Abstract
Cultured dermal substitutes have been used for the treatment of chronic skin ulcers; however, the biological risks of animal‐derived materials in the culture process such as foetal bovine serum (FBS) have been reported. In this study, we prepared an autologous fibroblast‐seeded artificial dermis (AFD) using animal‐product‐free medium supplemented with 2% patient autologous serum and without any animal‐derived materials such as trypsin in the culturing process. We applied the AFD in five patients with diabetic ulcers and investigated its safety and efficacy. As the primary endpoint, we defined ‘wound bed improvement' according to the percentage of granulation area to the whole wound area on day 21, and 60% or higher was regarded as improved. The mean age of the patients was 60·6 years and the mean duration of the ulcer was 22·6 months. In the evaluation of the primary endpoint, the ‘wound bed’ was improved in all patients [proportion of improvement: 100%, 95% confidence interval (CI): 48% to 100%]. Three patients had complete wound healing within 12 weeks after application and two patients had >80% wound healing at 12 weeks. Side effects were not serious. Our AFD may be a safe and effective treatment of diabetic ulcers.
Keywords: Animal‐product‐free medium; Autologous fibroblast‐seeded cultured dermal substitute; Autologous serum; Diabetic foot ulcer
Introduction
Diabetic foot ulcer, mainly due to neuropathy and arterial occlusive disease, is one of the most common complications of diabetic patients and is a leading cause of major amputations of the lower limbs. Throughout the world, more than 250 million people are reported to be suffering from diabetes mellitus and the number still increasing (1). The prevalence of foot ulcers ranges from 4% to 10% among patients with diabetes mellitus 2, 3 with an annual population‐based incidence of 1% to 5% 1, 2, 3, and the lifetime incidence may be as high as 25% 2, 3. More than 15% of all ulcers result in some form of amputation 2, 3. In the treatment of diabetic foot ulcers, it is necessary to evaluate and improve the blood supply, if needed, to reduce the ulcer and to perform appropriate local ulcer treatment 1, 2, 3. Recently, several new wound therapies have been developed based on the understanding of the wound‐healing process and cell technology 4, 5. Among these new therapies, tissue‐engineered skin substitutes have made marked advances in the field of wound healing and the treatment of chronic wounds such as diabetic ulcers 6, 7, 8, 9. Generally, most of the cells used for these skin substitutes are derived from allogeneic neonatal foreskin, and a cocktail of cytokines and growth factors secreted from living skin substitutes is considered to promote wound healing 8, 9, 10.
We developed bilayered acellular artificial skin composed of an upper silicone sheet and a lower collagen sponge (11) by modifying the material described by Yannas and Burke (12). In our previous study, we reported that an autologous fibroblast‐seeded artificial dermis (AFD) promoted the epithelisation of wounds and prevented wound contraction compared with an allogeneic fibroblast‐seeded collagen sponge (13). In the conventional culturing process of cultured skin substitutes, foetal bovine serum (FBS) has been used as a nutrient source 8, 9; however, the use of FBS increases safety concerns because it might transmit prion or viral diseases 14, 15 and cause unfavourable immune and local inflammatory reactions to bovine proteins (16). In our previous study, we reported that animal‐product‐free medium (HFDM‐1; Cell Science & Technology Institute, Inc., Miyagi, Japan), a animal component‐free medium for human fibroblasts, supplemented with 2% autologous serum could expand autologous primary fibroblasts and those after seeding to collagen sponges as well as Dulbecco's modified eagle medium (DMEM) with 10% FBS (17).
In this study, we prepared AFD cultured using HFDM‐1 supplemented with 2% patient autologous serum and without using animal‐derived materials such as trypsin in the culturing process. We then investigated the safety and efficacy of our AFD in the treatment of diabetic ulcers.
Materials and methods
Study design
This study was a prospective, open‐labelled, proof‐of‐concept clinical trial conducted in Kyoto University Hospital. Patient registration and data management were performed in an independent data centre at the Translational Research Center, Kyoto University Hospital. The study protocol was approved by the ethics committee of Kyoto University Graduate School and Faculty of Medicine and was conducted according to the Declaration of Helsinki.
The eligibility criteria were chronic (at least 1‐month duration), non‐healing (without granulation tissue and skin graft not expected to take), full‐thickness, lower extremity ulcers resulting from diabetes mellitus, at least 20 years of age and adequate arterial blood supply assessed by measuring skin perfusion pressure (SPP) of 25 mmHg or greater at a site of 1 cm proximal or distal to those ulcers. Written informed consent was obtained from all the patients.
The exclusion criteria were systemic infection including hepatitis B, hepatitis C, human immunodeficiency virus and syphilis; ulcers with local infection based on clinical examination, ulcer larger than 70 × 50 mm2; ulcers with large tendon or bone exposure; osteomyelitis and cellulitis; malignant disease; uncontrolled diabetes mellitus (defined by HbA1c ≥12%) or requiring continued use of oral corticosteroid therapy (> 5 mg/day prednisolone equivalent); patients receiving immunosuppressive agents; pregnant women; nursing mothers and patients with hypersensitivity to any component of AFD or local anaesthetics and antiseptics.
Preparation of autologous fibroblast‐seeded AFD
After enrolment, a skin biopsy (1 cm × 5 mm) and 20 ml blood were taken from each patient. All cell‐processing procedures for autologous fibroblasts and fibroblast‐seeded AFD were performed in the Center for Cell and Molecular Cell Therapy, which is a cell‐processing laboratory following current good manufacturing practices in Kyoto University Hospital. Patient autologous serum was also prepared, and blood was taken again if needed. In the culturing process, no animal‐derived material was used. The skin specimens were cut into small pieces with surgical scissors, placed in a 10‐cm tissue culture dish and cultured using HFDM‐1 supplemented with 2% autologous serum. Outgrowing fibroblasts were dissociated using TripLE Select (Life Technologies Co., Carlsbad, CA) and passaged. The fibroblasts were seeded onto AFD (Pelnac S size: 82 × 60 mm2; Gunze Ltd., Kyoto, Japan) at a density of 1·0 × 105 cells/cm2 and cultured for 10 days before grafting.
Autologous graft treatment and subsequent therapy
The ulcer was surgically debrided to remove necrotic tissue and chronic granulation tissue. AFD was then cut according to the shape of the wound, grafted onto the debrided wound and sutured to the marginal skin. AFD was covered with non adherent paraffin and framycetin gauze (Sofratulle; Sanofi‐Aventis K.K., Tokyo, Japan) and sterile cotton pads and gauze. This study used a single application of AFD. After the application of AFD, dressings were changed as necessary. No topical agent accelerating wound healing, such as basic fibroblast growth factor (bFGF) spray (18) (bFGF spray and Fibrast spray; Kaken Pharmaceutical, Tokyo, Japan), was applied until day 21 after AFD application. The use of antibiotic ointment and dressing was allowed. Patients were hospitalised until day 21 to ensure stabilisation of the applied AFD. On day 21 after application, the sutures and silicone sheet of AFD were removed and subsequent therapy including skin graft and any topical agent could be started. Patients were followed up until 12 weeks after application to observe the adverse events related to the application of AFD.
Evaluation of treatment
Using a digital camera, digital images of the wounds were taken with a calibrator (CASMATCH; BEAR Medic Corp., Tokyo, Japan) placed on the skin adjacent to the wound at enrolment, before grafting, just after grafting and 3, 5, 7, 9 and 12 weeks after grafting. The colour and size of the images were adjusted using CASMATCH and image editing software (Adobe Photoshop CS4; Adobe Systems Inc., CA) to assess the wound and granulation areas. As with the primary endpoint, the granulation area on day 21 was independently measured under blinding by a central review.
The primary endpoint in this study was ‘wound bed improvement on day 21’. Granulation tissue is a wound connective tissue that is formed at the beginning of wound healing (19). This highly fibrous tissue is usually pink in colour because numerous small capillaries invade granulation tissue to supply oxygen and nutrients. The appearance of granulation tissue is a good sign of healing because it means that the healing process of the wound is starting 19, 20, 21, 22. The area of granulation tissue was measured in this study, and the percentage of wound bed improvement was defined as the value (%) calculated from the granulation areas on day 21 divided by the wound area on day 21 multiplied by 100, and the wound bed was regarded as improved when the value was 60% or higher. Although a value of 50% or more as the cut‐off for wound bed improvement was proposed by the Japanese Society of Pressure Ulcers 21, 22, 23, we used 60% as a stricter cut‐off value because it is sufficient to show that the wound has begun to heal.
The secondary endpoints were the area of target ulcers on day 21, time to complete wound closure, time to skin grafting, area of the skin graft after 2 and 4 weeks and adverse events.
Statistical analysis
This study was designed to test the hypothesis that the wound bed improvement rate on day 21 is >5%. We calculated that 11 patients were required for a power of 90% with a one‐sided alpha of 5% based on the one‐sided binomial test with a null hypothesis of 5% and alternative hypothesis of 35%. Two interim analyses were planned using five and eight patients for safety and efficacy. Changes in the mean wound area from just after application of AFD to day 21 were examined by the paired t‐test. All patients were included in the efficacy and safety analyses. All data management and statistical analysis were conducted in the Department of Clinical Trial Design and Management, Translational Research Center, with the use of SAS version 9.2 (SAS Institute, Cary, NC).
Results
Five patients with diabetic ulcers were enrolled in the study between September 2008 and December 2009 and included in the first interim analysis. The baseline data of patients, outcome of the primary endpoint and wound healing at 12 weeks are shown in Table 1. All patients were male and the mean (±SD, standard deviation) age was 60·6 (±11·1) years. The mean duration of ulcer was 22·6 (±26·6) months. In all patients, AFD can be produced using HFDM‐1 supplemented with 2% autologous serum. All patients received a single application of AFD and were followed up until 12 weeks after application. In the evaluation of the primary endpoint, granulation tissue had formed over >70% of the wound area on day 21 and the ‘wound bed’ was improved in all five patients [proportion of improvement: 100%, 95% confidence interval (CI): 48% to 100%]. This number of patients with wound bed improvement exceeded the number required for significance in the planned final analysis of 11 patients. Regarding a secondary endpoint, the mean wound area (3·42 ± 2·36 cm2) on day 21 was significantly reduced compared with the area (7·07 ± 4·92 cm2) just after application of AFD (P = 0·03). The wounds of three patients healed completely during the trial (proportion of complete wound closure: 60%, 95% CI: 15% to 95%). Only one patient underwent skin grafting on day 29 and the area of the skin graft was 46% at 2 weeks and 17% at 4 weeks.
Table 1.
Baseline data of patients and outcome
| Case | Age | Sex | Complications | Lesions | Before application | 21 days after application | 12 weeks after application | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration of ulcer (months) | Area of ulcer (cm2) | SPP (mmHg) | Area of ulcer (cm2) | Granulated area (cm2) | Wound improvement (%) | Healing | ||||||
| 1 | 51 | M | CRF on HD, DR | Right foot | 75 | 7.46 | 104.5 | 53.88 | 2.7 | 70 | Improved | 90% Reduction of wound size with skin graft |
| 2 | 65 | M | CRF on HD, PMR, DR | Left foot | 16 | 0.7 | 62 | 0.3 | 0.3 | 100 | Improved | Complete wound closure without skin graft |
| 3 | 47 | M | CRF on HD, DR | Left foot | 5 | 9.2 | 59.5 | 5.6 | 4.6 | 82 | Improved | 80% Reduction of wound size with skin graft |
| 4 | 67 | M | CRF, DR | Right foot | 13 | 4.3 | 75 | 1.1 | 0.9 | 82 | Improved | Complete wound closure without skin graft |
| 5 | 73 | M | CRF, DR, OMI | Left foot | 4 | 13.7 | 74.5 | 6.2 | 4.8 | 77 | Improved | Complete wound closure without skin graft |
| Mean 60·6 | 22.6 | 7.07 | 7.51 | 3.42 | 2.66 | 82.2 | ||||||
SPP, skin perfusion pressure; DM, diabetes mellitus; CRF, chronic renal failure; HD, haemodialysis; PMR, polymyalgia rheumatica; DR, diabetic retinopathy; OMI, old myocardial infarction.
There were 30 adverse events in the five patients. One serious adverse event (reflux oesophagitis) was regarded as not being related to AFD. The most frequent adverse events included hypoglycaemic attack, nausea and vomiting. Two adverse events (one wound infection and one wound pain) may have been related to the products used in the study, but they recovered quickly.
Interim analyses of the five patients showed that the AFD was safe, with promising efficacy, and we therefore stopped this study.
Case presentations
A 51‐year‐old man (case 1) with a diabetic ulcer on his right heel was enrolled in this trial (Figure 1A). The duration of his ulcer was 75 months and SPP at a site 1‐cm proximal to the ulcer was 105 mmHg. Skin (10× 5 mm2) from the groin region and 20 ml of blood were taken, and autologous fibroblasts and AFD were prepared. In the culturing process, 11 ml of autologous serum was used and the culture period was 46 days. The necrotic tissue was debrided three times under local anaesthesia and after the third debridement, AFD was applied to the wound (Figure 1B). Twenty‐one days after application, the granulation area was evaluated as 70% of the wound area and the ‘wound bed’ was regarded as improved (Figure 1C). Four weeks after application, full‐thickness skin was grafted (Figure 1D) and the central part of the graft covered the wound bed (Figure 1E). This ulcer had healed nearly 12 weeks after application (Figure 1F).
Figure 1.
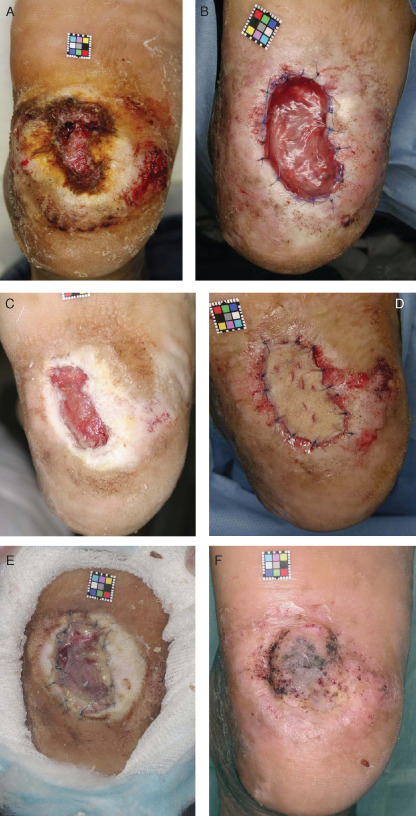
A case of diabetic ulcer of the right heel (case 1). (A) The ulcer area was 7·46 cm2. (B) AFC was applied and sutured to the marginal skin after the third surgical debridement. (C) By 21 days after application, granulated tissue had formed in 70% of the wound area. (D) 28 days after application, a full‐thickness skin graft was applied. (E) By 14 days after skin grafting, the central part of the graft had taken on the wound bed. (F) 12 weeks after application, the ulcer was nearly healed.
A 67‐year‐old man (case 4) had a stump ulcer for 13 months after right transmetatarsal amputation because of gangrene (Figure 2A). SPP at a site 1 cm proximal to the ulcer was 75 mmHg. In the culturing process in this case, the amount of autologous serum was 11 ml and culture period was 46 days. After debridement, AFD was applied (Figure 2B). Twenty‐one days after application, the granulated area was evaluated as 82% of the wound area and the ‘wound bed’ was regarded as improved (Figure 2C). This ulcer healed completely 77 days after application (Figure 2D).
Figure 2.
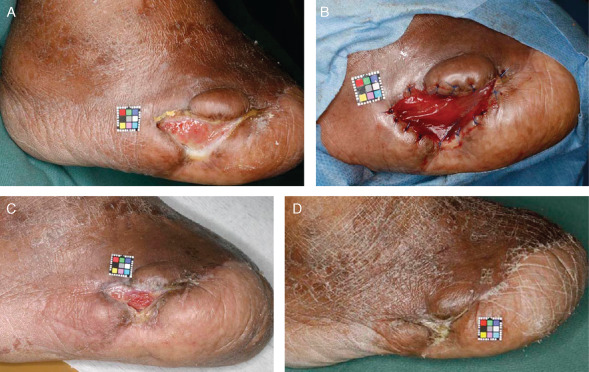
A case of stump ulcer after transmetatarsal amputation (Case 4). (A) The ulcer area was 4·3 cm2. (B) Artificial dermis was applied and sutured to the marginal skin after debridement. (C) By 21 days after application, the ulcer was filled with granulation tissue. (D) By 9 weeks after application, the ulcer had healed completely.
Discussion
A bilayered living human skin equivalent (Apligraf; Organogenesis Inc., Canton, MA) and a human fibroblast‐derived dermal substitute (Dermagraft; Advanced BioHealing Inc., La Jolla, CA) are representative bioengineered products and have been reported to be effective for the treatment of diabetic foot ulcers 8, 9, 24. Autologous dermal and epidermal tissue‐engineered grafts (Hyalograft 3D autograft and Laserskin autograft, respectively; Anika Therapeutics srl, Abano Terme, Italy) have been used to treat chronic ulcers, and it was reported that a 50% reduction in the diabetic ulcer area was achieved significantly faster in the treatment group (6). The complete ulcer healing rate at 12 weeks was reported to be 24% to 56% 24, 25, 26, 27, 28, 29. In this study, three of the five patients showed complete healing at 12 weeks, indicating that the effectiveness of our AFD was comparable with that of skin equivalent products.
As the primary endpoint in this study, we defined ‘wound bed improvement on day 21’ according to the granulation area. Skin equivalent products are usually applied once or twice a week until complete wound healing. In this study, AFD was applied only once in consideration of safety because this was the first clinical trial of our AFD; therefore, we needed to assess whether hard‐to‐heal ulcers would heal as do acute wounds. We assessed all cases as ‘wound bed’ improved and 60% healed within 12 weeks, so this index may be used to predict the probability of wound healing.
FBS has been used in the culture process of conventional products of dermal substitutes. Allogeneic human serum can be used for the culture of fibroblasts as an alternative to FBS, but the possibility of disease transmission and immune reactions remains; therefore, the use of patient autologous serum is desirable to avoid the risk of problems associated with FBS. Diabetic patients usually have systemic complications such as a poor nutritional condition or chronic renal failure, and it is frequently difficult to take a large amount of autologous serum. In this study, the average amount of autologous serum needed for the preparation of AFD was 11·6 ml and this amount would not be an issue in diabetic patients, even those on haemodialysis.
Considerable efforts have been made to eliminate animal‐derived materials from the culturing process, and several animal‐product‐free media for mesenchymal stem cells have been reported (30). An animal‐product‐free medium for fibroblast cultivation may be available in the near future.
In conclusion, we prepared autologous fibroblast‐seeded AFD using HFDM‐1 supplemented with 2% autologous serum without using animal‐derived materials and applied it in the treatment of five diabetic ulcers. In this study, side effects were not serious and three patients were completely healed within 12 weeks after application. Although this study was a preliminary clinical trial with a small sample size, our dermal substitutes may provide a safe and effective treatment for diabetic ulcers.
References
- 1. Vuorisalo S , Venermo M , Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg 2009. ; 50 : 275 – 91. Review. [PubMed] [Google Scholar]
- 2. Singh N , Armstrong DG , Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005. ; 293 : 217 – 28. [DOI] [PubMed] [Google Scholar]
- 3. Wu SC , Driver VR , Wrobel JS , Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manag 2007. ; 3 : 65 – 76. [PMC free article] [PubMed] [Google Scholar]
- 4. Game FL , Hinchliffe RJ , Apelqvist J , Armstrong DG , Bakker K , Hartemann A , Löndahl M , Price PE , Jeffcoate WJ. A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev . 2012 ; 28 ( Suppl 1 ): 119 – 41. [DOI] [PubMed] [Google Scholar]
- 5. Priya SG , Jungvid H , Kumar A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng Part B Rev 2008. ; 14 ( 1 ): 105 – 18. Review. [DOI] [PubMed] [Google Scholar]
- 6. Uccioli L , Giurato L , Ruotolo V , Ciavarella A , Grimaldi MS , Piaggesi A , Teobaldi I , Ricci L , Scionti L , Vermigli C , Seguro R , Mancini L , Ghirlanda G. Two‐step autologous grafting using HYAFF scaffolds in treating difficult diabetic foot ulcers: results of a multicenter, randomized controlled clinical trial with long‐term follow‐up. Int J Low Extrem Wounds 2011. ; 10 : 80 – 5. [DOI] [PubMed] [Google Scholar]
- 7. Ehrenreich M , Ruszczak Z. Update on tissue‐engineered biological dressings. Tissue Eng 2006. ; 12 : 2407 – 24. Review. [DOI] [PubMed] [Google Scholar]
- 8. Marston WA , Hanft J , Norwood P , Pollak R. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003. ; 26 : 1701 – 5. [DOI] [PubMed] [Google Scholar]
- 9. Cavorsi J , Vicari F , Wirthlin DJ , Ennis W , Kirsner R , O'Connell SM , Steinberg J , Falanga V. Best‐practice algorithms for the use of a bilayered living cell therapy (Apligraf) in the treatment of lower‐extremity ulcers. Wound Repair Regen 2006. ; 14 : 102 – 9. Review. [DOI] [PubMed] [Google Scholar]
- 10. Wong T , McGrath JA , Navsaria H. The role of fibroblasts in tissue engineering and regeneration. Br J Dermatol 2007. ; 156 : 1149 – 55. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki S , Kawai K , Ashoori F , Morimoto N , Nishimura Y , Ikada Y. Long‐term follow‐up study of artificial dermis composed of outer silicone layer and inner collagen sponge. Br J Plast Surg 2000. ; 53 : 659 – 66. [DOI] [PubMed] [Google Scholar]
- 12. Yannas IV , Orgill DP , Burke JF. Template for skin regeneration. Plast Reconstr Surg 2011 ; 127 ( Suppl 1 ): 60S – 70S. Review. [DOI] [PubMed] [Google Scholar]
- 13. Morimoto N , Saso Y , Tomihata K , Taira T , Takahashi Y , Ohta M , Suzuki S. Viability and function of autologous and allogeneic fibroblasts seeded in dermal substitutes after implantation. J Surg Res 2005. ; 125 : 56 – 67. [DOI] [PubMed] [Google Scholar]
- 14. Tonti GA , Mannello F. From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera? Int J Dev Biol 2008. ; 52 : 1023 – 32. Review. [DOI] [PubMed] [Google Scholar]
- 15. Stute N , Holtz K , Bubenheim M , Lange C , Blake F , Zander AR. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol 2004. ; 32 : 1212 – 25. [DOI] [PubMed] [Google Scholar]
- 16. Tuschong L , Soenen SL , Blaese RM , Candotti F , Muul LM. Immune response to fetal calf serum by two adenosine deaminase‐deficient patients after T cell gene therapy. Hum Gene Ther 2002. ; 13 : 1605 – 10. [DOI] [PubMed] [Google Scholar]
- 17. Morimoto N , Takemoto S , Kanda N , Ayvazyan A , Taira MT , Suzuki S. The utilization of animal product‐free media and autologous serum in an autologous dermal substitute culture. J Surg Res 2011. ; 171 : 339 – 46. [DOI] [PubMed] [Google Scholar]
- 18. Uchi H , Igarashi A , Urabe K , Koga T , Nakayama J , Kawamori R , Tamaki K , Hirakata H , Ohmura T , Furue M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol 2009. ; 19 : 461 – 8. [DOI] [PubMed] [Google Scholar]
- 19. Shaw TJ , Martin P. Wound repair at a glance. J Cell Sci 2009. ; 122 : 3209 – 13. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin P. Wound healing–aiming for perfect skin regeneration. Science 1997. ; 276 : 75 – 81. [DOI] [PubMed] [Google Scholar]
- 21. Sanada H , Iizaka S , Matsui Y , Furue M , Tachibana T , Nakayama T , Sugama J , Furuta K , Tachi M , Tokunaga K , Miyachi Y ; Scientific Education Committee of the Japanese Society of Pressure Ulcers. Clinical wound assessment using DESIGN‐R total score can predict pressure ulcer healing: pooled analysis from two multicenter cohort studies. Wound Repair Regen 2011. ; 19 : 559 – 67. [DOI] [PubMed] [Google Scholar]
- 22. Sanada H , Moriguchi T , Miyachi Y , Ohura T , Nakajo T , Tokunaga K , Fukui M , Sugama J , Kitagawa A. Reliability and validity of DESIGN, a tool that classifies pressure ulcer severity and monitors healing. J Wound Care 2004. ; 13 : 13 – 8. [DOI] [PubMed] [Google Scholar]
- 23. Matsui Y , Furue M , Sanada H , Tachibana T , Nakayama T , Sugama J , Furuta K , Tachi M , Tokunaga K , Miyachi Y. Development of the DESIGN‐R with an observational study: an absolute evaluation tool for monitoring pressure ulcer wound healing. Wound Repair Regen 2011. ; 19 : 309 – 15. [DOI] [PubMed] [Google Scholar]
- 24. Langer A , Rogowski W. Systematic review of economic evaluations of human cell‐derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res . 2009. ; 9 : 115. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veves A , Falanga V , Armstrong DG , Sabolinski ML ; Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001. ; 24 : 290 – 5. [DOI] [PubMed] [Google Scholar]
- 26. Brem H , Balledux J , Bloom T , Kerstein MD , Hollier L. Healing of diabetic foot ulcers and pressure ulcers with human skin equivalent: a new paradigm in wound healing. Arch Surg 2000. ; 135 : 627 – 34. [DOI] [PubMed] [Google Scholar]
- 27. Curran MP , Plosker GL. Bilayered bioengineered skin substitute (Apligraf): a review of its use in the treatment of venous leg ulcers and diabetic foot ulcers. BioDrugs 2002. ; 16 : 439 – 55. Review. [DOI] [PubMed] [Google Scholar]
- 28. Dinh TL , Veves A. The efficacy of Apligraf in the treatment of diabetic foot ulcers. Plast Reconstr Surg 2006. ; 117 ( 7 Suppl ): 152S – 157S. Review. [DOI] [PubMed] [Google Scholar]
- 29. Marston WA , Hanft J , Norwood P , Pollak R. Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003. ; 26 : 1701 – 5. [DOI] [PubMed] [Google Scholar]
- 30. Miwa H , Hashimoto Y , Tensho K , Wakitani S , Takagi M. Xeno‐free proliferation of human bone marrow mesenchymal stem cells. Cytotechnology 2012. ; 64 : 301 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]


