Abstract
This study examined the effect of monochromatic infrared energy (MIRE) on diabetic wound healing. Fifteen diabetic rats were given MIRE intervention on their skin wounds located on the dorsum and compared with 15 control diabetic rats. Assessments were conducted for each group at weeks 1, 2 and 4 post wounding (five rats at each time point) by calculating the percentage of wound closures (WCs) and performing histological and immunohistochemical staining on sections of wound tissue. Evaluations of WCs and histological examinations of reepithelialisation, cellular content and granulation tissue formation showed no significant difference between the MIRE and the control group at each time point. Through semi‐quantitative immunohistochemical staining, the deposition of type I collagen in the MIRE group was found to have improved when compared with the control group at the end of week 2 (P = 0.05). No significant differences in the myofibroblast population were detected between the two groups. In conclusion, MIRE appeared to promote collagen deposition in the early stage of wound healing in diabetic rats, but the overall wound healing in the MIRE group was not significantly different from that of the control group.
Keywords: Collagen, Diabetic rat, Monochromatic infrared energy, Myofibroblast, Wound healing
Introduction
Diabetes mellitus is a chronic metabolic disease that causes various complications. Foot ulcer is one of the most serious complications of diabetes, and it is estimated that around 10–25% of patients with diabetes would develop foot ulcers. Among patients with diabetic foot ulcers, over half would eventually receive a lower limb amputation 1, 2, 3. Therefore, it is crucial to develop a better approach to managing diabetic ulcers to prevent skin complications and the amputation of limbs.
Normal wound healing involves a series of sequential stages, including haemostasis, inflammation, remodelling of granulation tissue and reepithelialisation (4). Immune cells such as neutrophils and macrophages arrive at the wound site, and phagocytose bacteria at the damaged tissues at the site. The damaged epithelial layers then reepithelialise, and the granulation tissue composed of inflammatory cells, fibroblasts and myofibroblasts, as well as newly developed blood vessels, forms at the wound site. Collagen deposition takes place at a later stage, which allows the wound to be remodelled by replacing the newly synthesised type III collagen with type I collagen. The differentiation of fibroblasts into myofibroblasts is a vital cellular activity that influences wound healing. With its contractile ability to pull the edges of a wound together, myofibroblast plays an important role in enhancing the closure of wounds. Larger myofibroblast populations are found in highly vascularised wound tissues and smaller ones in wounds with poor vascularity 5, 6.
The mechanism of healing in chronic wounds such as diabetic ulcers is distorted, resulting in delays in the wound‐healing process (7). A recent review paper stated that delays in the healing of diabetic foot ulcers could be due to microvascular dysfunction (8), which was supported by previous research findings 9, 10, 11. It is believed that neuropathy contributes to the risk of diabetic foot ulcers, while impairment in local angiogenesis may lead to diminished peripheral blood flow, which is one of the major causes of delay in the healing of wounds for people with diabetes (1). Experiments using diabetic animal models have also shown that abnormal granulation remodelling and a reduction in collagen synthesis and deposition might also contribute to the delays in diabetic wound healing 12, 13.
Monochromatic infrared energy (MIRE) is a relatively new electrophysical therapy device that has been shown to improve sensation and reduce pain in patients with diabetic peripheral neuropathy. Each treatment pad was embedded with gallium aluminum arsenide diodes that emit near infrared with a uniform wavelength of 890 nm (14). It was postulated that the delivery of a near‐infrared wavelength of 890 nm increases microcirculation and enhances the release of nitric oxide, thus promoting tissue healing 15, 16. In two clinical studies, it was reported that MIRE resulted in significant restoration of peripheral sensation in diabetic patients 17, 18. However, contradictory findings have also been reported, in which MIRE did not produce significant improvements in skin sensation, pain intensity or quality of life 14, 19.
There are several case reports indicating that MIRE appears to promote the healing of diabetic ulcers 15, 20. Horwitz et al. (15) showed that MIRE promoted the healing of ulcers in two patients with type 1 diabetes. They delivered MIRE for 30 minutes on two patients using different protocols (one patient received treatment on alternate day for 5 months; but the other patient received treatment once a week for 3 months). They showed complete wound closure (WC) in both patients after the treatment period. In another case report, four patients with diabetic foot ulcers that had failed to heal for at least 1 month showed complete healing after receiving MIRE treatment for a period ranging from 21 to 52 days, with the frequency of treatment being three times per week (20). However, there have been very few previous studies examining the mechanisms of MIRE on promoting diabetic wound healing. Recently, our group showed that MIRE increased microcirculation by enhancing capillary blood cell velocity and superficial skin blood flow in healthy subjects (21). Thus, we hypothesised that MIRE may help to promote diabetic wound healing. In this study, we examined whether MIRE would promote the cutaneous wound healing in streptozotoxin (STZ)‐induced diabetic rat model.
Materials and methods
Diabetic rat model
The study was conducted by Department of Rehabilitation Sciences, The Hong Kong Polytechnic University. All animal experiments were conducted with the approval of the Animal Subjects Ethics Sub‐Committee of the Hong Kong Polytechnic University. The study protocol complied with the guideline of The Hong Kong Polytechnic University and all animals received humane care. Thirty male Sprague–Dawley rats (10 weeks, 280–320 g) supplied by the Centralised Animal Facilities of the same university were housed in a temperature‐controlled animal holding room with a 12‐hour light/dark cycle. The rats were allowed to acclimate to their environment for 1 week before the experiment. Each rat was then given a single intraperitoneal injection of STZ (Sigma‐Aldrich, St Louis, MO; 55 mg/kg in citrate buffer) and its blood glucose was measured 1 week after the injection. Rats with a blood glucose level of >300 mg/dl (16.7 mmol/l) were defined as having been successfully induced with diabetes 22, 23.
Excisional skin wounds
After anaesthesia administered via an intraperitoneal injection of a mixture of ketamine and xylazine (100 mg and 10 mg/kg body weight, respectively; Alfasan International, Woerden, Holland), the rats were shaved on the dorsum and cleansed with a betadine solution. A 2 × 2 cm full‐thickness square‐shaped skin wound was then created on each rat (24). The wound was covered with sterile gauze to avoid exposure and prevent infection.
MIRE intervention
The MIRE intervention was given on postoperative day 2. Thirty diabetic rats were randomly allocated into either the MIRE group (n = 15) or the control group (n = 15) without treatment. MIRE with a wavelength of 890 nm (Anodyne Therapy Professional System 480; Anodyne Therapy, Tampa, FL) was used and the intensity was set at level 6, that is 85% of full power, so that the temperature of the wound bed would not exceed 37°C after 30 minutes of treatment (14). The animals received 30 minutes of MIRE three times a week before they were euthanised. The animals in the control group were handled according to the same procedure as that used on the MIRE group, except that the MIRE machine was not switched on in their case.
Assessment of wound healing
The wound area (WA) was assessed immediately after the creation of the wound (day 0) and also on postwounding days 7, 14, 21 and 28. Photographs of the wounds were taken using a digital camera (Nikon coolpix P5100; Nikon Corp, Tokyo, Japan). The WA on day 0 (WA0) and the WA on the date of assessment (WAx) were calculated using computer software called the Verge Videometer Measurement Documentation (VeV MD) system (VERG Inc., Winnipeg, MB). The percentage of WC (%) was then calculated using the following formula: WC % = (WA0−WAx)× 100%/WA0.
Histological staining
At postwounding days 7, 14 and 28, five rats were randomly selected from each of the control and the MIRE groups and were euthanised by giving them an overdose of ketamine and xylazine. These time points were chosen according to the reported timeline of the wound‐healing process in animals and humans 15, 20, 25, 26. For histological assessment, the wound tissue was excised in full‐thickness with subcutaneous fat and including a margin of at least 5 mm of healed skin. The specimens were fixed in 4% paraformaldehyde in phosphate‐buffered saline (PBS, pH 7.4) and were processed for embedding in paraffin. Sections with a thickness of 5 µm were cut for routine haematoxylin and eosin (H & E) staining and immunohistochemistry.
In the histological examination, the levels of reepithelialisation, cellular content and granulation tissue in each wound were rated using the previously reported scoring scales 26, 27 (Table 1). The scoring was completed by two blinded evaluators who were specialised in the pathology of laboratory animals. The deposition and distribution of type I and type III collagen were examined by immunofluorescence staining. The presence of myofibroblast was examined using immunofluorescence staining for α‐smooth muscle actin (α‐SMA), which represents the actin isoform typical of vascular smooth muscle cells as well as of the fibroblast/myofibroblast phenotypic transition 28, 29.
Table 1.
Scoring system for histological and immunohistochemical examinations *
| Items | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Reepithelialisation (by H & E staining) | None to minimal | Minimal to moderate | Almost completely reepithelialised | Completely reepithelialised, thin layer | Thicker epithelial layer |
| Cellular content (by H & E staining) | None to minimal | Few fibroblasts | More fibroblasts | Predominately fibroblast | Mature fibroblast in dermis |
| Granulation tissue (by H & E staining) | None to spare | Thin layer at edges | Thin layer across wound | Uniformly thick | Clearing |
| Collagen deposition (by immunofluorescence staining) | None | Few collagen fibres | Moderate amount of collagen fibre | Extensive collagen fibre | Dense and organised |
In the process of immunofluorescence staining, tissue sections were deparaffinised and rehydrated through graded alcohol into water and finally washed with PBS. After incubating with 5% normal horse serum in PBS for 1 hour to prevent non specific binding, the tissue sections were incubated with individual primary antibodies (α‐SMA, 1:50000; Sigma‐Aldrich; type I collagen, 1:250 and type III collagen, 1:100, both from Novus Biologicals, LLC, Littleton, CO) at 4°C overnight. The sections were subsequently incubated with appropriate Alexa Fluor dye‐conjugated secondary antibodies (1:300; Molecular Probes Inc., Eugene, OR). Finally, the sections were mounted with Vectashield containing DAPI (Vector Lab, Burlingame, CA) for nuclei visualisation. Images of six randomised views of each slide under magnifications of 200× or 400× were taken using a Nikon 80i Eclipse epifluorescent microscope (Nikon Instruments Inc, Melville, NY) and a SPOT RT‐SE™ digital camera (Diagnostic Instruments Inc., Sterling Heights, MI). An antigen‐retrieval approach for collagen III was performed by autoclaving the slides with tissue sections in 0.01 M sodium citric solution (pH 6) at 120°C for 20 minutes before the step of blocking.
The histological and immunohistochemical staining processes were performed at least three times for each section of tissue.
Statistical analysis
Descriptive data were presented as the mean ± SEM. An independent t‐test was conducted to compare the difference between the MIRE and the control groups. Average score of the staining calculated for each group at each time point was tested using the Mann–Whitney U‐test to determine whether there were differences between the groups. The level of significance was set at P≤ 0.05 for all outcome measures.
Results
Wound closure
The photographs of representative wounds taken at different time points from each experimental group are shown in Figure 1A. Overall, no significant differences were found between the MIRE and the control groups in terms of the percentage of WC throughout the 4‐week experimental period (Figure 1B).
Figure 1.
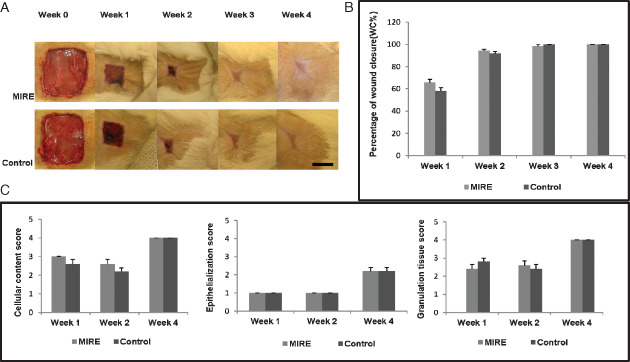
Comparisons of the wounds of the monochromatic infrared energy rats and control rats at various time points in terms of (A) healing status, (B) percentage of wound closure (WC), (C) average score of cellular content, epithelialisation and granulation tissue. Scale bar = 1 cm.
Wound reepithelialisation, cellular content and granulation tissue formation
In H & E staining, no significant differences were found between the MIRE and the control groups in the mean score for wound reepithelialisation, granulation tissue formation and cellular contents throughout the 4‐week experimental period (Figure 1C).
Collagen deposition
With immunofluorescence staining, positively stained type I and type III collagen fibres were rarely detected in either the MIRE group or the control group in week 1 post wounding. In week 2, collagen I fibres were observed across the entire area of the wound in the MIRE group (Figure 2A) in contrast to sparse collagen I fibres distributed in a dispersed manner in a thin layer under the wound in the control group (Figure 2B). The score for collagen I fibre in the MIRE group was significantly greater than that in the control group (collagen I: 3 ± 0 versus2.4 ± 0.245, P = 0.05) in week 2 (Figure 2C). However, by week 4, dense and well‐aligned type I collagen fibres were seen in the wounds of both the MIRE and control groups, with no significant difference between the groups (Figure 2C). No significant difference in the immunoreactivity of type III collagen fibres was found between the MIRE and the control group throughout the experimental period (Figure 2C).
Figure 2.
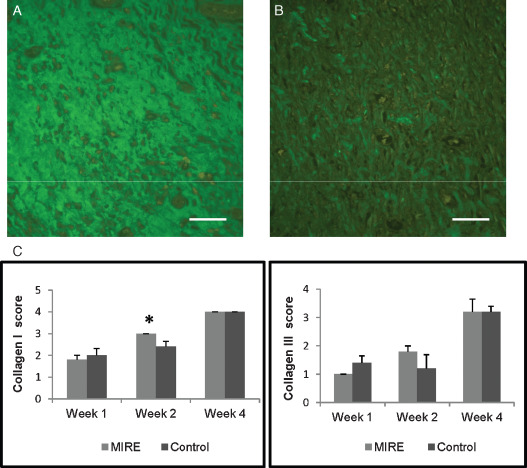
Comparisons of collagen deposition between the monochromatic infrared energy (MIRE) rats and control rats. (A) Collagen I deposition of wounds in the MIRE rats at week 2; (B) Collagen I deposition of wounds in the control rats at week 2. (C) The average scores for collagen I and collagen III deposition labelled by immunofluorescence staining. * represents significant group difference (P≤ 0.05) in collagen I found between the MIRE and control rats at week 2. Scale bar = 50 µm.
Myofibroblast formation
With immunofluorescence staining, α‐SMA‐positive myofibroblasts were detected in the granulation tissue of the dermal layer in both the MIRE (Figure 3A) and the control groups (Figure 3B) by week 1 post wounding, indicating that myofibroblasts were present at the early healing stage of the diabetic wound in a rat model. Although only a marginally significant difference in the myofibroblast population score was found between the control and the MIRE groups (P = 0.058), myofibroblasts appeared to be less populous in the former than in the latter (Table 2).
Figure 3.
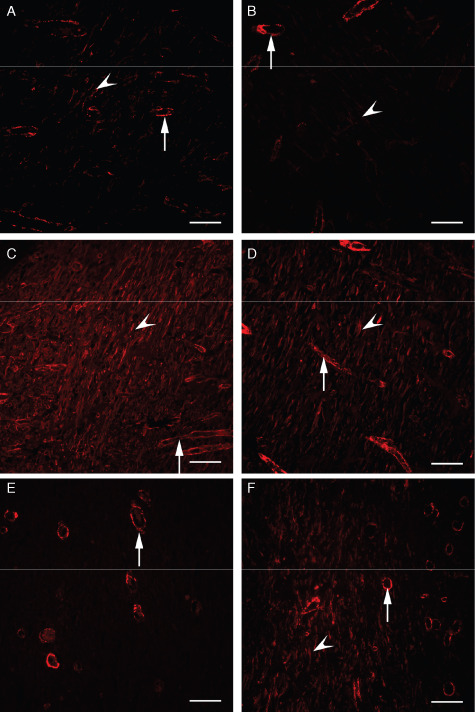
Comparisons of myofibroblast distribution by immunofluorescence staining against α‐smooth muscle actin (SMA; arrows: blood vessels; arrowheads: α‐SMA labelled myofibroblasts). (A) Monochromatic infrared energy (MIRE) group at week 1; (B) Control group at week 1; (C) MIRE group at week 2; (D) Control group at week 2; (E) Disappearance of myofibroblasts in four out of five wounds in the MIRE group and all wounds in the control group at week 4; (F) Existence of myofibroblasts in one of the five rats in the MIRE group at week 4. Scale bar = 50 µm.
Table 2.
Comparing the population and intensity of the myofibroblasts in the MIRE group and control group
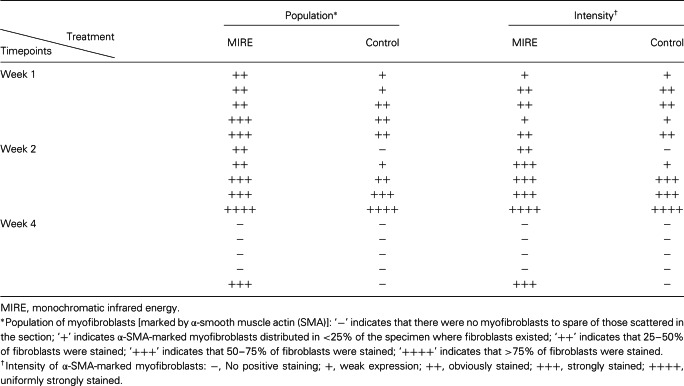
In week 2, the population of myofibroblasts and the intensity of their α‐SMA staining had increased markedly in both the MIRE and the control groups (Table 2 and Figure 3C and D) as compared with week 1. The myofibroblast population and intensity score in the control group were somewhat lower than that in the MIRE group but the differences were not statistically significant. In week 4, when the wound‐healing process is supposed to be approaching the final stage, myofibroblasts were no longer seen in any of the wound tissue sections in the control group (Figure 3E) or the MIRE group, with the exception of those for one animal (Figure 3F and Table 2), which we regard as an individual variation.
Discussion
Delayed wound healing is a common complication of diabetes. Diabetic wounds commonly involve impaired cellular infiltration, inadequate formation of granulation tissue, reduced angiogenesis and inadequate deposition of collagen (30). Previous studies have shown that MIRE produces sensory restoration in people with diabetic peripheral neuropathy 31, 32, 33. In addition, we have recently showed that MIRE increases microcirculation in healthy subjects (21). Therefore, we explored the possibility that MIRE might also help to promote wound healing by using the diabetic rat model.
Using H & E staining, we have examined the extent of reepithelialisation, cellular content and granulation tissue formation, all of which are well‐accepted outcome measures for monitoring the progress of wound healing. On the basis of the treatment protocols adopted in this study, results from our histological examinations did not support that MIRE was effective in promoting wound healing in our diabetic rat model. However, it was noted that the deposition of type I collagen fibre was significantly more at week 2 post wounding upon MIRE intervention as compared with the control. In addition, myofibroblast formation was also found to have been promoted in the wounds treated with MIRE, although the effect did not reach statistical significance. Unlike fibroblasts, myofibroblasts possess the ability to contract, through the presence of α‐SMA in their cytoskeleton, allowing the cells to contract and pull the wound tissues together to close the wound. It is also thought that myofibroblasts promote wound healing by secreting more collagen fibre and accelerating the process of tissue repair 28, 34. We speculated that an increase in myofibroblast formation in the MIRE groups resulted in an increase in collagen content observed in week 2. A previous study using non diabetic Wistar rats showed that myofibroblasts were absent in the dermal layer until the sixth day post wounding, peaked at the 15th, but decreased thereafter and disappeared 30 days after the wounding (12). Darby et al. (35) reported a delay in the appearance of myofibroblasts in diabetic wounds as compared with non diabetic wounds in mice. In our study, myofibroblasts showed up in the experimental rats as early as 1 week post wounding. Although there was no statistical significance, more myofibroblasts were present in the MIRE‐treated wounds than in those of the control group at week 1 postwounding, and the myofibroblast population and intensity score for the former group were apparently higher by week 2 than for the latter group, indicating that MIRE may account for the earlier formation of myofibroblasts.
This study is the first to explore the effect of MIRE on diabetic wound healing in an animal model. To examine the optimal intensity of the anodyne, we conducted a pilot study (unpublished data) to measure the temperature at the centre of each wound using a sensitive electronic thermometer. When the intensity of MIRE was set at level 10 (maximum), the temperature of the wound bed rose to above 38°C by the end of 30 minutes of treatment. This may have an adverse effect on the fresh diabetic wound. On the basis of our pilot study, we set the intensity at level 6 to control the maximum temperature to below 37°C so as to avoid detrimental effects on the fresh wound due to overheating. Therefore, we believe that the unsatisfactory effect of MIRE reported in this study was not because of suboptimal intensity. However, further studies are needed to verify whether other treatment protocols of MIRE, for example, using different levels of intensity, longer treatment durations or different pad placements, would be effective in promoting diabetic wound healing.
Although several clinical studies have focussed on the effects of MIRE in reversing diabetic peripheral neuropathy 14, 18, 19, the findings are still controversial. Some recent findings have suggested that MIRE is not effective in improving the condition of diabetic peripheral neuropathy 14, 19, 25, 36.
Likewise, this study showed that with our present protocols, a MIRE treatment did little to enhance the wound healing process for diabetic ulcers in the SD rat model.
Conclusion
This study showed that MIRE promotes the deposition of type I collagen and likely enhances the differentiation of fibroblasts into myofibroblasts during the early stage of cutaneous wound healing in diabetic rats. However, no significant effect was observed in the overall wound healing process in terms of WC, reepithelialisation, cellular content and granulation tissue formation.
Acknowledgements
This work was supported by the General Research Fund provided by Research Grants Council of the Hong Kong SAR Government (PolyU5600/11M).
References
- 1. Brem H, Tomic‐Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007;117:1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harlin SL, Willard LA, Rush KJ, Ghisletta LC, Meyers WC. Chronic wounds of the lower extremity: a preliminary performance measurement set. Plast Reconstr Surg 2008;121:142–74. [DOI] [PubMed] [Google Scholar]
- 3. Jones RN, Marshall WP. Does the proximity of an amputation, length of time between foot ulcer development and amputation, or glycemic control at the time of amputation affect the mortality rate of people with diabetes who undergo an amputation? Adv Skin Wound Care 2008;21:118–23. [DOI] [PubMed] [Google Scholar]
- 4. Bitto A, Minutoli L, Altavilla D, Polito F, Fiumara T, Marini H, Galeano M, Calo M, Lo Cascio P, Bonaiuto M, Migliorato A, Caputi AP, Squadrito F. Simvastatin enhances VEGF production and ameliorates impaired wound healing in experimental diabetes. Pharmacol Res 2008;57:159–69. [DOI] [PubMed] [Google Scholar]
- 5. Alizadeh N, Pepper MS, Modarressi A, Alfo K, Schlaudraff K, Montandon D, Gabbiani G, Bochaton‐Piallat ML, Pittet B. Persistent ischemia impairs myofibroblast development in wound granulation tissue: a new model of delayed wound healing. Wound Repair Regen 2007;15:809–16. [DOI] [PubMed] [Google Scholar]
- 6. Thorey IS, Hinz B, Hoeflich A, Kaesler S, Bugnon P, Elmlinger M, Wanke R, Wolf E, Werner S. Transgenic mice reveal novel activities of growth hormone in wound repair, angiogenesis, and myofibroblast differentiation. J Biol Chem 2004;279:26674–84. [DOI] [PubMed] [Google Scholar]
- 7. King L. Impaired wound healing in patients with diabetes. Nurs Stand 2001;15:39–45. [DOI] [PubMed] [Google Scholar]
- 8. Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev 2009;25:604–14. [DOI] [PubMed] [Google Scholar]
- 9. Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des 2005;11:2301–9. [DOI] [PubMed] [Google Scholar]
- 10. Tooke JE. Possible pathophysiological mechanisms for diabetic angiopathy in type 2 diabetes. J Diabetes Complicat 2000;14: 197–200. [DOI] [PubMed] [Google Scholar]
- 11. Wiernsperger N, Nivoit P, De Aguiar LG, Bouskela E. Microcirculation and the metabolic syndrome. Microcirculation 2007;14:403–38. [DOI] [PubMed] [Google Scholar]
- 12. Darby I, Skalli O, Gabbiani G. Alpha‐smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990;63:21–9. [PubMed] [Google Scholar]
- 13. Verhofstad MH, Bisseling TM, Haans EM, Hendriks T. Collagen synthesis in rat skin and ileum fibroblasts is affected differently by diabetes‐related factors. Int J Exp Pathol 1998;79:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lavery LA, Murdoch DP, Williams J, Lavery DC. Does anodyne light therapy improve peripheral neuropathy in diabetes? Diabetes Care 2008;31:316–21. [DOI] [PubMed] [Google Scholar]
- 15. Horwitz LR, Burke TJ, Carnegie D. Augmentation of wound healing using monochromatic infrared energy. Exploration of a new technology for wound management. Adv Wound Care 1999;12:35–40. [PubMed] [Google Scholar]
- 16. Hunter S, Langemo D, Hanson D, Anderson J, Thompson P. The use of monochromatic infrared energy in wound management. Adv Skin Wound Care 2007;20:265–6. [DOI] [PubMed] [Google Scholar]
- 17. Arnall DA, Nelson AG, Lopez L, Sanz N, Iversen L, Sanz I, Stambaugh L, Arnall SB. The restorative effects of pulsed infrared light therapy on significant loss of peripheral protective sensation in patients with long‐term type 1 and type 2 diabetes mellitus. Acta Diabetol 2006;43:26–33. [DOI] [PubMed] [Google Scholar]
- 18. Leonard DR, Farooqi MH, Myers S. Restoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy. Diabetes Care 2004;27:168–72. [DOI] [PubMed] [Google Scholar]
- 19. Clifft JK, Kasser RJ, Newton TS, Bush AJ. The effect of monochromatic infrared energy on sensation in patients with diabetic peripheral neuropathy: a double‐blind, placebo‐controlled study. Diabetes Care 2005;28:2896–900. [DOI] [PubMed] [Google Scholar]
- 20. Nather A, Sim YE, Chew L, Neo SH. Anodyne therapy for recalcitrant diabetic foot ulcers: a report of four cases. J Orthop Surg (Hong Kong) 2007;15:361–4. [DOI] [PubMed] [Google Scholar]
- 21. Mak MC, Cheing GL. Immediate effects of monochromatic infrared energy on microcirculation in healthy subjects. Photomed Laser Surg 2012;30:193–9. [DOI] [PubMed] [Google Scholar]
- 22. Kuo YR, Wang CT, Wang FS, Chiang YC, Wang CJ. Extracorporeal shock‐wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ‐induced diabetes. Wound Repair Regen 2009;17:522–30. [DOI] [PubMed] [Google Scholar]
- 23. Witte MB, Kiyama T, Barbul A. Nitric oxide enhances experimental wound healing in diabetes. Br J Surg 2002;89:1594–601. [DOI] [PubMed] [Google Scholar]
- 24. Athanasiou A, Karkambounas S, Batistatou A, Lykoudis E, Katsaraki A, Kartsiouni T, Papalois A, Evangelou A. The effect of pulsed electromagnetic fields on secondary skin wound healing: an experimental study. Bioelectromagnetics 2007;28:362–8. [DOI] [PubMed] [Google Scholar]
- 25. Franzen‐Korzendorfer H, Blackinton M, Rone‐Adams S, McCulloch J. The effect of monochromatic infrared energy on transcutaneous oxygen measurements and protective sensation: results of a controlled, double‐blind, randomized clinical study. Ostomy Wound Manage 2008;54:16–31. [PubMed] [Google Scholar]
- 26. Kawalec JS, Hetherington VJ, Pfennigwerth TC, Dockery DS, Dolce M. Effect of a diode laser on wound healing by using diabetic and nondiabetic mice. J Foot Ankle Surg 2004;43:214–20. [DOI] [PubMed] [Google Scholar]
- 27. Yu W, Naim JO, Lanzafame RJ. Effects of photostimulation on wound healing in diabetic mice. Lasers Surg Med 1997;20:56–63. [DOI] [PubMed] [Google Scholar]
- 28. Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 1999;250:273–83. [DOI] [PubMed] [Google Scholar]
- 29. Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha‐smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 1986;103: 2787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown RL, Breeden MP, Greenhalgh DG. PDGF and TGF‐alpha act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res 1994;56:562–70. [DOI] [PubMed] [Google Scholar]
- 31. DeLellis SL, Carnegie DH, Burke TJ. Improved sensitivity in patients with peripheral neuropathy: effects of monochromatic infrared photo energy. J Am Podiatr Med Assoc 2005;95:143–7. [DOI] [PubMed] [Google Scholar]
- 32. Harkless LB, DeLellis S, Carnegie DH, Burke TJ. Improved foot sensitivity and pain reduction in patients with peripheral neuropathy after treatment with monochromatic infrared photo energy – MIRE. J Diabetes Complicat 2006;20: 81–7. [DOI] [PubMed] [Google Scholar]
- 33. Powell MW, Carnegie DE, Burke TJ. Reversal of diabetic peripheral neuropathy and new wound incidence: the role of MIRE. Adv Skin Wound Care 2004;17:295–300. [DOI] [PubMed] [Google Scholar]
- 34. Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 2003; 200:500–3. [DOI] [PubMed] [Google Scholar]
- 35. Darby IA, Bisucci T, Hewitson TD, MacLellan DG. Apoptosis is increased in a model of diabetes‐impaired wound healing in genetically diabetic mice. Int J Biochem Cell Biol 1997;29:191–200. [DOI] [PubMed] [Google Scholar]
- 36. Nawfar SA, Yacob NB. Effects of monochromatic infrared energy therapy on diabetic feet with peripheral sensory neuropathy: a randomised controlled trial. Singapore Med J 2011;52:669–72. [PubMed] [Google Scholar]


