Abstract
Several different advanced treatments have been used to improve healing in chronic wounds, but none have shown sustained success. The application of topical growth factors (GFs) has displayed some potential, but the varying results, high doses and high costs have limited their widespread adoption. Many treatments have ignored the evidence that wound healing is driven by interactions between extracellular matrix proteins and GFs, not just GFs alone. We report herein that a clinical Good Manufacturing Practice‐grade vitronectin:growth factor (cVN:GF) complex is able to stimulate functions relevant to wound repair in vitro, such as enhanced cellular proliferation and migration. Furthermore, we assessed this complex as a topical wound healing agent in a single‐arm pilot study using venous leg ulcers, as well as several ‘difficult to heal’ case studies. The cVN:GF complex was safe and re‐epithelialisation was observed in all but 1 of the 30 patients in the pilot study. In addition, the case studies show that this complex may be applied to several ulcer aetiologies, such as venous leg ulcers, diabetic foot ulcers and pressure ulcers. These findings suggest that further evaluation is warranted to determine whether the cVN:GF complex may be an effective topical treatment for chronic wounds.
Keywords: Chronic wound, Growth factors, Topical treatment, Vitronectin, Wound healing
INTRODUCTION
Delays to wound healing are common in many major diseases, including cancer (1), cardiovascular disease (2) and diabetes (3), and also in patients with severe burn injury (4). Venous leg ulcers, diabetic foot ulcers, arterial leg ulcers and pressure ulcers are important chronic wounds with specific underlying aetiologies. Although different therapeutic strategies have been used to improve impaired wound healing, there are still very few effective adjuncts to standard treatments.
Our research has focussed on the development of multimeric complexes containing growth factors (GFs) and extracellular matrix (ECM) proteins, namely insulin‐like growth factors (IGFs), IGF‐binding proteins (IGFBPs), epidermal growth factor (EGF) and the ECM protein, vitronectin (VN), which we term vitronectin:growth factor (VN:GF) complexes. We have previously shown that VN:GF complexes significantly enhance proliferation and migration of human keratinocyte cell lines 5, 6 and primary human keratinocytes 5, 7, 8, and reduce the requirement for serum and high concentrations of insulin in the ex vivo expansion and propagation of cells for human skin (7) and corneal grafts (9). These functions have been shown to be dependent on the coactivation of VN‐binding integrins and the IGF‐1 receptor (IGF‐1R) 10, 11, which lead to activation of the ERK 1/2/MAPK and AKT/PI3K pathways 11, 12, 13. We have showed that the VN:GF complex is able to enhance the re‐epithelialisation of wounds created in a three‐dimensional in vitro human skin equivalent model 8, 14, 15 and in an in vivo porcine burns model (8). Moreover, the VN:GF complexes retain their biological activity in the presence of chronic wound exudates (8), even though these fluids contain increased concentrations of proteolytic enzymes 16, 17.
In view of these data, we hypothesised that the VN:GF complex might hold potential as an effective treatment for chronic wounds. We report herein the production of clinical Good Manufacturing Practice (GMP)‐grade VN:GF (cVN:GF) complex that is functionally equivalent to the Promega‐based VN:GF (pVN:GF) complex incorporating VN purified from serum, as used in our previous studies 5, 6, 8, 18. In addition, two pilot human studies were undertaken: a study in patients with venous leg ulcers and a series of case studies in individuals with a range of chronic wound aetiologies.
MATERIALS AND METHODS
GMP production of cVN:GF complex
The cVN:GF complex is a sterile combination of recombinant VN, IGFBP‐3, IGF‐I and EGF delivered in a solution comprising 0·64 g/l disodium hydrogen phosphate, 0·17 g/l potassium dihydrogen phosphate, 6·9 g/l sodium chloride, 0·17 g/l potassium chloride pH 6·9. Each of four recombinant proteins were produced under GMP conditions using proprietary methods. The following expression systems were used: Spodoptera frugiperda (SF9) insect cells (expresSF+, Protein Sciences Inc., Meriden, CT), Escherichia coli MM294 ΔOmpT (BR067) (Hospira, Adelaide, Australia) and Escherichia coli BL21 pLysS cells (Invitrogen, Mulgrave, Victoria, Australia). All proteins were purified and tested for biological activity compared with a control protein, for example, VN (purified from human plasma, Promega, Madison, WI). Following manufacture, all proteins were combined in a vial fill and then irradiated in a frozen state with dry ice and subjected to a minimum dose of ≥25 kGy exposure by passage through a Co‐60 γ ray source.
Cell culture
The MCF‐7 human breast carcinoma cell line (HTB‐22, ATCC, Manassas, VA) used in this study was cultured in DMEM/Ham's F‐12 (DMEM/F12) media (1:1) (Invitrogen) containing 10% foetal calf serum (FCS) (HyClone, South Logan, UT), 0·1 mg/ml streptomycin, 50 units/ml penicillin and 1 mg/ml gentamycin (Sigma‐Aldrich, Castle Hill, NSW, Australia). This cell line was chosen as it is used in the GMP release criteria for the cVN:GF complex, and has previously been used in several VN:IGFBP:IGF assays 10, 11. Furthermore, over the past decade, we have used the MCF‐7 cell line as a model migration system because of the inability of these cells to migrate in the absence of serum or a suitable chemoattractant. In addition, we have performed numerous studies in primary keratinocyte cells and both MCF‐7 and HaCaT cell lines 6, 8, 9, 11, 18. These experiments indicate that similar trends are observed between the two epithelial tissue derived cell lines in response to VN:GF complexes. All cultures were passaged every 2–3 days by trypsin/EDTA detachment. For use in bioassays, cells were serum starved by incubation in serum‐free media (SFM) and phenol‐red free DMEM/F12 (1:1) for 4 hours prior to trypsinisation and seeding into tissue culture vessels.
Transwell™ migration assays
Migration assays were performed as previously described by our laboratory 5, 6, 19. EGF was included in these and subsequent studies as our intended application is to promote keratinocyte cell growth in chronic leg ulcers (8), and the media used for primary‐cultured keratinocytes also contained EGF (20). Briefly, 1 ml experimental treatments, SFM, VN alone (1 µg/ml), pVN:GF complex (VN 1 µg/ml: IGFBP‐3 1 µg/ml: IGF‐I 0·3 µg/ml: EGF 0·3 µg/ml) and cVN:GF complex (VN 16 µg/ml: IGFBP‐3 1 µg/ml: IGF‐I 0·3 µg/ml: EGF 0·3 µg/ml) were added to the lower chamber of Transwell™ plates and proteins were allowed to bind the culture wells for 3 hours at 37°C, 5% CO2. The wells were then washed twice with SFM + 0·5% BSA (Fraction V, RIA and ELISA grade, Calbiochem, San Diego, CA) after which SFM + 0·05% BSA was added to the lower chambers. Cells which have been serum starved for 4 hours were harvested via trypsinisation and seeded at a density of 2 × 105 cells/well in SFM + 0·05% BSA into the upper chamber of Transwell™ inserts (12 µm, Corning, Lowell, MA). Plates containing the Transwell™ inserts were then incubated for 5 hours at 37°C, 5% CO2. Cells which had migrated to the lower surface of the membrane were fixed in 10% paraformaldehyde and stained with 0·023% crystal violet in phosphate buffered saline (PBS). The number of cells which had migrated to the lower surface of the porous membrane was then quantified by extracting the crystal violet stain in 10% acetic acid and determining the optical density of these extracts at 595 nm (21). Data from three experiments (each experiment tested either duplicate or triplicate samples) were pooled and then analysed.
Cell proliferation assays
The ability of VN:GF complexes to promote MCF‐7 cell proliferation was assessed in cell growth assays using a 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfo‐phe nyl)‐2H‐tetrazolium, inner salt (MTS)‐based assay system (CellTiter, Promega) (22). Briefly, 100 µl experimental treatments, FCS, VN alone (0·83 µg/ml), pVN:GF complex (VN 0·83 µg/ml: IGFBP‐3 0·83 µg/ml: IGF‐I 0·28 µg/ml: EGF 0·28 µg/ml) and cVN:GF complex (VN 13·28 µg/ml: IGFBP‐3 0/83 µg/ ml: IGF‐I 0·28 µg/ml: EGF 0·28 µg/ml) were added to the wells. Although the cells were cultured in FCS‐containing media, all experimental treatments were formulated in SFM. Thus the VN:GF treatments were SFM plus the VN:GF complex, whereas the FCS treatments were SFM plus FCS. Three replicate samples of the controls (FCS and SFM) were tested, while six replicate samples were tested for both Promega‐based VN:GF complexes (pVG) and clinical‐based VN:GF complexes (cVG) in a single experiment. Subsequently, 5000 cells in SFM (50 µl) were added to each well and incubated at 37°C, 5% CO2 for 96 hours. MTS reagent was then added to the wells and after 2 hours of incubation at 37°C, 5% CO2 (to allow the tetrazolium salt colour to develop) absorbance was read at 490 nm in a Beckman Coulter microplate reader (Beckman Coulter, Fullerton, CA) and the results were expressed as percentage above SFM.
Venous leg ulcer pilot study
Patients.
A total of 31 patients (aged 35–97 years, 17 males and 14 females) with confirmed chronic venous leg ulcers were recruited for this study. The patients met the following clinical criteria: a venous refilling time of <25 seconds on photoplethysmography; ulcer size between 1 and 25 cm2 as determined by Visitrak planimetry (Smith and Nephew, Hull, UK); ulcer extended through both the epidermis and the dermis with no exposed deep fascia, muscle, tendon or bone; ulcer had been present for >1 month; ulcer located between and including the knee and ankle and a viable wound bed was present with areas of granulation tissue. Patients with clinically infected ulcers, uncontrolled diabetes (baseline glycosylated haemoglobin >12%), severe rheumatoid arthritis or receiving corticosteroids or other immune suppressive drugs were excluded. All patients gave their written, informed consent. The protocol was approval by the South Metropolitan Area Health Service Human Research Ethics Committee (Western Australia) and followed the principles of the Declaration of Helsinki.
Study protocol and wound evaluation.
Patients were treated with the cVN:GF complex treatment (VN 120 µg, IGFBP‐3 7·5 µg, IGF‐I 2·5 µg, EGF 2·5 µg in PBS containing 1·3 mM acetic acid) twice a week for 3 weeks according to the treatment protocol and were monitored for an additional week following the final application. Before each application, the wound was cleansed with sterile saline. Next, the test fluid was applied to the leg ulcer in a horizontal position and remained uncovered for 20 minutes or until the fluid had dried onto the wound. After each treatment application, Acticoat‐3 (Smith and Nephew) was applied to the ulcer as a primary dressing, followed by four‐layer compression bandages (Profore, Smith and Nephew) giving 40 mmHg pressure at the ankle. Acticoat‐3 is an antimicrobial silver dressing product which is commonly used in venous leg ulcer treatment 23, 24. Baseline data were obtained on the first day of treatment, with the patients then assessed at weekly intervals. At the beginning of each week of treatment, and on the final day of observation, the surface area of the wound was estimated by Visitrak planimetry (Smith and Nephew) and photographed digitally to enable the surface area to be later calculated using Able Image Analyzer® software (version 3·6, http://able.mulabs.com/). Adverse events in relation to deterioration of the wound or the surrounding tissue, as well as any other symptoms or signs of deterioration in general health, were recorded at each assessment.
Chronic wound case studies
Patients.
Eight patients were recruited and comprised three wound types: diabetic foot ulcer wounds (n = 4), chronic venous leg ulcers (n = 3) and a pressure ulcer (n = 1). The patients met the following clinical criteria: age 18–85 years; ulcer size < 5 cm × 5 cm; ulcer present for >1 month; and confirmed venous disease (venous refilling time of <25 seconds on photoplethysmography), diabetes or localised skin changes on an area subjected to pressure. In addition, patients with diabetic foot ulcers had adequate vasculature [toe pressure >55 mm of mercury or an ankle‐brachial index (ABI) > 0·5], appropriate plantar pressure redistribution and a haemoglobin A1C of <12%. Patients exhibiting an ulcer with confirmed venous disease were required to have an ABI of >0·65 and be able to tolerate compression therapy. In the case of pressure ulcers, patients with a Braden scale ≤12 were required to have a therapeutic surface to be entered into this study. All patients gave their written, informed consent. The protocol was approval by the ethical committee of the Women's College Hospital Toronto (Canada) and followed the principles of the Declaration of Helsinki.
Study protocol and wound evaluation.
Patients were treated with the cVN:GF complex treatment (VN 120 µg, IGFBP‐3 7·5 µg, IGF‐I 2·5 µg, EGF 2·5 µg in PBS containing 1·3 mM acetic acid) once a week for 12 weeks according to the treatment protocol. At each weekly treatment, the patients' bandages and dressings were removed and the wounds were cleansed and debrided. Next, the test fluid was applied to the leg ulcer in a horizontal position and covered with a semi‐occlusive dressing, for example, Mepilex (Mölnlycke, Gothenburg, Sweden). Venous leg ulcers were treated with compression therapy and pressure off‐loading was carried out in the case of diabetic foot ulcers and pressure ulcers. The characteristics of the wound base and surrounding skin, as well as digital photographs showing wound size, were recorded at each patient visit.
Statistical analysis
The results for migration and proliferation assays are given as mean ± standard error of mean, and two‐tailed Student's t tests (independent) were performed to test between the pVN:GF complexes and cVN:GF complexes. The level of statistical significance was P < 0·05. The descriptive data of the clinical subjects are expressed as medians and interquartile ranges; however, the ulcer surface areas are also expressed as a mean and standard deviation, to allow comparison with published data. Ulcer size was analysed over time (days 0, 7, 14 and 24) using one‐factor repeated measures analysis of variance. The effect of ulcer duration (≤12 weeks or >12 weeks) and duration of compression therapy (12 weeks or >12 weeks) on ulcer size over time were analysed by two‐factor repeated measures analysis of variance (subject group and time of evaluation).
RESULTS
Cell migration and proliferation is equivalent between the cVN:GF complex and the pVN:GF complex treatments in in vitro functional assays
The cVN:GF complex was compared with the pVN:GF complex, VN alone and SFM controls in cell migration assays (Figure 1A). The results, expressed as percentage of the pVN:GF complex treatment (100%), indicate that the cVN:GF complex treatment stimulated migration to the same extent (101%). We also assessed the ability of the cVN:GF complex to stimulate cell proliferation (Figure 1B). The cVN:GF complex induced similar responses as found with the pVN:GF complex (86·4%) and was not significantly different to the FCS control (112·6%) (P > 0·05). Of note, the cVN:GF complex treatment was γ irradiated to produce a sterile product. These results show that irradiation does not have a deleterious effect on the ability of the complex to induce cell migration or proliferation, as well as indicate that the cVN:GF material is equivalent to the non GMP product.
Figure 1.
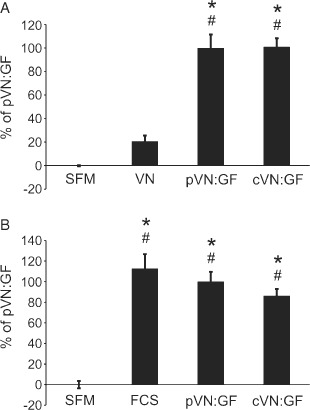
Clinical Good Manufacturing Practice‐grade vitronectin:growth factor (cVN:GF) complex is functionally equivalent to Promega‐based vitronectin:growth factor (pVN:GF) complex in MCF‐7 cells. (A) cVN:GF complex stimulates cell migration in MCF‐7 cells. Migration assays were performed with treatments of serum‐free media (SFM), vitronectin (VN) alone, pVN:GF complex and cVN:GF complex. Data are expressed as percentage of pVN:GF complex. Values shown are mean ± standard error of mean (SEM) (n = 8). (B) cVN:GF complex stimulates cell proliferation in MCF‐7 cells. Proliferation assays were performed with treatments of SFM, foetal calf serum (FCS), pVN:GF complex and cVN:GF complex. Data are expressed as percentage of pVN:GF complex. Values shown are mean ± SEM; n = 3 for SFM and FCS controls and n = 6 for pVN:GF complex and cVN:GF complex. Asterisks indicate significantly different treatments (P < 0·01) to VN alone, while hashes indicate treatments were not significantly different (P > 0·05) to pVN:GF complex.
Treatment of venous leg ulcers with cVN:GF complexes is associated with consistent reduction in wound surface area
This initial study was primarily focussed on safety and involved a 4‐week single‐arm study aimed at identifying any potential adverse reactions, and at assessing the wound healing response. Thirty patients (14 females and 16 males) completed the study. One patient withdrew for reasons unconnected with this study. Six patients had more than one ulcer on the leg; however, these patients were only entered into this study if one ulcer was separated from the other ulcers by at least 3 cm. Only one ulcer per subject was evaluated.
The patient demographics are shown in Table 1. The initial ulcer area is shown as both median and mean values. The median gives a better indication of the sizes of the ulcers; however, because many studies report mean values, this is added for comparison. Of the 30 subjects, 7 had not had compression bandages applied to their legs before this study, and of the 23 who had received compression; this had been for a mean of 9·47 months. All but one target ulcer (96·7% or 29/30 ulcers) reduced in size from the start of this study (day 0) to the last day of observation (day 24). Five ulcers healed during the study period. The healing trajectory for five representative ulcers, along with the mean healing trajectory for all ulcers, is shown in Figure 2A, with the ulcer size expressed as a percentage of the original ulcer surface area. Figure 2B contains the images of the initial ulcer (surface area) and at 24 days for the five subjects mentioned previously. Wound healing trajectories were compared between clinically relevant ulcer classifications at the start of treatment with the cVN:GF complex: target ulcer duration (≤12 weeks or >12 weeks) (Figure 2D); length of compression therapy (≤12 weeks or >12 weeks) (Figure 2E) and target ulcer size (1·0–2·5 cm2/2·5–25·0 cm2) (Figure 2F).
Table 1.
Patient characteristics for venous ulcer pilot study *
| Age | 71·2 years [standard deviation (SD): 14·3 years] |
| Gender (male:female) | 16:14 |
| Initial ulcer area | 6·55 cm2 (SD: 6·24 cm2; median/range: 3·59 cm2/0·87–19·86 cm2) |
| Area at completion of study | 3·75 cm2 (SD: 4·90 cm2; median/range: 1·41 cm2/0–17·36 cm2) |
| Ulcer duration | 11·12 months (SD: 20·5 months; median/range: 6·0 months/1–113 months) |
| Previous compression | 9·47 months (SD: 19·79 months; median/range: 3·5 months/0–108 months) |
*Values for ulcer sizes, ulcer duration and duration of compression are expressed as median and range, as well as mean and standard deviation, as these data are not normally distributed. Data are based on the wound size as determined by digital analysis. For individual patient measurements refer to Figure S1.
Figure 2.
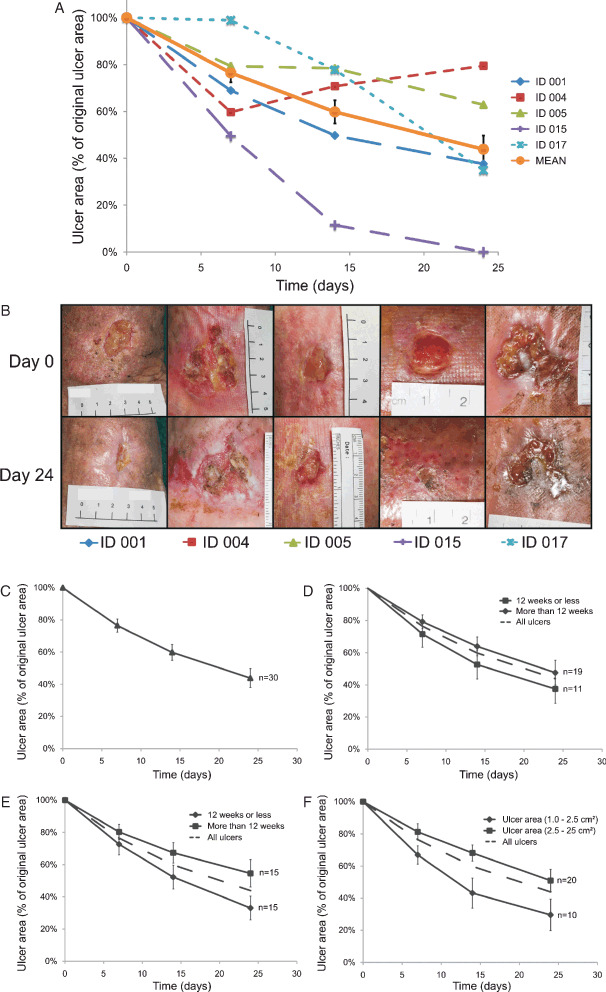
Effect of topical application of clinical Good Manufacturing Practice‐grade vitronectin:growth factor complex on chronic venous leg ulcers. The ulcer areas were photographed digitally for surface area calculation. (A) Wound healing response (percentage original wound area) of five representative patient ulcer areas and overall mean (n = 30) is shown. (B) Corresponding patient ulcer photos, left to right: ID 001, ID 004, ID 005, ID 015 and ID 017. (C) Wound healing response (mean percentage original wound area) was not significantly different at any time point between clinically relevant ulcer classifications: target ulcer duration (≤12 weeks or >12 weeks) (D); length of compression therapy (≤12 weeks or >12 weeks) (E) and target ulcer size (1·0–2·5 cm2/2·5–25·0 cm2) (F). The dotted line indicates the mean value of all ulcers (n = 30). Values shown are mean ± standard error of mean.
The changes in ulcer sizes at the four times of measurement (days 0, 7, 14 and 24) (Figure S1, supporting information) were analysed using repeated measures analysis of variance. The analysis showed that there was a significant effect of time on the ulcer sizes, indicating that the ulcer sizes significantly reduced over the course of this study [F(1·11, 5·55) = 18·31, P < 0·01]. There was no significant effect resulting from ulcer duration [F(8,5) = 1·06, P = 0·50] or the duration of compression therapy before commencing this study [F(7,5) = 1·03, P = 0·51], indicating that neither of these two factors had a significant impact on the ulcer sizes over the course of this study.
All adverse events that occurred during this study were documented and classified according to their severity and their causal relationship to treatment with the cVN:GF complex. A total of 35 adverse events were reported and no severe adverse events were reported. Twenty‐one of these events were general systemic events that were considered unlikely to be related to the cVN:GF complex. These comprised dizzy spells (2), tiredness, common cold, nausea and vomiting (2), tonsillitis, migraine (2), skin rash or itchiness not near the ulcer (4), bradycardia, vertebral fracture and musculoskeletal pains (6). Seven events involved inflammation or infection of the toes or feet, not near the ulcer sites. There were two reports of maceration or breakdown of the skin adjacent to the ulcers, one of pain with application of the bandages, one of itching at the ulcer site and three of a burning sensation with the dressing application. Only two events were considered to be possibly related to the application of the cVN:GF complex, and these were itching at the ulcer site and one case of burning with application of the dressing.
Chronic wound case studies
Following the initial study, a series of case studies were also conducted to examine the effectiveness of the cVN:GF in a broader range of ulcer aetiologies, including diabetic foot ulcers; venous leg ulcers and pressure wounds. Seven patients (two females and five males) completed the study. Patient demographics, ulcer aetiology, clinical result and medical/other complicating factors are summarised in Table 2. The median age of subjects was 60 years (range 26–73 years). The patients were chosen because of the ‘difficult to heal’ nature of their wounds as determined by an experienced clinician. The results of these cases are summarised in Table 2, and representative ulcer photos are shown in Figure 3. All wounds showed signs of improvement during the study period. In addition, the patient with a pressure ulcer (myelomeningocoele – spina bifida) healed completely within 6 weeks.
Table 2.
Patient characteristics for case study
| Patient Id no. | Ulcer pathology | Age (years) | Gender (male/female) | Original wound area at 12 weeks (%) |
|---|---|---|---|---|
| CA1 | Diabetic | 73 | Male | 71 |
| CA2 | Diabetic | 60 | Male | 68 |
| CA4 | Diabetic | 70 | Male | 85 |
| CA6 | Venous | 52 | Female | 74 |
| CA8 | Venous | 71 | Male | 61 |
| CA9 | Diabetic | 37 | Female | 26 |
| CA10 | Pressure | 26 | Male | 0, week 6 |
Figure 3.
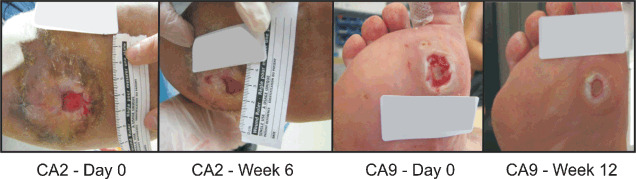
Effect of topical application of clinical Good Manufacturing Practice‐grade vitronectin:growth factor complex on chronic wounds. Representative diabetic patient ulcer photos, left to right: CA2 at day 0, CA2 at week 6, CA9 at day 0, CA9 at week 12.
The following adverse events were observed during these chronic wounds case studies: back pain, nausea, upper respiratory tract infection, ankle, leg or foot pain (not at the target ulcer site), dizzy spells, cramping, vomiting, abdominal skin rash, skin rash on legs, eczema, soft tissue inflammation (not at the target ulcer site) and an infection in a patients toe (not at the target ulcer site). These events all improved over the course of the studies. An unassessable adverse event related to application of a primary wound dressing (Acticoat‐7, Smith and Nephew) was a burning sensation. One patient with a venous ulcer, CA7, withdrew from the study at week 6 because of acquisition of an injury resulting from wearing new footwear. Nevertheless, CA7s wound area had reduced by 30% at week 5. None of these adverse events were considered by the clinical evaluation team to be related to the cVN:GF complex treatment.
DISCUSSION
The data reported here show that coupling GFs to an ECM protein holds promise as a wound healing therapeutic modality. Specifically, the two pilot studies show for the first time that the treatment of chronic wounds in humans with the cVN:GF complex was associated with consistent reduction in wound surface area, even in long‐standing wounds.
Prior to this report, we had showed that this complex showed promising results in an in vivo porcine deep partial thickness burn model (8). For this study, we successfully manufactured the VN:GF complex under GMP conditions to produce a clinical GMP‐grade product (cVN:GF) that could be used on human patients. We then showed that this cVN:GF complex showed equivalent functional activity to the pVN:GF complex treatment that had been used in our previous studies 5, 6, 8, 18.
The single‐arm pilot study of the cVN:GF complex treatment in patients with chronic venous leg ulcers showed the wound surface areas reduced to a mean 44% of the original wound area by day 24. This study was designed principally as a safety study, and confirmed that the cVN:GF complex treatment was well tolerated by patients and compatible with venous ulcer healing. Moreover, all but 1 of 30 patients responded to the treatment with a reduction in wound size and 5 patients healed completely by the end of the treatment period. We recognise that the lack of a control group limits the conclusions that can be made about the effect of the cVN:GF complex treatment on venous ulcer healing rate. However, it is noteworthy that as the duration of the ulcer had no significant effect on the change in ulcer, the cVN:GF treatment may be beneficial in the treatment of long‐standing ulcers. Indeed, prior clinical studies have identified that increased ulcer duration is associated with decreased healing 25, 26. Kurd et al. also documented that 15–30% of chronic venous leg ulcers remain unhealed with appropriate compression therapy after a year of treatment (27) illustrating that new adjunctive therapies are required. The results reported here suggest that the cVN:GF complex treatment may therefore have potential for application in combination with compression therapy to reduce healing times, improve patient quality of life and reduce costs.
In the chronic wound case studies, all wounds improved following treatment with the cVN:GF complex treatment. The results in these ‘difficult to heal’ chronic ulcers suggest that the cVN:GF complex treatment might be an appropriate strategy for difficult to heal chronic wounds where the underlying cause has been corrected. Of interest, Kurd et al. reported that the ability to predict the likelihood of healing depended on how the patient improves by the fourth week of care (27). Therefore, we propose that the cVN:GF complex treatment could be initiated for non healing ulcers at this point. In addition, these case studies show that the cVN:GF complex treatment may be beneficial to multiple ulcer aetiologies.
Several previous studies have used GFs to treat chronic wounds, often with varying results 28, 29, 30, 31, 32, 33, 34, 35. The main problem in these trials appears to be the large doses and frequent application needed to show efficacy. Using a 25 cm2 wound as an example, previous GF treatments over 1 week have been: 500 mg fibroblast growth factor (FGF) (30); 1·8 mg granulocyte colony‐stimulating factor (G‐CSF) (33); 3 g keratinocyte growth factor 2 (KGF‐2) (36) and 5·4 mg vascular endothelial growth factor (VEGF) (37). Compared with the 132·5 µg cVN:GF complex treatment used in this study, previous treatments have used seven to 10 000 times this amount. Not only are large doses of GF expensive, we propose that these supra‐physiological doses can lead to the down‐regulation of GF receptors in cells present in the wound bed (8). This in turn can lead to down‐regulation of intracellular signalling, which has previously been described as an outcome of prolonged GF exposure (38) and may lead to impaired cellular function.
In summary, we report herein the first studies using a clinical GMP‐grade VN:GF complex as a wound healing agent in chronic wounds. The cVN:GF complex was safe and associated with consistent reduction in wound surface area. These findings suggest that the cVN:GF complex may be an effective topical treatment for difficult to heal chronic wounds once the underlying aetiology has been treated, along with other potential tissue repair and regeneration applications. While encouraging, randomised controlled trials are nevertheless required to determine the effect of the topical VN:GF complex treatment on the healing of chronic wounds compared with current best clinical practices, and to determine which wounds will benefit most from treatment with the topical VN:GF complex.
ACKNOWLEDGEMENTS
We would like to thank our funding sources: the Tissue Repair and Regeneration Program from the Institute of Health and Biomedical Innovation, Queensland University of Technology and the publically listed company, Tissue Therapies. Some of the authors (ZU, DRvL and DIL) have a duality of interest in terms of the fact that they hold shares in Tissue Therapies, a biotechnology start‐up company that has been spun out of the Queensland University of Technology to commercialise this technology.
Supporting information
SUPPORTING INFORMATION
The following Supporting information is available for this article:
Figure S1. Venous ulcer pilot study individual patient data.
Additional Supporting Information may be found in the online version of this article:
Supporting info item
REFERENCES
- 1. Wang J, Boerma M, Fu Q, Hauer‐Jensen M. Radiation responses in skin and connective tissues: effect on wound healing and surgical outcome. Hernia 2006;10:502–6. [DOI] [PubMed] [Google Scholar]
- 2. Valencia IC, Falabella H, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol 2001;44:401–21. [DOI] [PubMed] [Google Scholar]
- 3. Jeffcoate WJ, Price P, Harding KG. Wound heating and treatments for people with diabetic foot ulcers. Diabetes Metab Res Rev 2004;20:S78–89. [DOI] [PubMed] [Google Scholar]
- 4. Atiyeh BS, Hayek SN, Gunn SW. New technologies for burn wound closure and healing – review of the literature. Burns 2005;31:944–56. [DOI] [PubMed] [Google Scholar]
- 5. Hollier B, Harkin DG, Leavesley D, Upton Z. Responses of keratinocytes to substrate‐bound vitronectin: growth factor complexes. Exp Cell Res 2005;305:221–32. [DOI] [PubMed] [Google Scholar]
- 6. Hyde C, Hollier B, Anderson A, Harkin D, Upton Z. Insulin‐like growth factors (IGF) and IGF‐binding proteins bound to vitronectin enhance keratinocyte protein synthesis and migration. J Invest Dermatol 2004;122:1198–206. [DOI] [PubMed] [Google Scholar]
- 7. Dawson RA, Upton Z, Malda J, Harkin DG. Preparation of cultured skin for transplantation using insulin‐like growth factor I in conjunction with insulin‐like growth factor binding protein 5, epidermal growth factor, and vitronectin. Transplantation 2006;81:1668–76. [DOI] [PubMed] [Google Scholar]
- 8. Upton Z, Cuttle L, Noble A, Kempf M, Topping G, Malda J, Xie Y, Mill J, Harkin DG, Kravchuk O, Leavesley DI, Kimble RM. Vitronectin: growth factor complexes hold potential as a wound therapy approach. J Invest Dermatol 2008;128: 1535–44. [DOI] [PubMed] [Google Scholar]
- 9. Ainscough SL, Barnard Z, Upton Z, Harkin DG. Vitronectin supports migratory responses of corneal epithelial cells to substrate bound IGF‐I and HGF, and facilitates serum‐free cultivation. Exp Eye Res 2006;83:1505–14. [DOI] [PubMed] [Google Scholar]
- 10. Van Lonkhuyzen DR, Hollier BG, Shooter GK, Leavesley DI, Upton Z. Chimeric vitronectin: insulin‐like growth factor proteins enhance cell growth and migration through co‐activation of receptors. Growth Factors 2007;25:295–308. [DOI] [PubMed] [Google Scholar]
- 11. Hollier BG, Kricker JA, Van Lonkhuyzen DR, Leavesley DI, Upton Z. Substrate‐bound insulin‐like growth factor (IGF)‐I‐IGF binding protein‐vitronectin‐stimulated breast cell migration is enhanced by coactivation of the phosphatidylinositide 3‐kinase/AKT pathway by alpha v‐integrins and the IGF‐I receptor. Endocrinology 2008;149:1075–90. [DOI] [PubMed] [Google Scholar]
- 12. Klemke RL, Yebra M, Bayna EM, Cheresh DA. Receptor tyrosine kinase signaling required for integrin alpha‐v‐beta‐5‐directed cell motility but not adhesion on vitronectin. J Cell Biol 1994;127:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamboliev IA, Gerthoffer WT. Modulatory role of ERK MAPK‐caldesmon pathway in PDGF‐stimulated migration of cultured pulmonary artery SMCs. Am J Physiol Cell Physiol 2001;280:C1680–8. [DOI] [PubMed] [Google Scholar]
- 14. Topping G, Malda J, Dawson R, Upton Z. Development and characterisation of human skin equivalents and their potential application as a burn wound model. Primary Intention 2006;1:14–21. [Google Scholar]
- 15. Xie Y, Rizzi SC, Dawson R, Lynam E, Richards S, Leavesley DI, Upton Z. Development of a three‐dimensional human skin equivalent wound model for investigating novel wound healing therapies. Tissue Eng C 2010;16:1111–23. [DOI] [PubMed] [Google Scholar]
- 16. Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase‐9 (MMP‐9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol 2008;158:951–61. [DOI] [PubMed] [Google Scholar]
- 17. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–5. [DOI] [PubMed] [Google Scholar]
- 18. Kricker JA, Towne CL, Firth SM, Herington AC, Upton Z. Structural and functional evidence for the interaction of insulin‐like growth factors (IGFs) and IGF binding proteins with vitronectin. Endocrinology 2003;144:2807–15. [DOI] [PubMed] [Google Scholar]
- 19. Noble A, Towne C, Chopin L, Leavesley D, Upton Z. Insulin‐like growth factor‐II bound to vitronectin enhances MCF‐7 breast cancer cell migration. Endocrinology 2003;144:2417–24. [DOI] [PubMed] [Google Scholar]
- 20. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975;6:331–43. [DOI] [PubMed] [Google Scholar]
- 21. Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA. Intergrin beta‐1‐mediated and beta‐3‐mediated endothelial‐cell migration is triggered through distinct signaling mechanisms. J Cell Biol 1993;121:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium formazan assay for cell growth assays in culture. Cancer Commun 1991;3:207–12. [DOI] [PubMed] [Google Scholar]
- 23. Sibbald RG, Contreras‐Ruiz J, Coutts P, Fierheller M, Rothman A, Woo K. Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers. Adv Skin Wound Care 2007;20:549–58. [DOI] [PubMed] [Google Scholar]
- 24. Miller CN, Newall N, Kapp SE, Lewin G, Karimi L, Carville K, Gliddon T, Santamaria NM. A randomized‐controlled trial comparing cadexomer iodine and nanocrystalline silver on the healing of leg ulcers. Wound Repair Regen 2010;18:359–67. [DOI] [PubMed] [Google Scholar]
- 25. Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420–5. [DOI] [PubMed] [Google Scholar]
- 26. Moffatt C, Doherty D, Smithdale R, Franks P. Clinical predictors of leg ulcer healing. Br J Dermatol 2010;162:51–8. [DOI] [PubMed] [Google Scholar]
- 27. Kurd SK, Hoffstad OJ, Bilker WB, Margolis DJ. Evaluation of the use of prognostic information for the care of individuals with venous leg ulcers or diabetic neuropathic foot ulcers. Wound Repair Regen 2009;17:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uchi H, Igarashi A, Urabe K, Koga T, Nakayama J, Kawamori R, Tamaki K, Hirakata H, Ohura T, Furue M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol 2009;19:461–8. [DOI] [PubMed] [Google Scholar]
- 29. Richard JL, Parer‐Richard C, Daures JP, Clouet S, Vannereau D, Bringer J, Rodier M, Jacob C, Comte‐Bardonnet M. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot, randomized, double‐blind, placebo‐controlled study. Diabetes Care 1995;18:64–9. [DOI] [PubMed] [Google Scholar]
- 30. Robson MC, Phillips LG, Lawrence WT, Bishop JB, Youngerman JS, Hayward PG, Broemeling LD, Heggers JP. The safety and effect of topically applied recombinant basic fibroblast growth‐factor on the healing of chronic pressure sores. Ann Surg 1992;216:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robson MC, Phillips TJ, Falanga V, Odenheimer DJ, Parish LC, Jensen JL, Steed DL. Randomized trial of topically applied, repifermin (recombinant human keratinocyte growth factor‐2) to accelerate wound healing in venous ulcers. Wound Repair Regen 2001;9:347–52. [DOI] [PubMed] [Google Scholar]
- 32. Marques da Costa R, Jesus FM, Aniceto C, Mendes M. Double‐blind randomized placebo‐controlled trial of the use of granulocyte‐macrophage colony‐stimulating factor in chronic leg ulcers. Am J Surg 1997;173:165–8. [DOI] [PubMed] [Google Scholar]
- 33. de Lalla F, Pellizzer G, Strazzabosco M, Martini Z, Du Jardin G, Lora L, Fabris P, Benedetti P, Erle G. Randomized prospective controlled trial of recombinant granulocyte colony‐stimulating factor as adjunctive therapy for limb‐threatening diabetic foot infection. Antimicrob Agents Chemother 2001;45:1094–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hirshberg J, Coleman J, Marchant B, Rees RS. TGF‐beta3 in the treatment of pressure ulcers: a preliminary report. Adv Skin Wound Care 2001;14: 91–5. [DOI] [PubMed] [Google Scholar]
- 35. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo‐controlled double‐blind study. Diabetes Care 1998;21:822–7. [DOI] [PubMed] [Google Scholar]
- 36. Robson MC, Phillips LG, Heggers JP, McPherson MA, Heiner LS. Clinical studies on growth factors in pressure sores: preliminary report. Prog Clin Biol Res 1991;365:95–102. [PubMed] [Google Scholar]
- 37. Hanft JR, Pollak RA, Barbul A, van Gils C, Kwon PS, Gray SM, Lynch CJ, Semba CP, Breen TJ. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care 2008;17:30–2,4–7. [DOI] [PubMed] [Google Scholar]
- 38. Rubin C, Gur G, Yarden Y. Negative regulation of receptor tyrosine kinases: unexpected links to c‐Cbl and receptor ubiquitylation. Cell Res 2005;15:66–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
The following Supporting information is available for this article:
Figure S1. Venous ulcer pilot study individual patient data.
Additional Supporting Information may be found in the online version of this article:
Supporting info item


