Google Scholar]
- 3. Su WP , Schroeter AL , Pewrry HO , Powell FC. Histopathologic and immunopathologic study of pyoderma gangrenosum. J Cutan Pathol 1986. ; 13 : 323 – 30. [DOI] [PubMed] [Google Scholar]
- 4. Powell FC , Collins S. Pyoderma gangrenosum. Clin Dermatol 2000. ; 18 : 283 – 93. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong PM , Ilyas I , Pandey R , Berendt AR , Conlon CP , Simpson AH. Pyoderma gangrenosum. A diagnosis not to be missed. J Bone Joint Surg 1999. ; 81 : 893e4. [DOI] [PubMed] [Google Scholar]
- 6. Mandal A , Addison P , Stewart K , Neligan P. Vacuum‐assisted closure therapy in pyoderma gangrenosum. Eur J Plast Surg 2006. ; 28 : 529 – 31. DOI: 10.1007/s00238-005-0014-1. . [DOI] [Google Scholar]
- 7. Reichrath J , Bens G , Bonowitz A , Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence‐based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005. ; 53 : 273 – 83. [DOI] [PubMed] [Google Scholar]
- 8. Fioramonti P , Onesti MG , Fino P , Di Ronza S , Sorvillo V , Persichetti P. Feasibility of conservative medical treatment for pyoderma gangrenosum. In Vivo 2012. ; 26 : 157 – 9. [PubMed] [Google Scholar]
- 9. Barańska‐Rybak W , Kakol M , Naesstrom M , Komorowska O , Sokołowska‐Wojdyło M , Roszkiewicz J. A retrospective study of 12 cases of pyoderma gangrenosum: why we should avoid surgical intervention and what therapy to apply. Am Surg 2011. ; 77 : 1644 – 9. [PubMed] [Google Scholar]
- 10. Lindberg‐Larsen R , Fogh K. Traumatic pyoderma gangrenosum of the face: pathergy development after bike accident. Dermatology 2009. ; 218 : 272 – 4. [DOI] [PubMed] [Google Scholar]
- 11. Sebastian VA , Carroll BT , Jessen ME. Pyoderma gangrenosum associated with chronic idiopathic myelofibrosis after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg 2010. ; 10 : 135 – 7. [DOI] [PubMed] [Google Scholar]
- 12. Wadia F , Malik M , Porter M. Post operative wound breakdown cause by pyoderma gangrenosum after bilateral simultaneous total knee arthroplasty. J Arthroplasty 2007. ; 22 : 1232e5. [DOI] [PubMed] [Google Scholar]
- 13. Nakajima N , Ikeuchi M , Izumi M , Kuriyama M , Nakajima H , Tani T. Successful treatment of a wound breakdown caused by pyoderma gangrenosum after total knee arthroplasty. Knee 2010. ; 18 : 453 – 5 . [DOI] [PubMed] [Google Scholar]
- 14. Verma SB. Atypical pyoderma following total knee replacement surgery e first report in dermatologic literature. Ann Brazil Dermatol 2009. ; 84 : 689e91. [DOI] [PubMed] [Google Scholar]
- 15. Hill DS , O'Neill JK , Toms A , Watts AM. Pyoderma gangrenosum: a report of a rare complication after knee arthroplasty requiring muscle flap cover supplemented by negative pressure therapy and hyperbaric oxygen. J Plast Reconstr Aesthet Surg 2011. ; 64 : 1528 – 32. [DOI] [PubMed] [Google Scholar]
- 16. Ghersi MM , Ricotti C , Nousari CH , Newman MI. Negative pressure dressing in the management of pyoderma gangrenosum ulcer. Arch Dermatol 2007. ; 143 : 1249 – 51. [DOI] [PubMed] [Google Scholar]
- 17. Niezgoda JA , Cabigas EB , Allen HK , Simanonok JP , Kindwall EP , Krumenauer J. Managing pyoderma gangrenosum: a synergistic approach combining surgical débridement, vacuum‐assisted closure, and hyperbaric oxygen therapy. Plast Reconstr Surg 2006. ; 117 : 24e – 28e. [DOI] [PubMed] [Google Scholar]
- 18. Argenta L , Morykwas M. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997. ; 38 : 563. [PubMed] [Google Scholar]
- 19. Fraccalvieri M , Zingarelli E , Ruka E , Antoniotti U , Coda R , Sarno A , Bocchiotti MA , Bruschi S. Negative pressure wound therapy using gauze and foam: histological, immunohistochemical and ultrasonography morphological analysis of the granulation tissue and scar tissue. Preliminary report of a clinical study. Int Wound J 2011. ; 8 : 355 – 64. DOI: 10.1111/j.1742-481X.2011.00798.x. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fraccalvieri M , Ruka E , Bocchiotti MA , Zingarelli E , Bruschi S. Patient's pain feedback using negative pressure wound therapy with foam and gauze. Int Wound J 2011. ; 8 : 492 – 9. DOI: 10.1111/j.1742-481X.2011.00821.x. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borgquist O , Gustafsson L , Ingemansson R , Malmsj ö M. Tissue in growth into foam but not into gauze during negative pressure wound therapy. Wounds 2009. ; 21 : 302 – 9. [PubMed] [Google Scholar]
- 22. Dabade TS , Davis MDP. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet's syndrome). Dermatol Ther 2011. ; 24 : 273 – 84. [DOI] [PubMed] [Google Scholar]
- 23. Bassetto F , Lancerotto L , Salmaso R , Pandis L , Pajardi G , Schiavon M , Tiengo C , Vindigni V. Histological evolution of chronic wounds under negative pressure therapy. J Plast Reconstr Aesthet Surg 2012. ; 65 : 91 – 9. [DOI] [PubMed] [Google Scholar]


