Abstract
Diabetic lower extremity wounds cause substantial burden to healthcare systems, costing tens of thousands of dollars per episode. Negative pressure wound therapy (NPWT) devices have been shown to be cost‐effective at treating these wounds, but the traditional devices use bulky electrical pumps that require a durable medical equipment rental‐based procurement process. The Spiracur SNaP™ Wound Care System is an ultraportable NPWT system that does not use an electric pump and is fully disposable. It has superior healing compared to standard of care with modern dressings and comparable healing to traditional NPWT devices while giving patients greater mobility and giving clinicians a simpler procurement process. We used a mathematical model to analyse the costs of the SNaP™ system and compare them to standard of care and electrically powered NPWT devices. When compared to standard of care, the SNaP™ system saves over $9000 per wound treated and more than doubles the number of patients healed. The SNaP system has similar healing time to powered NPWT devices, but saves $2300 in Medicare payments or $2800 for private payers per wound treated. Our analysis shows that the SNaP™ system could save substantial treatment costs in addition to allowing patients greater freedom and mobility.
Keywords: Cost and cost analysis, Diabetic foot ulcers, Negative pressure wound therapy, SNaP system, Wound care
INTRODUCTION
Diabetic lower extremity wounds cause substantial pain and economic costs (1). A 2004 study estimated that diabetic ulcer‐related costs averaged over $13 000 per episode and were close to $30 000 for higher severity ulcers (2), not counting the associated psychosocial, quality of life and lost productivity costs 2, 3. Substantial evidence has been published supporting the use of negative pressure wound therapy (NPWT) as a safe and effective modality in the treatment of diabetic lower extremity wounds, including at least three prospective randomised controlled trials 4, 5, 6, 7. In 2003, Eginton et al. reported in a randomised crossover‐design trial comparing wound healing between conventional moist dressings and NPWT in diabetic foot ulcers (7). They found that NPWT treatment resulted in a significantly greater decrease in wound volume and depth compared to moist gauze dressings (59% versus 0% and 49% versus 8%, respectively) (7). Another randomised control study was published by Armstrong et al. in 2005 in The Lancet (6). This study examined 162 diabetic patients at 18 centres in the USA who had partial foot amputation up to the transmetatarsal level. Subjects were randomly assigned to either NPWT or standard of care. A greater proportion of patients in the NPWT group had healed wounds than the control group (56% versus 39%) by the end of the study (P < 0·040). In addition, they found that NPWT‐treated wounds also healed faster (P < 0·05) with faster granulation tissue formation (P < 0·002). Most recently, Blume et al. published their multicentre randomised controlled trial in 342 diabetic foot wound patients with outcomes that supported the findings of the Armstrong study (5). In this trial, NPWT was compared to advanced moist wound therapy (AMWT) with standard off‐loading therapy as needed. Blume et al. found that a greater proportion of foot ulcers completely healed with NPWT (73 of 169, 43·2%) than with AMWT (48 of 166, 28·9%) within the 112‐day active treatment phase (P = 0·007). The Kaplan–Meier median estimate for 100% wound closure was 96 days (95% CI 75·0–114·0) for NPWT and was not determinable for AMWT (P = 0·001).
Although numerous NPWT systems are available on the market today, including the KCI Wound VAC® (San Antonio, TX) and the Smith and Nephew Renasys® (St Petersburg, FL) systems, these systems have a number of drawbacks. Some of these shortcomings relate to their size and bulk, time‐consuming dressing application process, noise level, the need for an electrical power source, difficult procurement process and associated administrative costs. Outpatient payment typically involves a complex combination of both Medicare part A for home health care and part B for the durable medical equipment rental and supplies 8, 9. In addition, these powered systems were originally designed for very large complex wounds such as complete midline abdominal wound dehiscence or large sarcoma resection sites, and are not ideally suited for use on smaller wounds such as diabetic foot ulcers. According to Margolis et al. who examined data from over 31 000 diabetic neuropathic foot ulcers, the mean and median size of diabetic foot ulcers is only 5·886 and 1·18 cm2, respectively (10). In spite of the compelling evidence for the benefits of NPWT for the treatment of diabetic lower extremity wounds, it is often impractical to treat the smaller sized ulcers in active patients with current bulky electrically powered NPWT systems. This is especially true in the outpatient wound care clinic setting where many of these patients receive care, and procurement of rental‐based NPWT systems is difficult and time‐consuming. These considerations translated into few diabetic ulcer patients receiving therapy with NPWT.
The Spiracur SNaP™ Wound Care System is a novel ultraportable NPWT system. It is intended to have superior healing compared to standard of care incorporating modern dressings and comparable healing to other negative pressure devices while allowing the patient to have greater freedom and mobility. It is small enough to be worn discreetly under clothing and is not electrically powered, so it is silent and does not need to be plugged into an electricity outlet for battery recharging. Instead of a rental‐based model of procurement, the SNaP system is designed to be available off‐the‐shelf for use.
This is a new device with a different payment pattern than other powered negative pressure wound therapies, and because it is so small and portable, it may be used on patients instead of modern dressings in cases where powered negative pressure device use is impractical. We analysed the costs of the Spiracur SNaP™ Wound Care System and compared them to the cost of modern dressings and powered negative pressure devices.
METHODS
We analysed costs and effectiveness for three wound care therapies for diabetic lower extremity wounds: modern dressings, powered negative pressure and the SNaP™ Wound Care System. Because powered negative pressure has different costing for Medicare and private payers, we analysed powered negative pressure under two scenarios: Medicare and private payers. The cost analyses are designed to take into account all healthcare costs.
Because head‐to‐head clinical trials with full economic outcomes do not exist, this analysis takes a decision analytic modelling approach. We use an economic model with peer‐reviewed data to simulate outcomes for treatment with different therapies. The model uses the best available data on each of the therapies and uses a modelling approach to predict the outcomes. This approach is recommended by Gold and Drummond 11, 12. We searched the PubMed database for studies of cost‐effectiveness and NPWT for relevant studies with information on the effectiveness of negative pressure wound therapy. Studies in the NPWT literature examined a wide variety of wound types, compared to different standards of care, and used differing methodologies 2, 3, 4, 5, 6, 7, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24. Additionally, we found articles referenced by KCI, the manufacturer of the leading NPWT devices (25).
To measure costs and effectiveness, we first estimated the proportion of patients expected to heal over a period of 16 weeks. The healing process is modelled as exponential decay of individuals remaining in therapy each week. This is a similar method to another study that examined costs of NPWT, but that study used linear healing rates (16). We believed that the exponential process more closely matches Markov processes used in other comparative effectiveness studies. We chose a 16‐week time horizon because it is astandard for NPWT trials and may be clinically meaningful 5, 6, 19. Most studies of NPWT served as useful background, but we used healing and complication rates from the existing literature on diabetic foot wounds and recent studies of the SNaP™ Wound Care System 5, 6, 17, 19, 20, 22, 26, 27 (Table 1). We assumed equal efficacy between the SNaP™ Wound Care System and powered NPWT devices based on preliminary studies of SNaP™ Wound Care System and ongoing clinical trials 19, 20, 26. Our base case analysis is based on a study directly comparing the SNaP system with modern dressing protocols, but we examine other levels of effectiveness based on other large studies of other powered NPWT devices in sensitivity analysis 3, 5.
Table 1.
Effectiveness parameters *
| Modern dressings | Powered NPWT | SNaP™ Wound Care System | Notes/source | |
|---|---|---|---|---|
| Effects of therapy (over 16 weeks) | ||||
| Percent healed (fraction per 16 weeks) | 35·7% | 83·1% | 83·1% | (19) |
| Amputations (fraction per 16 weeks) | 10·4% | 3·7% | 3·7% | 5, 6, 22 |
| Debridements (fraction per 16 weeks) | 44·7% | 28·6% | 28·6% | (17) |
| Skin grafts (rate per day unhealed) | 0·0219 | 0·0099 | 0·0099 | (19) |
| Osteomyelitis (rate per day unhealed) | 0·15% | 0·15% | 0·15% | (27) |
*‘Fraction per 16 weeks’ is the percentage of patients that heal or experience complications during the 16 weeks of therapy. The ‘rate per day unhealed’ is the rate at which these complications or procedures occur for each day that a patient is not healed. If patient wounds heal, the rate of skin grafts and osteomyelitis is zero. Data from sources were converted to a 16‐week probability or daily rate. Amputation rates are weighted based on the sample sizes from the source studies.
Costs of therapy include direct costs of the therapy and also other healthcare costs for individuals with diabetic lower extremity wounds (Table 2). We calculated average daily, weekly, monthly and bi‐monthly costs for each therapy. Costs are based on the literature comparing NPWT to modern dressings and from Medicare reimbursement rates. These costs were applied to the duration of therapy for the fraction of patients still remaining on therapy. This is a similar method to that used in another NPWT cost study (16), except in that study, all costs were weekly. In addition, the analysis accounts for treatment failure and long‐term costs associated with treatment failure. These long‐term costs were derived from a study of the overall long‐term costs of diabetic lower extremity ulcers (2). When necessary, costs were updated to 2010 dollars using the GDP deflator.
Table 2.
Cost parameters
| Modern dressings | Powered NPWT | SNaP™ Wound Care System * | Timing | Notes | |
|---|---|---|---|---|---|
| Equipment and supplies | |||||
| Strap/holster | – | – | $15 | One‐time | |
| Equipment rental | – | $1553 † | Monthly | Median State payment for HCPCS E2402 § | |
| All‐inclusive daily charge | – | $125 ‡ | – | Daily | Includes cost of pump, dressings and canisters |
| Canister/cartridge | – | $8·67 † | $225 | 3× per week for powered NPWT, 2× per week for SNaP™ Wound Care System | For NPWT, HCPCS A7000 |
| Dressings | 5·66 | 24·91 † | $75 | 3× per week for modern dressings, 3× per week for NPWT, 2× per week for SNaP™ Wound Care System | For modern dressings, AWP DuoDerm® 4′′× 4′′ (32), for NPWT, HCPCS A6550 |
| Dressing changes | |||||
| Home nursing cost | $85 | $85 ‡ | $85 | 2× per week for modern dressings, 2× per week for NPWT, 1× per week for SNaP™ Wound Care System | |
| Home health care | – | $2313 † | – | Bi‐monthly | (28) Required for Medicare reimbursement § |
| Clinic visit cost | $79·05 | $76·29 | $76·29 | Weekly | CPT 15852 for modern dressings and 97605 for NPWT |
| Additional healthcare costs | |||||
| Fraction of patient days inpatient | 10% | 10% | 10% | (17) | |
| Fraction of inpatient days in hospital | 20% | 20% | 20% | ||
| Hospitalisation | $1800 | $1800 | $1800 | Daily | |
| Skilled nursing facility | $250 | $250 | $250 | Daily | 33, 34 |
| Complications | |||||
| Secondary amputation | $7594 | $7594 | $7594 | Per amputation | Average of CPT's 28810, 28820, 28825, (weighted 5%/10%/5%) and DRG's 616, 617, 618 (weighted 10%/10%/60%). |
| Does not include long‐term costs | |||||
| Osteomeylitis | $8647 | $8647 | $8647 | Per episode | DRG's 539, 540, 541, weighted 40%/40%/20% |
| Debridement | $130 | $130 | $130 | Per debridement | CPT codes 97597 and 97598 |
| Skin graft | $1790 | $1790 | $1790 | Per skin graft | Cost of Apligraf®(32), plus average of CPT's 1560, 15610, 15620, 15630, 15760 |
| Long‐term costs | |||||
| Cost per patient with unhealed wound at end of 16 weeks | $14 809 | $14 809 | $14 809 | Per unhealed patient | Based on costs and severity level mix from (2) |
NPWT, negative pressure wound therapy; AWP, average wholesale price; HCPCS, healthcare common procedure coding system; CPT, current procedural terminology; DRG, diagnosis‐related group.
*Costs for SNaP™ Wound Care System strap/holster, cartridge and dressing are estimates provided by Spiracur.
†Only for Medicare reimbursement.
‡Only for private payers.
§Paid even if therapy is only required for a fraction of the period.
Costs are allocated as they would be paid by the payers. For Medicare reimbursement for powered NPWT, some payments are made monthly or bi‐monthly, whether the device or service is used for the full‐time period or a fraction thereof. For example, Medicare pays about $1553 per month for rental of the device, whether the device is used for the full month or a fraction. We model the healing as a weekly exponential decay, so some Medicare powered NPWT patients will have device rental payments during weeks 2, 3 and 4 of a month even if they are healed. Home healthcare payments for Medicare operate in a similar fashion, but based on a 60‐day period. All other costs (SNaP costs, other health care, complications, etc.) are only paid if the patient is still not healed.
For powered NPWT therapy paid by Medicare, patients typically receive home health care. Home healthcare payments have wide variation, depending on the clinical, functional and service utilisation for the patient. This may vary widely for wound care patients. Although payments for individual patients may vary, our objective is to evaluate the effect of treating average wound care patients or a large pool of patients, so we want to use an appropriate average cost. We use the standardised 2010 60‐day episode payment rate of $2312·94 (28).
Using these values for effectiveness and costs, we calculated costs accrued during the 16 weeks of therapy and the total costs per patient including long‐term costs for unhealed patients. The total costs include the costs of 16 weeks of therapy and also include long‐term costs for those patients who do not heal within the 16‐week therapy time window. To obtain those long‐term costs, we take data from a study on the overall ulcer‐related costs of health care per episode (2). These costs include things like pharmacy costs, further therapy, outpatient care, hospital readmissions and further surgical procedures. Those authors break down costs by severity level of the ulcer. The severity level mix may be different for patients who still have an ulcer after 16 weeks of failed treatment. Arguments could be made that patients are more or less severe, so we assume the same severity level mix as in that paper. So, we add $14 809 per patient who does not heal after 16 weeks. We include long‐term costs category to capture the full longer term costs that someone must pay for if the patient's wound does not heal after 16 weeks. Finally, we calculated effectiveness measures such as days in therapy, amputations, debridements, skin grafts, days in hospital and days in skilled nursing facilities during the 16 weeks of therapy.
RESULTS
Base case
Base case results are shown in Table 3 and 1, 2. The SNaP™ Wound Care System has better effectiveness than modern dressings and equal effectiveness to powered negative pressure wound therapy. The SNaP™ Wound Care System saves $9699 (42%) over modern dressings, $2774 (17%) over powered NPWT for a private payer and $2296 (15%) over powered NPWT for Medicare.
Table 3.
Base case results*
| Modern dressings | Powered NPWT | SNaP™ Wound Care System | |
|---|---|---|---|
| Effectiveness | |||
| Fraction healed% | 35·7 | 83·1 | 83·1 |
| Average days in therapy | 87 | 53 | 53 |
| Amputations | 0·10 | 0·037 | 0·037 |
| Debridements | 0·45 | 0·29 | 0·29 |
| Skin grafts | 1·9 | 0·23 | 0·23 |
| Days in hospital | 5·1 | 1·3 | 1·3 |
| Days in skilled nursing facility | 20 | 5·1 | 5·1 |
| Private payer | Medicare | |||
|---|---|---|---|---|
| Costs | ||||
| 16‐Week therapy | $13 557 | $13 651 | $13 173 | $10 878 |
| Total costs | $23 079 | $16 154 | $15 676 | $13 380 |
| Breakdown of total costs | ||||
| Equipment and supplies | $218 | $6779 | $4347 | $4663 |
| Dressing changes | $3102 | $1908 | $3861 | $1250 |
| Additional health care | $4883 | $3037 | $3037 | $3037 |
| Complications | $5353 | $1928 | $1928 | $1928 |
| Long‐term costs | $9522 | $2503 | $2503 | $2503 |
*All numbers are averages per patient treated. Effectiveness results are during 16 weeks of therapy.
Figure 1.
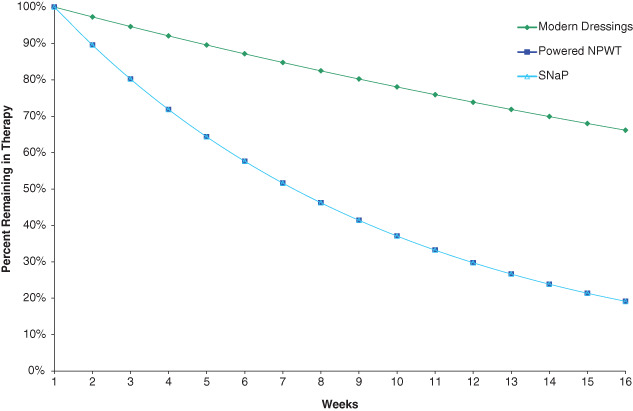
Fraction of patients remaining in therapy over the course of 16 weeks. Projected Kaplan–Meier curve showing the fraction of patients remaining on therapy. The lines compare the three treatment choices. The lines for powered negative pressure and SNaP are superimposed because of equal effectiveness.
Figure 2.
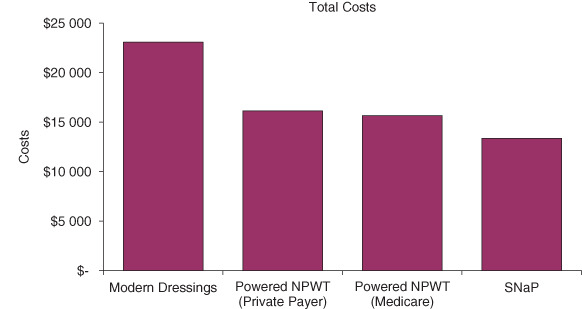
Cost results. NPWT, negative pressure wound therapy.
When examining costs by category, the Spiracur SNaP™ Wound Care System costs $4445 more for the equipment and supplies than modern dressings. But, it saves $1853 in dressing changes, $1846 in additional healthcare costs, $3425 in costs of complications and $7020 in long‐term costs for patients who do not heal. The low cost of the SNaP™ Wound Care System equipment and dressing changes saves costs versus powered NPWT devices. Because the SNaP™ Wound Care System only has two dressing changes per week versus three for powered negative pressure, the SNaP™ Wound Care System saves $659 for a private payer. And, because the SNaP™ Wound Care System does not require bi‐monthly home healthcare payments, it saves $2612 for an average Medicare patient in costs of dressing changes.
Sensitivity of results to assumptions of therapy effectiveness
The base case analysis used healing rate data from a new study of the SNaP™ Wound Care System (19). This study shows very promising results for the SNaP™ Wound Care System versus matched controls using modern dressings. However, larger randomised controlled trials of powered negative pressure therapy versus modern dressings have shown smaller improvements in performance versus modern dressings 5, 6. Of the patients using modern dressings in both of those studies, 37·9% healed within 16 weeks and 53·7% of patients on powered negative pressure therapy healed during that timeframe. If we assume that the negative pressure systems have the same conservative performance, we find that the SNaP™ Wound Care System still saves costs versus modern dressings and powered NPWT (Table 4). It saves $420 versus modern dressings, $3928 versus powered negative pressure for a private payer and $2201 versus powered negative pressure for Medicare.
Table 4.
Results assuming lower performance of negative pressure devices*
| Modern dressings | Powered NPWT | SNaP™ Wound Care System | |
|---|---|---|---|
| Effectiveness | |||
| Fraction healed (%) | 37·9 | 53·7 | 53·7% |
| Average days in therapy | 86 | 73 | 73 |
| Amputations | 0·10 | 0·037 | 0·037 |
| Debridements | 0·45 | 0·29 | 0·29 |
| Skin grafts | 1·9 | 0·25 | 0·25 |
| Days in hospital | 4·7 | 2·9 | 2·9 |
| Days in skilled nursing facility | 19 | 12 | 12 |
| Private payer | Medicare | |||
|---|---|---|---|---|
| Costs | ||||
| 16‐Week therapy | $13 379 | $19 227 | $17 500 | $15 299 |
| Total costs | $22 575 | $26 084 | $24 356 | $22 156 |
*All numbers are averages per patient treated. Effectiveness results are during 16 weeks of therapy.
There is ongoing debate within the wound care community about the effectiveness of NPWT 29, 30. Because of this and because there are few studies of the effectiveness of the SNaP™ Wound Care System, we conducted further analysis to see how the effectiveness of all of these wound therapies would affect total costs. Figure 3 shows how the total costs of the all of the therapies would rise if they were less effective at healing and how costs would drop if they were more effective. For any given level of effectiveness, modern dressings have lower total costs. However, if negative pressure wound therapies are sufficiently more effective, they can have lower overall total costs. The SNaP™ Wound Care System would have to heal about 10–20% more patients over 16 weeks than modern dressings for it to have lower total costs. Powered NPWT would have to heal about 5–15% more patients over 16 weeks than the SNaP Wound Care System for it to have lower total costs.
Figure 3.
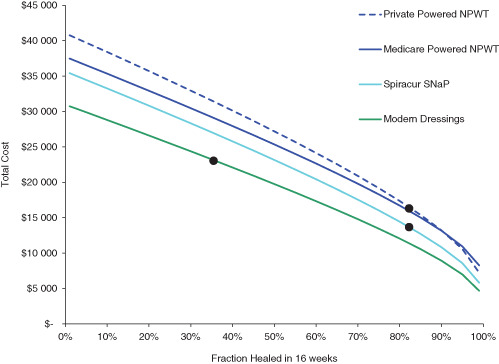
Total costs of therapy as a function of healing effectiveness. Each line represents total costs for a given therapy at a given level of effectiveness (defined as the fraction of patients healed within 16 weeks on therapy). As the therapy effectiveness increases, total costs decrease. The effectiveness used in the base case analysis for each therapy is shown as black dots in the figure.
DISCUSSION
The SNaP™ Wound Care System saves costs over modern dressings because of its superior healing which avoids longer treatment time and the associated costs of treatment and complications. It saves costs versus powered negative pressure devices mainly because it has fewer dressing changes and does not require patients to have home health care. Also, the costs of the equipment and supplies are substantially lower when compared to private payer costs for powered negative pressure wound therapy. Although initial data support rapid healing from the SNaP Wound Care System, our analysis showed that the SNaP™ Wound Care System can save costs versus modern dressings or powered NPWT even if healing is slower, similar to that seen in larger trials of powered negative pressure systems.
Incentives for adopting therapies that are less resource‐intensive may be affected by payment structures. For example, Medicare home healthcare payments are paid per episode, not per nursing visit. So, Medicare does not have an incentive to encourage the use of therapies that reduce the resource utilisation to deliver care if it pays for at‐home nursing based on this payment structure. However, home health care providers may have incentives to encourage the use of therapies that require lower resource utilisation (like the SNaP™ Wound Care System) if they are paid per episode, but have costs based on individual nursing visits.
It is interesting that the most costly treatment regimen is standard of care that uses modern dressings. This is explained in part by the increased utilisation demanded by frequent dressing changes, but, more importantly by the inferior clinical effectiveness, incurring more costs for complication, procedures and hospitalisations and long‐term care. This underscored the need for payers to assess the ‘bottom line’ of cost savings when investing in improved outcomes and quality of care.
This analysis is subject to limitations of the data it uses as inputs. Because the SNaP™ Wound Care System is new, we have less cost and effectiveness data for it than for modern dressings or powered negative pressure wound therapy. Studies to compare the effectiveness of SNaP with other negative pressure therapies are ongoing and we await full results. Future research should gather more cost and effectiveness data on the SNaP™ Wound Care System and incorporate that into future analyses.
This analysis does not account for all costs and effects of the therapies. The cost model assumes that the therapies are used until the patient heals or for 16 weeks, whichever comes first. This type of monotherapy is similar to clinical trials of therapies. However, in real‐life conditions, patients might begin with negative pressure treatment and then transition to less‐expensive modern dressings once the wounds become smaller. We did not account for costs of pain medications during treatment. Additionally, we accounted for the costs of amputation procedures, but not rehabilitation and longer term cost because of amputation. We did not include costs of patients being unable to go back to work. Initial indications show that the SNaP™ Wound Care System may have shorter dressing changes than for powered NPWT devices or modern dressing changes. However, we did not account for savings in those labour costs. Finally, recent research shows that use of powered negative pressure may cause an increase in anxiety. The authors of that study identify restriction of activities as a possible cause of that anxiety (31). The ultraportable SNaP™ Wound Care System may give patients less anxiety than powered negative pressure devices. However, we also did not quantify intangibles like mobility, freedom, pain and anxiety. If these costs and effects were added, the SNaP™ Wound Care System may have additional benefits and cost savings as compared to modern dressings and powered NPWT devices.
The SNaP™ Wound Care System is a promising new wound care technology that should provide healing similar to powered negative pressure devices, but also allow patients greater freedom and mobility. The results of larger trials should provide more precision on exactly how effective the system is. Because of the unique design of the device, it may allow for the practical use of NPWT for patients who otherwise would have only received therapy with modern dressings. This could not only favourably impact patient outcomes, but also result in tremendous healthcare cost savings. This is in addition to the thousands of dollars per patient that could be saved if the SNaP™ Wound Care System was used as a direct substitute for electrically powered NPWT systems or modern dressing protocols.
ACKNOWLEDGEMENTS
DH has received sponsorship of this research from Spiracur, Inc. PS is a consultant for Spiracur.
REFERENCES
- 1. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 2. Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower‐extremity ulcers among patients with diabetes. Diabetes Care 2004;27:2129–34. [DOI] [PubMed] [Google Scholar]
- 3. Wu SC, Armstrong DG. Clinical outcome of diabetic foot ulcers treated with negative pressure wound therapy and the transition from acute care to home care. Int Wound J 2008;5 Suppl 2:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 5. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum‐assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631–6. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 7. Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative‐pressure wound dressings for diabetic foot wounds. Ann Vasc Surg 2003; 17:645–9. [DOI] [PubMed] [Google Scholar]
- 8. LCD for Negative Pressure Wound Therapy Pumps (L27025) . Baltimore (MD): Centers for Medicare & Medicaid Services; 10 p.
- 9. Schaum KD. Medicare Part B negative pressure wound therapy pump policy. A partner for Medicare Part A PPS. Home Healthc Nurse 2002;20:57–8. [DOI] [PubMed] [Google Scholar]
- 10. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: the association of wound size, wound duration, and wound grade on healing. Diabetes Care 2002;25:1835–9. [DOI] [PubMed] [Google Scholar]
- 11. Gold MR. Cost‐effectiveness in health and medicine. New York: Oxford University Press, 1996. [Google Scholar]
- 12. Drummond MF. Drummond MFMfteeohcp. Methods for the economic evaluation of health care programmes. 3rd edn. Oxford/New York: Oxford University Press, 2005. [Google Scholar]
- 13. Expert Working Group. Vacuum assisted closure: recommendations for use. A consensus document. Int Wound J 2008;5 Suppl 4:iii–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwien T, Gilbert J, Lang C. Pressure ulcer prevalence and the role of negative pressure wound therapy in home health quality outcomes. Ostomy Wound Manage 2005;51:47–60. [PubMed] [Google Scholar]
- 15. Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg 2008;95:685–92. [DOI] [PubMed] [Google Scholar]
- 16. Lavery LA, Boulton AJ, Niezgoda JA, Sheehan P. A comparison of diabetic foot ulcer outcomes using negative pressure wound therapy versus historical standard of care. Int Wound J 2007;4:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Apelqvist J, Armstrong DG, Lavery LA, Boulton AJ. Resource utilization and economic costs of care based on a randomized trial of vacuum‐assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg 2008;195:782–8. [DOI] [PubMed] [Google Scholar]
- 18. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–37; discussion 38. [DOI] [PubMed] [Google Scholar]
- 19. Lerman B, Oldenbrook L, Eichstadt SL, Ryu J, Fong KD, Schubart PJ. Evaluation of chronic wound treatment with the SNaP wound care system vs. modern dressing protocols. Plast Reconstr Surg 2010;126:1253–61. [DOI] [PubMed] [Google Scholar]
- 20. Armstrong DG. Comparison of negative pressure wound therapy with the SNaP Wound Care System vs. V.A.C. therapy system for the treatment of chronic lower extremity ulcers: a multicenter randomized controlled trial. Spiracur, Inc Working Paper, 2010. [DOI] [PubMed]
- 21. Moues CM, van den Bemd GJ, Meerding WJ, Hovius SE. An economic evaluation of the use of TNP on full‐thickness wounds. J Wound Care 2005;14:224–7. [DOI] [PubMed] [Google Scholar]
- 22. Frykberg RG, Williams DV. Negative‐pressure wound therapy and diabetic foot amputations: a retrospective study of payer claims data. J Am Podiatr Med Assoc 2007;97:351–9. [DOI] [PubMed] [Google Scholar]
- 23. Etoz A, Ozgenel Y, Ozcan M. The use of negative pressure wound therapy on diabetic foot ulcers: a preliminary controlled trial. Wounds 2004;16:264–9. [Google Scholar]
- 24. McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum‐assisted closure versus saline‐moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Manage 2000;46:28–32, 34. [PubMed] [Google Scholar]
- 25. V.A.C. Therapy Economic Value . Available at URL http://www.kci1.com/cs/Satellite?c=KCI_General_C&childpagename=KCI1/KCILayout&cid=1229624973003&p=1229538260417&packedargs=locale%3Den_US&pagename=KCI1Wrapper [accessed 16 June 2010].
- 26. SNaP Wound Care System Versus Traditional NPWT Device for Treatment of Chronic Wounds . Available at URL http://clinicaltrials.gov/ct2/show/NCT00951080 [accessed 25 March 2010].
- 27. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 28. Medicare Program; Home Health Prospective Payment System Rate Update for Calendar Year 2010; Final Rule . 74 Federal Register Parts 409, 424, and 484 (10 November 2009): 58078–183. [PubMed]
- 29. Gregor S, Maegele M, Sauerland S, Krahn JF, Peinemann F, Lange S. Negative pressure wound therapy: a vacuum of evidence? Arch Surg 2008;143:189–96. [DOI] [PubMed] [Google Scholar]
- 30. Peinemann F, McGauran N, Sauerland S, Lange S. Negative pressure wound therapy: potential publication bias caused by lack of access to unpublished study results data. BMC Med Res Methodol 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keskin M, Karabekmez FE, Yilmaz E, Tosun Z, Savaci N. Vacuum‐assisted closure of wounds and anxiety. Scandinavian J Plast Reconst Surg Hand Surg/Nordisk plastikkirurgisk forening [and] Nordisk klubb for handkirurgi 2008;42:202–5. [DOI] [PubMed] [Google Scholar]
- 32. Red book: pharmacy's fundamental reference. Montvale, NJ: Thomson Healthcare, 2009. [Google Scholar]
- 33. Market survey of long‐term care costs. New York: Metropolitan Life Insurance Company, 2009. [Google Scholar]
- 34. Skilled Nursing Facility Services Payment System . Available at URL http://www.medpac.gov/documents/MedPAC_Payment_Basics_09_SNF.pdf [accessed 25 March 2010].


