Abstract
It is important for clinicians to understand which are the clinical signs, the patient characteristics and the procedures that are related with the occurrence of hypertrophic burn scars in order to carry out a possible prognostic assessment. Providing clinicians with an easy‐to‐ use tool for predicting the risk of pathological scars. A total of 703 patients with 2440 anatomical burn sites who were admitted to the Department of Plastic and Reconstructive Surgery, Burn Center of the Traumatological Hospital in Torino between January 1994 and May 2006 were included in the analysis. A Bayesian network (BN) model was implemented. The probability of developing a hypertrophic scar was evaluated on a number of scenarios. The error rate of the BN model was assessed internally and it was equal to 24·83%. While classical statistical method as logistic models can infer only which variables are related to the final outcome, the BN approach displays a set of relationships between the final outcome (scar type) and the explanatory covariates (patient's age and gender, burn surface area, full‐thickness burn surface area, burn anatomical area and wound‐healing time; burn treatment options such as advanced dressings, type of surgical approach, number of surgical procedures, type of skin graft, excision and coverage timing). A web‐based interface to handle the BN model was developed on the website www.pubchild.org (burns header). Clinicians who registered at the website could submit their data in order to get from the BN model the predicted probability of observing a pathological scar type.
Keywords: Bayesian networks; Hypertrophic scar; Interaction models; Prediction models; Web‐assisted decision‐making
Introduction
Burn injury is often a devastating event with long‐term physical and psychosocial effects. All deep second‐ and third‐degree burns are at risk of developing hypertrophic scars which can severely undermine the quality of life (1).
Burn scars, mainly hypertrophic or keloid scars, are cosmetically disfiguring and, as a consequence, force the scarred person to deal with the incidence of sleeping trouble, psychological stress, anxiety, social avoidance and depression (2). Only recently have researchers begun to delineate the complex biochemical signalling pathways that regulate the mechanisms of normal and abnormal scar formation 3, 4. Similarly, it is important for clinicians to understand which are the clinical signs, patient characteristics and procedures that are principally related to the occurrence of pathological scarring in order to carry out a prognostic assessment (5).
In previous studies, research issues focused on the risk factors for pathological burn scars 6, 7. In particular, Deitch et al. (8) reported that an important indicator of the occurrence of wound problems was the time required for the burn to heal. Furthermore, Baker et al. (9) pointed out that bacterial colonisation can increase the incidence of hypertrophic scarring of the burn wound and a prospective study of 70 consecutive burn patients showed that young age and black race are factors associated with pathological scarring (6). Also, anatomic sites like the neck and upper limb are at higher risk of pathological scarring than the abdomen and perineum (6).
Nevertheless, these studies do not provide a prognostic tool for prediction of outcome on the basis of the patient's demographic and clinical information. One of the major problems in scar management is the unforeseeability, together with the availability of completely unspecific therapeutic aids for scar treatment; and burn care specialists are still looking for methods to identify patients who might benefit from prophylactic programmes (10).
Bayesian networks (BNs) have gained popularity in health care over the past decade to support diagnostic reasoning in establishing the diagnosis for an individual patient 11, 12, 13, 14 and predicting treatment outcome (15). They provide a robust and coherent probabilistic reasoning for handling uncertainty in establishing diagnoses and to exploit knowledge on the evolution of processes over time. For these reasons and also due to an intuitive graphical representation, they have arisen in recent years as an ease‐of‐use prognostic instrument for risk prediction 16, 17. The aim of this study is to evaluate the factors associated with an increased risk for developing pathological scarring after burns. To achieve this goal, a representation of the relationships among the principal risk factors by means of a BN, which may help in assessing outcomes, is presented.
Materials and methods
Data source
Training sample
From January 1994 to 15 May 2006 at the Department of Plastic and Reconstructive Surgery, Burn Center of the Trauma Hospital in Torino, a standard reporting form for collecting data regarding the scarring process was used. Clinical histories were constructed for all burn patients by abstraction of details from the clinical notes made during their stay in hospital as inpatients, and the details recorded of return for clinic attendances as outpatients or return as inpatients for corrective surgery. The cohort consists of 703 patients and 2440 anatomical burn sites. The scar type was defined on the basis of the morphologic classification described by Magliacani et al. (10) and the scar evolution, calculated in days from the manifestation until its complete remission, was assessed according to the classification groups described by Muir (18). The diagnosis of pathological or normotrophic scarring was based on clinical evaluation. The scars were considered normotrophic when they assumed characteristics similar to the surrounding healthy skin in terms of thickness, colour and pliability, or when they were slightly hypo‐ or hyper‐pigmented, or when they had an erythematous aspect, with or without itching, which disappeared in few weeks. On the contrary, pathological scars (hypertrophy, hypertrophy with contracture, contracture and atrophy) were diagnosed on the basis of the presence of typical signs (raised, erythematous and fibrous symptoms for hypertrophy; skin coarctation and disfigurement for contracture; thin and fragile for atrophy) and symptoms (pain, itching and dysesthesias for hypertrophy; reduced range of motion of involved joints and subjective sensation of constriction for contracture; itching for atrophy). Diagnoses were confirmed by two physicians.
Validation sample
From 15 May 2006 to 15 May 2007, the Department of Plastic and Reconstructive Surgery, Burn Center in Torino used a standard form for the collection of data regarding the scarring process. The cohort was made up of 49 patients and included a total of 162 anatomical burn sites. All patients were enrolled in a surveillance programme after the completion of the burn wound‐healing phase to control and/or treat the post‐burn scarring as necessary.
Statistical methods
BNs offer a robust framework for probabilistic reasoning with uncertainity. They consist of a qualitative part, an acyclic directed graph, where the nodes represent stochastic variables, and a quantitative part, which is a set of conditional probability distribution (19). While the graphical structure of the network captures qualitative relationships among variables, their strength is quantified by the conditional probability distribution associated with each node.
The two BN components can be determined through elicitation from experts or can be inferred from data using machine learning algorithms or even by combining elicitation from experts and learning algorithm strategies (20). In this study, machine learning algorithms were implemented for building both the graphical structure and the conditional probability distributions. A Bayesian search procedure based on the thick–thin approach was carried out for deriving the acyclic directed graph and the expectation–maximisation algorithm was chosen to estimate the conditional distribution of each variable (21). The machine learning procedure was implemented using GeNie software (22) to develop the graphical structure of the BN and Netica software (23) for learning conditional distributions and for performing the validation phase.
As in many of the current software tools, these software environments require variables to assume a finite number of states, both for building the structure of the BN and for carrying out parameter learning.
Thus, continuous variables were discretised on the basis of quartiles [burn surface area (BSA)] and full thickness‐BSA (FT‐BSA), tertiles (excision and coverage timing (ECT)] and clinical considerations [age, wound‐healing time (WHT)]. In Table 1, the nodes of the BN along with their modalities are listed. Missing values are entered as unknown category. A sensitivity analysis was carried out for the outcome node (normal scar versus hypertrophic scar) in order to identify the most influential variables. For categorical states, sensitivity was calculated as the degree of entropy reduction or mutual information, which measures how much uncertainty about a specific event is expected to decrease when a new finding becomes available (24), and the expected reduction of real variance. Finally, an external validation using the validation sample was performed in order to evaluate the performance of the BN as a prediction and classification tool.
Table 1.
Description of the variables in the Bayesian network *
| Variable description | Node acronym | Variable type | States description |
|---|---|---|---|
| Gender | Gender | Discrete | Female, male |
| Age | Age | Continuous | <15; 15–40; 40–65; >65 |
| Burn surface area | BSA | Continuous | <23; 23–47; 47–72; >72 |
| Full thickness‐burn surface area | FT‐BSA | Continuous | <19; 19–38; 38–57; >57 |
| Aetiology | Aetiology | Discrete | Chemical; contact; electrical; flame; scalds; pressure; flash; sunburns; steam |
| Burnt area | Burnt area | Discrete | Abdomen; lower limb; upper limb; neck; perineum; head; chest |
| Burn treatment | Burn treatment | Discrete | Medical; surgical |
| Number of surgical procedure | NO | Discrete | 0; 1; 2; 3; 4; 5; 6 |
| Type of surgical approach | TSA | Discrete | Alexander; dermal abrasion; flap; excision; excision and auto skin graft (SG); excision and xeno SG; excision and allo SG |
| Type of skin graft | TSG | Discrete | 1:2; 1:4; 1:6; sheet |
| Wound healing time | WHT | Continuous | 3 weeks; 3–6 weeks; >6 weeks |
| Excision and coverage timing (ECT) (from burn trauma to the first surgical procedure) | ECT | Continuous | <5 days; 5–20 days; >20 days |
| Scar type | Scar type | Discrete | Normotrophic; hypertrophic; hypertrophic with contracture; contracted; atrophic |
*Continuous variables were discretised on the basis of quartiles (BSA and FT‐BSA) and tertiles (age, WHT and ECT]. Categories 1:2; 1:4; 1:6 of the type of skin graft indicates the meshing ratio.
Results
The univariable analysis of predictors of post‐burn pathological scarring is given in Table 2.
Table 2.
Clinical characteristics of type of scar of the study population are displayed by demographic, clinical and treatment as percentage (N) *
| No pathological scars | Pathological scars | OR (CI 95%) | ||||
|---|---|---|---|---|---|---|
| Normotrophic | Hypertrophic | Hypertrophic with contracture | Contracted | Atrophic | ||
| Gender (male) | 65(692) | 61(551) | 59(227) | 60(53) | 75(3) | 1·24 (0·99; 1·56) P = 0·06 |
| Age | 38(25−54) | 38(24−54) | 36(24−52) | 41(22 − 59) | 31(15 − 51) | 0·94 (0·81; 1·10) |
| Burn surface area (BSA) (%) | 18(10−35) | 20(10−35) | 30(15−45) | 15(10 − 30) | 10(8 − 14) | 1·15 (0·97; 1·36) |
| Full thickness‐BSA (%) | 8(0 − 20) | 10(4 − 20) | 20(10−30) | 10(5 − 25) | 10(7 − 11) | 1·54 (1·22; 1·94) P = 0·001 |
| Aetiology | ||||||
| Chemical | 2(22) | 3(21) | 1(5) | 1(1) | 50(1) | 0·91 (0·43; 1·95) |
| Contact | 2(18) | 1(10) | 2(6) | 3(2) | 0(0) | 0·72 (0·27; 1·91) |
| Electrical | 3(29) | 1(6) | 0(0) | 1(1) | 0(0) | 0·17 (0·06; 0·49) P = 0·01 |
| Flame | 64(620) | 67(561) | 76(262) | 58(43) | 0(0) | Ref. |
| Scalds | 14(140) | 15(125) | 5(18) | 19(14) | 0(0) | 0·80 (0·59; 1·10) |
| Pressure | 0(0) | 0(3) | 1(2) | 0(0) | 0(0) | NA |
| Flash | 13(128) | 12(104) | 16(54) | 18(13) | 0(0) | 0·96 (0·65; 1·41) |
| Sunburns | 1(10) | 0(2) | 0(0) | 0(0) | 0(0) | 0·14 (0·04; 0·58) P = 0·02 |
| Steam | 1(6) | 1(6) | 0(0) | 0(0) | 50(1) | 0·84 (0·20; 3·49) |
| Burnt area | ||||||
| Abdomen | 9(98) | 7(67) | 1(5) | 0(0) | 0(0) | 0·41 (0·30; 0·58) P = 0·001 |
| Lower limb | 22(234) | 35(321) | 12(48) | 1(1) | 50(2) | 0·90 (0·70; 1·16) |
| Upper limb | 28(301) | 34(308) | 48(185) | 44(39) | 25(1) | Ref. |
| Neck | 7(71) | 3(26) | 13(49) | 18(16) | 0(0) | 0·72 (0·51; 1·03) |
| Perineum | 5(50) | 3(24) | 1(3) | 0(0) | 0(0) | 0·30 (0·19; 0·50) P = 0·001 |
| Head | 19(199) | 7(61) | 5(20) | 10(9) | 25(1) | 0·26 (0·19; 0·34) P = 0·001 |
| Chest | 10(105) | 11(98) | 19(75) | 26(23) | 0(0) | 1·05 (0·80; 1·39) |
| Burn treatment | ||||||
| Medical | 64(678) | 37(336) | 17(65) | 28(25) | 50(2) | 0·25 (0·20; 0·31) P = 0·001 |
| Surgical | 380(36) | 569(63) | 320(83) | 63(72) | 2(50) | Ref. |
| Number of surgical procedures | 2·10 (1·73; 2·56) | |||||
| 0 | 67(677) | 39(336) | 18(65) | 32(25) | 50(2) | |
| 1 | 25(256) | 43(373) | 48(168) | 40(31) | 50(2) | |
| 2 | 4(44) | 12(99) | 19(67) | 17(13) | 0(0) | |
| 3 | 1(15) | 4(35) | 9(31) | 8(6) | 0(0) | |
| 4 | 0(5) | 1(12) | 3(10) | 3(2) | 0(0) | |
| 5 | 1(7) | 1(5) | 3(9) | 0(0) | 0(0) | |
| 6 | 0(3) | 0(0) | 1(3) | 0(0) | 0(0) | |
| Type of surgical approach | ||||||
| Alexander | 1(4) | 1(7) | 1(4) | 0(0) | 0(0) | 1·05 (0·13; 8·83) |
| Dermal abrasion | 4(14) | 2(14) | 1(4) | 3(2) | 0(0) | 0·55 (0·26; 1·16) |
| Flap | 2(6) | 0(0) | 0(0) | 0(0) | 0(0) | NA |
| Excision | 2(7) | 4(23) | 4(12) | 2(1) | 0(0) | 1·97 (0·82; 4·69) |
| Excision and autograft | 89(335) | 91(515) | 93(299) | 95(60) | 100(2) | Ref. |
| Excision and xenograft | 1(5) | 0(1) | 0(0) | 0(0) | 0(0) | NA |
| Excision and allograft | 2(7) | 1(7) | 0(1) | 0(0) | 0(0) | NA |
| Type of skin graft | ||||||
| 1:2 | 27(59) | 30(114) | 21(44) | 31(12) | 0·97 (0·60; 1·55) | |
| 1:4 | 51(113) | 58(218) | 51(108) | 28(11) | Ref. | |
| 1:6 | 1(3) | 1(2) | 1(3) | 0(0) | 0·56 (0·12; 2·51) | |
| Sheet | 21(47) | 11(40) | 27(57) | 41(16) | 0·81 (0·49; 1·33) | |
| Wound‐healing time | 33(20−60) | 40(27−62) | 55(35−87) | 57(31−100) | 19(10 − 28) | 1·15 (1·02; 1·29) P = 0·04 |
| Excision and grafting timing | 10(5 − 19) | 10(6 − 17) | 7(3 − 16) | 10(4 − 16) | 9 | 0·90 (0·79; 1·02) |
*For age, burn surface area and full thickness‐BSA median and interquartile range are reported. In addition, odds ratio (OR) of pathological scar and 95% confidence interval (CI 95%) are shown.
The analysis demonstrates (i) a WHT more than 6 weeks, (ii) an area of FT‐BSA more than 57% and (iii) a number of surgical procedures more than four, as negative prognostic factors for pathological scarring; and (i) the aetiology of burn by sun or by electrical tools, (ii) some anatomical areas such as the abdomen and perineum and (iii) the burn treatment with advanced dressings, as positive prognostic factors for pathological scarring.
In Figure 1, the BN along with the conditional probability tables learnt using the EM algorithm is depicted. In Table 3, results of the sensitivity analysis are showed. Sensitivity analysis resulted in a list of variables ranked according to the capability they have to change the posterior probability of the outcome node (scar type) when new evidence is entered. Mutual information and beliefs of variance values provide an indication of the relative sensitivity of each variable. The value in each cell refers to the rank of each variable with respect to the outcome node. Variables with greater values have a greater impact in predicting the outcome.
Figure 1.
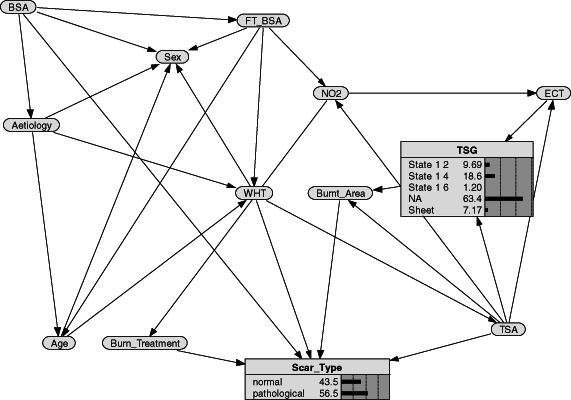
Bayesian network structure along with probability distributions.
Table 3.
Sensitivity analysis
| Node | Mutual information (%) | Variance of beliefs (%) |
|---|---|---|
| Type of surgical approach | 8·75 | 11·7 |
| Number of surgical procedures | 8·09 | 10·9 |
| Burn treatment | 8·05 | 10·9 |
| Excision and coverage timing (ECT) | 5·61 | 7·53 |
| Type of skin graft | 3·94 | 5·24 |
| Wound healing time | 3·04 | 4·18 |
| Burn area | 0·75 | 1·04 |
| Burn surface area (BSA) | 0·36 | 0·45 |
| Full thickness‐BSA | 0·1 | 0·13 |
| Age | 0· 08 | 0· 12 |
| Aetiology | 0·023 | 0·032 |
| Sex | 0·02 | 0·031 |
In Table 4, the probability of developing a hypertrophic scar is evaluated in different scenarios. The probability of observing the evidence is also reported. The error rate evaluated for the validation sample was 24·78%.
Table 4.
Predicted probabilities of developing hypertrophic scar along with the probability of observing the hypothesised scenario
| Patient | Age (years) | Burnt area | Surface area | Full thickness | Probability hypertrophic scar (%) | Probability of evidence (%) |
|---|---|---|---|---|---|---|
| 1 | <15 | Abdomen | <23 | <19 | 56·6 | 6·49 |
| 2 | 15–40 | Upper limb | <23 | 19–38 | 64·3 | 11·14 |
| 3 | >65 | Lower limb | <23–47 | <19 | 53·8 | 2·39 |
| 4 | <15 | Neck | <23 | >57 | 69·9 | 1·01 |
| 5 | 40–65 | Upper limb | 23–47 | >57 | 63·1 | 1·87 |
| 6 | >65 | Neck | >72 | >57 | 54·1 | 1·38 |
| 7 | <15 | Upper limb | <23 | <19 | 62·6 | 3·38 |
| 8 | 15–40 | Upper limb | >72 | 38–57 | 88·3 | 0·08 |
| 9 | >65 | Upper limb | >72 | <19 | 51·8 | 1 |
Web‐based interface for the BN model
Systems based on web technologies have become increasingly important in the clinical setting to perform real‐time processing and for monitoring frequencies and causes of health hazards (25).
A web‐based interface to handle the above BN model for predicting pathological scars has been developed on the website www.pubchild.org (burn header). Each clinician who registered to the website has to submit data in order to get from the BN model the predicted probability of observing a pathological scar type.
After registering to www.pubchild.org, users can enter data into the burns form section on the left menu. They have to set their evidence for each node: agent, aetiology, WHT, type of surgical approach, type of skin graft, number of surgical procedures, ECT, age, surgical scar treatment; they are allowed to enter as not available (NA) evidence also. The data from the user is sent through the Internet to the server. A first e‐mail notification which summarises data entered is sent to the user. Data entered is stored in a secured database maintained for studying purposes. The server web application is designed to handle incoming data and put them into each node of the BN model and update probability values of the scar type. Finally, a second e‐mail containing the BN predicted outcome along with its probability value will be sent. Security data transmission was ensured using https secure protocol. The server computer on which the web‐based data collection resides is up to date with the latest security patches for the operating system and web server software. The server computer has a firewall prohibiting access via network to all unnecessary services; access is restricted to port 443 on which encrypted information travels.
Discussion
In medicine, BN systems are used for aiding in prognosis decisional process: inferring the most probable outcome of an observed problem given a set of symptoms, patient history, physical signs and applied treatment. They can be especially useful because they can explore potentially causal dependencies among risk factors and can be converted into diagnostic and prognostic support tool (26).
One of the main advantages of BNs lies in their graphical nature, which displays the links between the variables entered in the model. Knowledge of the structure of a model can show the dependence or independence of variables and also suggest a direction of causality. A convenient feature of BNs is the ability to learn the graphical structure directly from the data, as well as parameters, thus allowing empirical validation of hypothesis.
The main limitation of BNs is the limited capability of many learning algorithms to deal with continuous variables. Discretisation of continuous variables is a potential drawback, because of the information loss at each node. However, if data is fundamentally discrete – as in this study where variables are inherently numerical‐discrete with a small number of values that appear over and over again, except age and WHT, which have been categorised according to the clinical considerations to privilege interpretability of the model – discretisation based on equal width interval binning or equal frequency binning has been shown to perform well enough, especially when the sample size is large, because discretisation techniques assume that data are noisy observations originating from an underlying discrete mechanism (27) (age, WHT).
The qualitative structure of the BN is shown in Figure 1. It points out that pathological scarring (scar type) is directly linked with several variables: if patient was treated with medical or surgical procedure (burn treatment), the burn anatomical area (burnt area), the WHT and the BSA of the patient.
Besides predictive purposes of the final outcome, the BN can also be used for explorative tasks by examining the correlations found by the modelling process. For this purpose, in our BN, WHT is related to FT‐BSA and aetiology, which in turn is related to the gender and the age of the patient. As the BN was built using learning algorithms, these relationships should be cautiously interpreted. For example, the direct link between aetiology, BSA, FT‐BSA and gender should not be considered as a causal relationship, whereas it should be explained by the fact that males and females have different aetiologies and different distribution of percentage of BSA and FT‐BSA.
The direct link between the normal/pathological scar and the WHT is in agreement with previous published studies. Since 1983, Deitch et al. (8) stated that the best predictor of the development of hypertrophic scar is the WHT, and Cubison et al. (28), analysing data on 337 children with scalds, suggested that the healing time should be factored into the decision of which treatment, conservative or not, to apply. This further justifies attempts to speed up the healing process even by means of expensive wound‐healing dressings.
In literature, patient age correlates with the occurrence of pathological scar development (6). Younger patients are at higher risk of developing keloid or hypertrophic scars, which may be due to the greater capacity of younger skin for collagen synthesis or greater skin tension in younger individuals. This could be explained by the fact that normal wound healing is characterised by an optimum balance between deposition and lysis of collagen. This fact can be observed also in the BN, which predicts a better prognosis for older patients. As shown in Table 4, young patients (see profile 4), less than 15 years, with burns on the neck and an FT‐BSA greater than 57% have a probability of a pathological scar of 69·9%. Patients older than 65 years (see profile 6) have probability of a pathological scar of about 54%. The same relationship between older age and minor probability of hypertrophic scar can be seen also for the other risk profiles.
Finally, it is remarkable that patients who are between 15 and 40 years with burns on upper limb with FT‐BSA ranging from 38 to 57 (see profile 8) have the probability of developing a pathological scar of about 88%; however, the probability of encountering such profiles is less than 1% (probability of evidence provided for profile 8 is equal to 0·08%).
As the sample was almost all white Italian patients, race was not considered as a risk factor, even if it is well documented in literature; for example, black individuals are predisposed to keloids as Hispanic and Asiatic race (8). Thus, further validation of the BN in different populations would be important. On the other hand, the use of Bayesian inference means that the BN can be readily updated when new data becomes available, providing a way to overcome data limitations by incorporating input data from different sources.
In conclusion, the BN output can support the physician in the prognosis of hypertrophic scars. In fact, this approach provides clinical scenarios which can be assessed along with their consequent outcome in terms of probability. This not only leads to more detailed prognostic information but also can help clinicians foresee complications and treat them as soon as possible. This is important also for improving, whether necessary, patients' communication through informed, well‐judged and not unaware messages about their prognosis.
References
- 1. Brusselaers N, Pirayesh A, Hoeksema H, Verbelen J, Blot S, Monstrey S. Burn scar assessment: a systematic review of objective scar assessment tools. Burns 2010;36:1157–64.[Epub 2010/05/22]. [DOI] [PubMed] [Google Scholar]
- 2. Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol 2003;4:245–72. [DOI] [PubMed] [Google Scholar]
- 3. Scott PG, Dodd CM, Ghahary A, Shen YJ, Tredget EE. Fibroblasts from post‐burn hypertrophic scar tissue synthesize less decorin than normal dermal fibroblasts. Clin Sci (Lond) 1998;94:541–7. [DOI] [PubMed] [Google Scholar]
- 4. Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen 2005;13:398–404. [DOI] [PubMed] [Google Scholar]
- 5. Gangemi EN, Gregori D, Berchialla P, Zingarelli E, Cairo M, Bollero D, Ganem J, Capocelli R, Cuccuru F, Cassano P, Risso D, Stella M. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg 2008;10:93–102.[Epub 2008/03/19]. [DOI] [PubMed] [Google Scholar]
- 6. McDonald WS, Deitch EA. Hypertrophic skin grafts in burned patients: a prospective analysis of variables. J Trauma 1987;27:147–50. [DOI] [PubMed] [Google Scholar]
- 7. Gangemi EN, Garino F, Berchialla P, Martinese M, Arecco F, Orlandi F, Stella M. Low triiodothyronine serum levels as a predictor of poor prognosis in burn patients. Burns 2008;34:817–24.[Epub 2008/02/05]. [DOI] [PubMed] [Google Scholar]
- 8. Deitch EA, Wheelahan TM, Rose MP, Clothier J, Cotter J. Hypertrophic burn scars: analysis of variables. J Trauma 1983;23:895–8. [PubMed] [Google Scholar]
- 9. Baker RH, Townley WA, McKeon S, Linge C, Vijh V. Retrospective study of the association between hypertrophic burn scarring and bacterial colonization. J Burn Care Res 2007;28:152–6. [DOI] [PubMed] [Google Scholar]
- 10. Magliacani G, Stella M, Castagnoli C, Trombotto C, Ondei S, Calcagni M. Post‐burn pathological scar: clinical aspects and therapeutic approach. An Medit Burns Club 1997;2:105–8. [Google Scholar]
- 11. Andreassen S, Rosenfalck A, Falck B, Olesen KG, Andersen SK. Evaluation of the diagnostic performance of the expert EMG assistant MUNIN. Electroencephalogr Clin Neurophysiol 1996;101:129–44. [DOI] [PubMed] [Google Scholar]
- 12. Heckerman DE, Horvitz EJ, Nathwani BN. Toward normative expert systems: Part I. The Pathfinder project. Methods Inf Med 1992;31:90–105. [PubMed] [Google Scholar]
- 13. Heckerman DE, Nathwani BN. Toward normative expert systems: Part II. Probability‐based representations for efficient knowledge acquisition and inference. Methods Inf Med 1992;31:106–16. [PubMed] [Google Scholar]
- 14. Kahn CE, Jr, Roberts LM, Shaffer KA, Haddawy P. Construction of a Bayesian network for mammographic diagnosis of breast cancer. Comput Biol Med 1997;27:19–29.[Epub 1997/01/01]. [DOI] [PubMed] [Google Scholar]
- 15. Lucas PJ, Boot H, Taal BG. Computer‐based decision support in the management of primary gastric non‐Hodgkin lymphoma. Methods Inf Med 1998;37:206–19.[Epub 1998/10/27]. [PubMed] [Google Scholar]
- 16. Berchialla P, Foltran F, Bigi R, Gregori D. Integrating stress‐related ventricular functional and angiographic data in preventive cardiology: a unified approach implementing a Bayesian network. J Eval Clin Pract 2012;15:637–643. [DOI] [PubMed] [Google Scholar]
- 17. Lucas PJ, van der Gaag LC, Abu‐Hanna A. Bayesian networks in biomedicine and health‐care. Artif Intell Med 2004;30:201–14. [DOI] [PubMed] [Google Scholar]
- 18. Muir IFK. On the nature of keloid and hypertrophic scars. Br J Plast Surg 1990;43:61–9. [DOI] [PubMed] [Google Scholar]
- 19. Jensen FV. Bayesian networks and decision graphs. New York: Springer, 2001. [Google Scholar]
- 20. Pearl J. Causality: models, reasoning and inference. Cambridge: Cambridge University Press, 2000. [Google Scholar]
- 21. Glymour C, Cooper GF. Computation, causation and discovery. Cambridge: MIT Press, 1999. [Google Scholar]
- 22. Decision Systems Laboratory – University of Pittsburgh . GeNIe 2.0. URL http://www.sis.pitt.edu/~genie/, 2006[accessed on 20 September 2011].
- 23. Norsys Software Corporation . Netica v3.18. URL http://www.norsys.com/netica.html, 2006. [accessed on 20 September 2011].
- 24. Pearl J. Probabilistic reasoning in intelligent systems: networks of plausible inference. San Mateo: Morgan Kaufmann Publisher, 1991. [Google Scholar]
- 25. Berchialla P, Stancu A, Scarinzi C, Snidero S, Corradetti R, Gregori D. Web‐based tool for injury risk assessment of foreign body injuries in children. J Biomed Inform 2008;41:544–56.[Epub 2008/02/23]. [DOI] [PubMed] [Google Scholar]
- 26. Foltran F, Berchialla P, Giunta F, Malacarne P, Merletti F, Gregori D. Using VLAD scores to have a look insight ICU performance: towards a modelling of the errors. J Eval Clin Pract 2010;16:968–75.[Epub 2010/08/21]. [DOI] [PubMed] [Google Scholar]
- 27. Fu LD, Tsamardinos I. A comparison of Bayesian network learning algorithms from continuous data. AMIA Annual Symposium proceedings/AMIA Symposium; 2005:960. [Epub 2006/06/17]. [PMC free article] [PubMed]
- 28. Cubison TC, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns 2006;32:992–9. [DOI] [PubMed] [Google Scholar]


