Abstract
Chronic venous leg ulcers are a major health issue and represent an often overlooked area of biomedical research. Nevertheless, it is becoming increasingly evident that new approaches to enhance healing outcomes may arise through better understanding the processes involved in the formation of chronic wounds. We have for the first time shown that the terminal purine catabolite uric acid (UA) is elevated in wound fluid (WF) from chronic venous leg ulcers with relative concentrations correlating with wound chronicity. We have also shown a corresponding depletion in UA precursors, including adenosine, with increased wound severity. Further, we have shown that xanthine oxidase, the only enzyme in humans that catalyses the production of UA in conjunction with a burst of free radicals, is active in chronic WF. Taken together, this provides compelling evidence that xanthine oxidase may play a critical role in the formation of chronic wounds by prolonging the inflammatory process.
Keywords: Chronic venous leg ulcers, Purines, Uric acid, Wound fluid and xanthine oxidase
INTRODUCTION
Chronic leg ulcers affect 1–3% of the elderly resulting in long‐term pain, immobility and decreased quality of life for a large proportion of sufferers (1, 2, 3). Venous stasis leg ulcers are the most frequently encountered chronic wounds in the clinical setting (4). The healing process in venous leg ulcers is typically persistent with median durations ranging from 6 (5) to 64 months (6) and an average duration of 12–13 months (7, 8). Most patients suffer from the condition for 15 or more years and 24% are hospitalised as a direct result of their ulcers (7).
Compression therapy is the primary form of treatment and prevention of venous leg ulcers. This form of treatment is critical given that these patients suffer with chronic venous insufficiency. However, this approach requires specialised expertise to apply effectively and many patients find the bandaging extremely uncomfortable leading to non compliance, which consequently prolongs healing time (9, 10). In addition, up to 15–30% of chronic venous leg ulcers do not respond to compression and remain unhealed, even after a year of treatment (11). Currently, there is an unmet need for an effective, low‐cost therapeutic and diagnostic test for chronic venous leg ulcers. Nevertheless, it is becoming increasingly evident that new approaches to enhance healing outcomes may arise through better understanding the processes involved in the formation and progression of chronic wounds.
Cell rupture as a consequence of mechanical or chemical disturbance results in the release of adenosine triphosphate (ATP) into the extracellular environment (12). ATP breaks down in a stepwise fashion to adenosine monophosphate, inosine monophosphate, adenosine, inosine and hypoxanthine, resulting in the accumulation of these metabolites in tissue (13). The final reactions in this pathway are the conversion by xanthine oxidase (XO) of hypoxanthine to xanthine, and subsequently xanthine to uric acid (UA), while simultaneously releasing the toxic superoxide radical (Figure 1). These metabolites have been extensively investigated in many disease states (14, 15, 16, 17) with elevated levels of hypoxanthine and xanthine in cerebrospinal fluid and serum regarded as reliable early indicators of inflammatory and ischaemic tissue damage (15, 16, 17). However, no comprehensive profiling of these small heterocyclic metabolites has been reported for chronic wounds.
Figure 1.
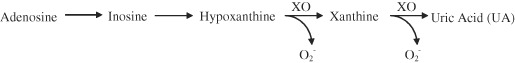
Purine catabolic pathway. Depletion of ATP culminating with the production of uric acid (UA) and superoxide (O ).
).
Xanthine oxidoreductase (XOR) is the only physiological enzyme in humans capable of catalysing the production of UA (18). A complex molybdo‐flavoenzyme, XOR is a homodimer with a molecular weight of approximately 150 kDa (19). XOR exists in two inter‐convertible forms, a dehydrogenase (XDH) which is predominant in normal tissues, and a proteolytically generated oxidase form (XO) that is present during tissue injury (18, 20). Unlike XDH, XO is unable to bind NAD+ and instead, consumes molecular oxygen as its primary electron donor liberating the toxic superoxide radical (18, 20). Superoxide itself is unstable, rapidly converting to form hydrogen peroxide (H2O2), either spontaneously or enzymatically through superoxide dismutase. H2O2 readily diffuses across membranes combining with metal ions like iron, generating the highly reactive hydroxyl radical (21, 22, 23). Sustained formation of oxidants may disturb the delicate redox balance within the wound environment exacerbating tissue injury (24).
Recent studies in our laboratory have shown an atypical accumulation of purine metabolites, in particular UA, in wound fluid (WF) collected from patients with chronic venous leg ulcers. To further investigate this, we established a sensitive and specific method for analysing purine metabolites in WF. We provide evidence that these purinogenic metabolites have enhanced turnover in clinically worse ulcers and that XO levels are increased with wound severity. From these findings we propose that XO may be a key contributor to persistent inflammation and that therapeutic inhibition of XO may restore wound healing.
MATERIALS AND METHODS
Patient recruitment and clinical data collection
Patients with chronic venous leg ulcers were recruited from three clinical sites in Brisbane, QLD; the clinics at the Princess Alexandra Hospital, the Royal Brisbane Hospital and Spiritus (formerly St Luke's Nursing Services). Ethical approval was obtained from the relevant institutions prior to the commencement of the study. The study was conducted according to Declaration of Helsinki principles and written informed consent was obtained from all patients before enrolment. Patients with a venous leg ulcer were diagnosed by the attending clinicians in charge of the wound clinics or by the physician who referred the patients to a community nursing service for care. Patients were eligible to partake in the study if they had an ankle‐brachial pressure index ≥0·8 and <1·3 and excluded if they had ulcers of non venous aetiology, and were immobilised (completely bed or wheelchair bound) or exhibited clinical signs of infection. It is acknowledged that even in the absence of clinical signs of infection there may be underlying bacterial bioburden; however, only ulcers exhibiting clinical signs of infection were excluded from the study. Ulcer assessment data, including ulcer characteristics, such as ulcer area, duration and the type of external compression therapy applied after treatment, were collected. A standardised approach was used for wound measurement which involved tracing the edge of the ulcer onto a plastic sheet and determining the area by planimetry using Visitrak™ (Smith and Nephew, Hull, UK). Wounds were also assigned a pressure ulcer scale of healing (PUSH) score to provide a more sensitive measure of healing, rather than examining area alone (25).
WF collection technique
WF was collected using a standard established collection technique which involves the aspiration of fluid from underneath an occlusive dressing (26). Briefly, the ulcers were washed with sterile water followed by the application of an occlusive dressing (Opsite, Smith and Nephew, Hull, UK) over the wound. WF was allowed to accumulate under the dressing and was recovered by washing the wound with approximately 1 ml of saline. WF was then centrifuged at 14 000 g for 10 minutes and stored at −80°C until further analysis. Protein concentrations of each WF sample were estimated using the Coomassie Plus Bradford Protein assay kit (Thermo Scientific, Rockford, IL). Samples were measured at 595 nm in a Bio‐Rad UV‐Visible Benchmarkplus microplate spectrophotometer (Bio‐Rad, Hercules, CA) and protein concentration determined against a bovine serum albumin standard curve (0·1–1 mg/ml).
HPLC/MRM detection of purine metabolites in WF
Relative concentrations of purine metabolites, adenosine, inosine, hypoxanthine, xanthine and UA in WF were quantified using a combination of high performance liquid chromatography (HPLC) with tandem Mass Spectrometry (MS) and multiple reaction monitoring (MRM). Briefly, a Polaris C18 analytical column 5 µm, 250 × 4·6 mm i.d (Varian) was used to fractionate purine metabolites in standard and sample solutions. The mobile phase consisted of 10 mM ammonium acetate pH 4·7 (buffer A); and buffer B, containing a 1:1 mixture of buffer A and methanol. Adenosine, inosine, hypoxanthine, xanthine and UA standards (Sigma Aldrich) and WF samples were injected and analysed in triplicate using a fully automated UltiMate 3000 nano, capillary and micro LC system (Dionex). The purine metabolites were eluted using a linear gradient from 0 to 100% buffer B over 5 minutes at a flow rate of 0·5 ml/min. MRM was performed using a 4000 QTRAP LC/MS/MS system (Applied Biosystems), a triple quadrupole/linear ion trap mass spectrometer, commonly used for metabolite identification (27). Analyses were performed using an ionspray voltage of 4·5 kV, the turbo spray temperature of 45°C and nitrogen was used as the collision gas.
Detection of xanthine oxidase activity in WF
XO enzyme activity was determined by concentrating pooled WF by ultracentrifugation using nanosep 3 K omega filters (PALL, Pensacola, FL) at 10 000 g for 10 minutes at 4°C. The retentate was resuspended with 10 mM Tris–HCl buffer, pH 8·0, containing either 10 µmol/l of xanthine or allopurinol, a specific inhibitor of XO, then incubated at 37°C for 2·5 hours in the ultrafiltration unit. Reaction mixtures were reprocessed on nanosep 3 K omega filters and the resulting filtrates were analysed using RP‐HPLC. Briefly, the filtrates were analysed on a Polaris® C18 analytical column 5 µm, 250 × 4·6 mm i.d. (Varian, Palo Alto, CA) using the BioLogic Duoflo chromatography system (Bio‐Rad, Hercules, CA) with UV detection at 254 and 280 nm. The mobile phase was 40 mmol/l KH2PO4 (potassium phosphate) buffer, pH 2·2 containing 2% methanol. The compounds were eluted using an isocratic flow rate maintained at 0·5 ml/min. The column was regenerated between runs using 80% acetonitrile/40 mmol/l potassium phosphate, pH 2·2 buffer.
Western blot analysis of xanthine oxidase in WF
The presence of XO in WF from ulcers with varying wound severity was determined by Western blotting using a commercially available anti‐XOR polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). This method carries high specificity that is necessary for the accurate detection of XO. In brief, WF samples containing 10 µg of total protein were subjected to reducing sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) (NuPAGE 4–12% gradient gels, Invitrogen Carlsbad, CA) before being transferred to nitrocellulose membranes (PALL). The membranes were blocked at room temperature in 5% BSA (Calbiochem, San Diego, CA) in Tris‐buffered saline containing 0·1% Tween‐20 (TBST) for approximately 10 minutes. Subsequently, the membranes were incubated with primary antibody (1:100) for 1 hour at room temperature and washed in 0·5% BSA in TBST before incubation with a secondary antibody (1:10 000, R&D Systems) for 30 min. After further washes the membranes were exposed to enhanced chemiluminesence (GE Healthcare, Buckinghamshire, UK) for 5 minutes. The signal was detected by exposing membranes to X‐ray film (FujiFilm Corporation, Tokyo, Japan) and then developed in an automated film developer (AGFA CP 1000, Mortsel, Belgium) to visualise the protein bands.
Statistical analysis
First, the median PUSH score (range 3–16) for the 70 WF samples analysed was calculated to be 10. WF samples were then split into two groups: PUSH scores 10–16 (n = 17) representing WF from the clinically severe ulcers, and 3–9 (n = 12) representing WF from the less severe ulcers. Statistical analysis was performed on data obtained from HPLC/ MRM purine profiling of WF samples from 29 patients using GraphPad Prism (Version 5). In cases where multiple WF samples were collected from a patient, the first WF sample collected was selected as the representative sample for statistical analysis. Statistical significance between these two groups was determined by the Mann–Whitney non parametric test.
RESULTS
Patient demographics
A subset of 29 patients, comprised of 18 males and 11 females with a mean age of 67·3 years (SD 12·6) were randomly selected. On admission into the study, the median ulcer duration was 26 weeks (range 1–520) while the median ulcer area was 4·9 cm2 (range 1–73 cm2). Of the 29 patients, 14 patients achieved complete wound closure within 24 weeks of the study. WF samples were collected from patients at various time points during the course of treatment depending on patient attendance, wound infection and more importantly, availability of sample. Overall, 70 WF samples were collected which included multiple samples from some patients and were used for the MRM purine profiling analysis. Limited sample volume (<1 ml) and protein concentration of WF made it particularly challenging to use the same cohort of samples for subsequent analysis.
Separation of purine catabolites using HPLC/MRM
Elevated levels of purine metabolites in biofluids are reliable indicators of inflammation and tissue damage (15, 16, 17). Therefore, monitoring changes of these purinogenic compounds in WF could provide valuable information regarding the healing status of these wounds. In view of this, a sensitive and specific procedure which involved combining HPLC with tandem MS and MRM was developed to profile purines in WF. Initially, the precursor and product ions for each compound were determined by directly injecting each purine standard into the QTRAP mass spectrometer (Table 1). Purine catabolites were resolved with a linear RP‐HPLC gradient and subsequently monitored by tandem MS with negative electrospray ionisation (ESI‐MS/MS). The HPLC–MRM run allowed for suitable separation of UA, hypoxanthine, xanthine, inosine and adenosine with approximate retention times of 10·4, 10·6, 10·9, 11·1 and 12·2 minutes, respectively. This separation allowed for enhanced selectivity for each purine compound in the MRM mode.
Table 1.
Multiple reaction monitoring transitions for the detection of purine metabolites in wound fluid
| Compound | M r | Precursor ion m/z | Product ion m/z | Declustering potential | Collision energy |
|---|---|---|---|---|---|
| Adenosine | 267·2 | 266 | 134 | 30 | 23 |
| Hypoxanthine | 136·1 | 135 | 92 | 25 | 23 |
| Inosine | 268·2 | 267 | 135 | 30 | 23 |
| Xanthine | 152·1 | 151 | 108 | 25 | 23 |
| Uric acid | 168·1 | 167 | 124 | 23 | 23 |
Calibration curves were determined between the ranges of 0·25 and 10 µM and were based on peak area counts. The calibration curves showed a linear response over the concentration range tested with correlation coefficients between (0·9978–0·9996) for all metabolites. The detection limit was classified as a signal to noise ratio of 3. In addition, an assessment of the analytical recovery was conducted by preparing two biological samples. The first, a reference sample, comprised of pooled WF and the second was the same sample supplemented with solutions of each purine standard at two concentrations, 10 and 2·5 µM, prior to filtration. Recovery was subsequently determined by subtracting the value of the reference sample from that in the spiked WF sample. The extraction yields for the different purine compounds were calculated to be in the range of 60–125% as shown in Table 2.
Table 2.
Recoveries of purine metabolites in wound fluid
| Compound | 10 µM purine standards | 2·5 µM purine standards | ||
|---|---|---|---|---|
| Mean recovery (µM) | Recovery (%) | Mean recovery (µM) | Recovery (%) | |
| Adenosine | 6·7 | 67 | 1·5 | 60 |
| Hypoxanthine | 10·5 | 105 | 2·7 | 108 |
| Inosine | 12·5 | 125 | 3·1 | 124 |
| Xanthine | 8·6 | 86 | 2·2 | 88 |
| Uric acid | 8·7 | 87 | 2·1 | 84 |
UA an important indicator of wound severity
The validated HPLC/MRM method was subsequently applied to determine the concentration of purine catabolites in 70 WF samples obtained from 29 patients. Relative concentrations of purine metabolites in WF were quantified based on peak area counts of each metabolite in relation to the respective standard curve. To account for the dilution of purine metabolites during sample collection, the concentration of each catabolite was normalised to WF protein concentration and expressed as micromoles per milligram of total protein content. The PUSH score for ulcer healing was also used to provide a clinical grading of wound severity (25). The PUSH score is a clinically recognised evaluation of wound characteristics, such as size, tissue type (i.e. epithelial, granulating, slough or necrotic) and amount of exudate (25). The final scores range from 1 to 17, with 17 representing the most severe wounds. Although the PUSH score was initially developed to assess pressure ulcers, it has been validated as a reliable assessment tool for other chronic wounds, including venous leg ulcers (28).
First, the amount of UA as a percentage of total purines in each WF sample was plotted against PUSH scores. The results obtained show that levels of UA increase as the severity of the wound increases (Figure 2A). To further confirm this trend, one WF sample was selected for each patient and the relative concentrations of UA were assessed against the two groupings of PUSH scores as indicated in Figure 2B. Relative concentrations of UA in clinically worse ulcers (PUSH scores 10–16) (n = 17) were significantly (P < 0·001) higher than that observed in the less severe ulcers (PUSH score 3–9) (n = 12).
Figure 2.
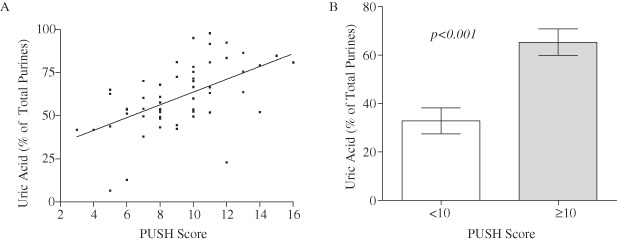
Elevated uric acid (UA) correlates with increased wound severity. (A) A scatter plot of the levels of UA expressed as a percentage of total purine versus PUSH scores. (B) Levels were expressed as amount of UA as a percentage of total purines ± SEM. The amount of UA was significantly increased (P < 0·001) in the clinically worse ulcers (10–16, n = 17) compared to the lower PUSH score group (3–9, n = 12). Statistical significance was determined by the Mann–Whitney non parametric test.
Reduced levels of purine precursors in clinically worse ulcers
Given that the levels of UA increased with wound severity, the amount of purine precursors, including adenosine, inosine, hypoxanthine and xanthine, in WF were assessed against PUSH score (Figure 3A). The results show that levels of purine precursors decreased as the PUSH score increased. Similarly, the levels of precursor purines were significantly higher (P < 0·0001) in WF from less severe ulcers (PUSH score 3–9) compared to the WF from clinically worse ulcers (PUSH score 10–16) (Figure 3B). That is, significantly higher levels of purines that serve as substrates for the enzyme XO in WF were observed in patients assigned a lower PUSH score compared to patients in the higher PUSH score group.
Figure 3.
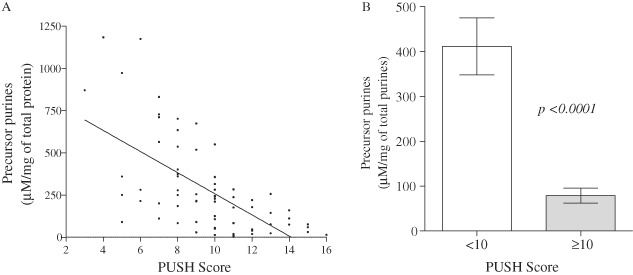
Reduced purine precursors correlates with increased wound severity. (A) A scatter plot of the levels of purine precursors versus PUSH scores. (B) Levels were expressed as concentration of the precursor purines (sum totals of adenosine, inosine, xanthine and hypoxanthine) (µM/mg of total protein) ± SEM. The amount of precursor purines was significantly higher (p < 0·0001) in the lower PUSH score group (3–9, n = 12) compared to the clinically worse ulcers (10–16, n = 17). Statistical significance was determined by the Mann–Whitney non parametric test.
Evidence of xanthine oxidase activity in WF
The accumulation of UA in WF suggests that XO is active at the wound site; hence, we conducted studies investigating levels of XO in WF. However, limitations associated with sample volume and protein concentration of WF made it necessary to pool patient samples for analysis. WF collected from a separate cohort of 15 patients with chronic venous leg ulceration was pooled and XO activity was then determined by supplementing pooled WF with the purine substrate xanthine and the production of UA was examined at 280 nm. The resulting chromatogram, as shown in Figure 4A, showed an additional peak at 16‐minute elution time in WF compared to human serum. This peak was identified as UA based on having an equivalent elution time to the commercially available standard. This oxidation of xanthine to UA was calculated to be approximately 58 nM of xanthine/minute/mg of protein. To further support this finding, XO activity was assayed using a potent XO inhibitor, allopurinol. Allopurinol is rapidly metabolised by XO to generate the active metabolite, oxypurinol. As depicted in Figure 4B, a peak consistent with the retention time of oxypurinol at 23 minutes was detected at 254 nm. These results show elevated levels of active XO in WF from patients with chronic venous leg ulcers relative to human serum.
Figure 4.
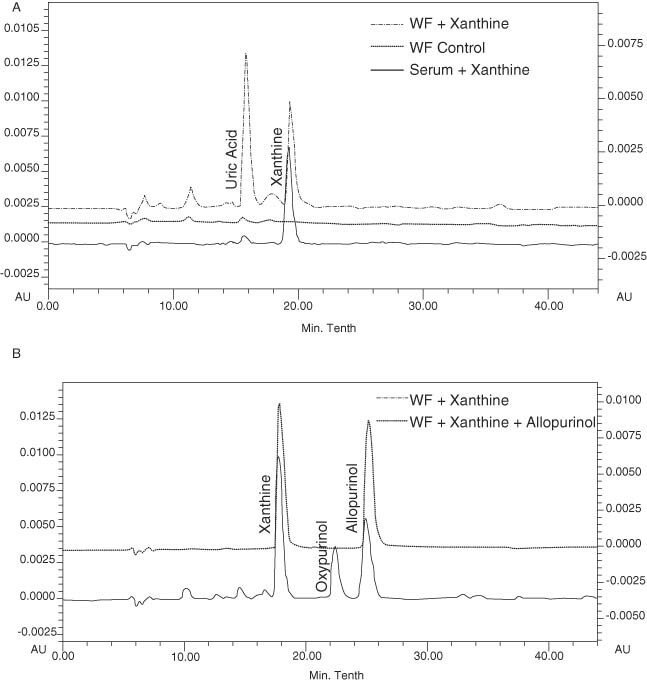
Evidence of xanthine oxidase in wound fluid. Overlaid traces demonstrating activity of xanthine oxidase (XO) in pooled wound fluid compared to human serum by (A) the production of UA and by (B) the production of the metabolite oxypurinol during inhibition with the XO inhibitor allopurinol.
XO levels in WF correlates with wound severity
HPLC/MRM analysis of purine metabolites in WF indirectly suggests that XO concentration corresponds to wound severity. Therefore, to investigate if wound severity correlates with XO levels, WF collected from seven patients with varying degrees of wound severity based on PUSH score were probed for the presence of XO by Western blotting. The immunoblot showed distinct bands in seven WF samples at an approximate molecular weight of 130 kDa (Figure 5). This band is consistent with previous literature that has reported the detection of a similar band in human tissue homogenates during reducing SDS‐PAGE analysis (29). The intensity of this band appears to increase as the relative PUSH scores increase (Figure 5). These results therefore support the purine profiling data indicating that XO levels are increased with wound severity.
Figure 5.
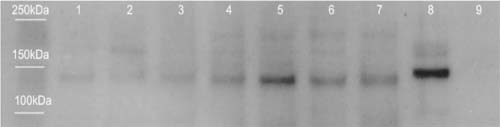
Western blot analysis of xanthine oxidase (XO) in wound fluid. The presence of XO in seven wound fluid samples with PUSH scores of 7, 8, 9, 9, 12, 15 and 15, respectively (Lanes 1–7) was determined using Western blotting. Homogenate of bovine liver (5 µg) was used a positive control (Lane 8) and a blank was run in Lane 9 as a negative control. Bands were detected in each sample at approximately 130 kDa.
DISCUSSION
Disruptions to the healing process result in the alteration of numerous biochemical factors in the wound environment that may be used to distinguish healing from non healing wounds. Consequently, there has been increased interest in characterising the various constituents in WF to detect factors that may correlate with wound chronicity, as well as be used to monitor response to treatment. Changes in small molecule metabolite concentrations in biological fluids provide valuable insights into the disruptions of ‘normal’ biological processes. These small molecules are downstream end products of gene and protein activity; therefore slight imbalances can create major fluctuations in metabolite concentrations that may be reflective of a disease state.
In this study, we developed and validated a reliable, simple and specific analytical assay for separation and simultaneous monitoring of low concentrations of purine metabolites in a complex biological fluid, such as WF. The combination of RP‐HPLC with MS/MS enabled specific analysis of these potential indicators of healing by MRM. The only inconsistency detected was that the extraction yields obtained for adenosine were surprisingly lower than expected (Table 2). This may reflect the greater instability of adenosine in WF relative to other purines (30, 31). One possible explanation for this anomaly is that adenosine is converted to catabolites further down the purine degradation pathway. Interestingly, the inosine recovery values obtained were >100% which may support this hypothesis.
Purine profiling of WF from chronic wounds showed for the first time the accumulation of purine metabolites, in particular UA. These results indicate that at least two important changes occur in the chronic wound environment; first, that the wound bed is poorly oxygenated resulting in a switch to anaerobic metabolism and consequent ATP breakdown (13, 18, 32). Second, the presence of UA in WF indicates that XO is active in the wound site, catalysing the conversion of hypoxanthine to xanthine and finally to UA. This was confirmed by enzyme activity assays that showed elevated levels of active XO in WF compared to human serum (Figure 4). Importantly, our data show significantly elevated levels of UA and a corresponding decrease in purine precursors in WF from clinically worse ulcers (2, 3). Interestingly, previous reports indicate that topical application of purine precursors accelerates wound healing in various animal and cell culture models (33, 34, 35). In particular, adenosine has been shown to play an important role in stimulating wound healing (36, 37). Taken together, these findings indirectly suggest that XO levels correlates to wound severity.
Western blot was used to show a direct relationship between XO and wound severity by detecting the presence of XO in WF with varying PUSH scores. The results show a trend whereby XO levels increase with wound severity (Figure 5), suggesting that elevated concentrations of XO at the wound site is directly associated with delayed healing. It should be noted that it is highly likely that the XO enzyme may also be present in acute wound fluid (AWF) with levels peaking during the inflammatory phase of wound healing. Thus, it is unlikely that XO is a unique marker found only in WF from chronic wounds; rather it is likely that this enzyme is significantly elevated in WF from chronic wounds compared to AWF or matching patient serum. Future studies measuring the activity of XO in AWF will assist in establishing if this enzyme is a useful biomarker of healing/non healing of all wounds.
Inflammation is the body's immediate response to physical trauma, eventually leading to tissue repair and restoration. Elevated levels of UA in the chronic wound environment are likely to play an inhibitory role in healing by promoting inflammation. This is exemplified by a recent study using transgenic mice that have increased turnover of UA (38). Kono et al. showed that depletion of UA inhibits the inflammatory response to cell death and that XO‐mediated generation of UA from dying cells is a damage‐associated molecular pattern that leads to the recruitment of neutrophils and amplified inflammation. Sustained production of UA in underperfused damaged tissues may also result in the precipitation of UA leading to the deposition of monosodium urate crystals around the wound environment, as is commonly observed in the case of gout (39). Suspensions of monosodium urate crystals are capable of producing an inflammatory response in both gouty and non gouty patients (40). Given that chronic wounds are characterised by a prolonged inflammatory phase, our results demonstrating elevated levels of UA in WF indicate that UA is likely to play an important role in perpetuating inflammation.
The production of UA is also associated with a burst of the highly reactive superoxide radical and the subsequent generation of another redox regulating oxidant, H2O2 (18, 20). Excessive release of these leukocyte‐induced oxidants can alter the structure of lipids, DNA and proteins, thereby disrupting their normal functions in wound healing (41). A wound environment rich in oxidants may further exacerbate the inflammatory response via the activation of redox‐sensitive transcription factors (42, 43, 44, 45). In vitro redox regulation studies indicate that activation of these transcription factors upregulates different genes involved in the inflammatory response, promoting the production of various pro‐inflammatory cytokines and MMP expression (46, 47). Excessive release of reactive oxygen species (ROS) in the wound environment has also been shown to cause cellular damage, lipid peroxidation and protein modification (48, 49). Redox imbalance in the extracellular environment can lead to protein oxidation, increasing their susceptibility to proteolysis, causing tissue damage and dysfunction (50). We propose that the presence of elevated concentrations of XO at the wound site is an overlooked source of ROS generation in chronic wounds and may also contribute to delayed wound healing.
In conclusion, the development of reliable, non invasive point of care diagnostics based on these findings may prove to be useful in monitoring the prognosis and progress of healing of chronic venous leg ulcers. Indeed, the observations that wound severity is related to XO catalysis of purine precursors to UA, provides not only a method of diagnosis, but also a potential therapeutic target. With respect to diagnostic applications, the severity of a wound could be monitored by detecting the levels of UA or the levels of one or more of the UA precursors in WF. The detection of elevated levels of UA would appear to be associated with a more severe wound, while elevated levels of precursor purines correlate to a less severe wound. Monitoring changes of purine metabolites in WF is therefore likely to provide valuable information regarding the healing patterns of chronic venous leg ulcers. More importantly, our data indicating enhanced turnover of purine precursors in clinically worse ulcers show that XO is a novel potential therapeutic target. Inhibition of XO using specific inhibitors, such as allopurinol, could simultaneously target the three major contributors that keep chronic wounds in a non healing state: oxidative stress, UA accumulation and purine precursor depletion. Thus, the outcomes of these studies are likely to benefit wound management through the development of a diagnostic test and the implementation of new therapeutics for the treatment of patients with chronic venous leg ulcers.
ACKNOWLEDGEMENTS
The authors thank Patricia Shuter, Megan Pratt, Robin Armstrong and Christina Parker for clinical assessments, collection of clinical data and wound fluid samples. We would also like to thank Mr Alun Jones (Institute of Molecular Biosciences, University of Queensland) for technical support with mass spectrometry. This project was supported by NH&MRC Project Grant Scheme (390102), Bluebox Proof of Concept Funding, Institute of Health and Biomedical Innovation Early Career Research Grant and Queensland Government Smart State Top‐Up Scholarship.
CONFLICT OF INTEREST
G.S and M.F are named inventors on aspects of this research that have been patented by the Queensland University of Technology.
REFERENCES
- 1. Baker SR, Stacey MC. Epidemiology of chronic leg ulcers in Australia. Aust N Z J Surg 1994;64: 258–61. [DOI] [PubMed] [Google Scholar]
- 2. Briggs M, Close SJ. The prevalence of leg ulceration: a review of the literature. EWMA J 2003;3:14–20. [Google Scholar]
- 3. Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46:381–6. [DOI] [PubMed] [Google Scholar]
- 4. Simon DA, Dix FP, McCollum CN. Management of venous leg ulcers. BMJ 2004;328:1358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lorimer KR, Harrison MB, Graham ID, Friedberg E, Davies B. Assessing venous ulcer population characteristics and practices in a home care community. Ostomy Wound Manage 2003;49:32–4, 8–40, 2–3. [PubMed] [Google Scholar]
- 6. Abbade LP, Lastoria S, de Almeida Rollo H, Stolf HO. A sociodemographic, clinical study ofpatients with venous ulcer. Int J Dermatol 2005; 44:989–92. [DOI] [PubMed] [Google Scholar]
- 7. Walker N, Rodgers A, Birchall N, Norton R, MacMahon S. Leg ulcers in New Zealand: age at onset, recurrence and provision of care in an urban population. N Z Med J 2002;115:286–9. [PubMed] [Google Scholar]
- 8. Edwards H, Courtney M, Finlayson K, Lindsay E, Lewis C, Shuter P, Chang A. Chronic venous leg ulcers: effect of a community nursing intervention on pain and healing. Nurs Stand 2005;19: 47–54. [DOI] [PubMed] [Google Scholar]
- 9. Walshe C. Living with a venous leg ulcer: a descriptive study of patients' experiences. J Adv Nurs 1995;22:1092–100. [DOI] [PubMed] [Google Scholar]
- 10. Chase S, Whittemore R, Crosby N, Freney D. Living with chronic venous ulcers: A descriptive study of knowledge and functional health status. J Commun Health Nurs 2000;17:1–13. [DOI] [PubMed] [Google Scholar]
- 11. Kurd SK, Hoffstad OJ, Bilker WB, Margolis DJ. Evaluation of the use of prognostic information for the care of individuals with venous leg ulcers or diabetic neuropathic foot ulcers. Wound Repair Regen 2009;17:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Vliet A, Bove PF. Purinergic signaling in wound healing and airway remodeling. Subcell Biochem 55:139–57. [DOI] [PubMed] [Google Scholar]
- 13. McCord JM. Oxygen‐derived free radicals in postischemic tissue injury. N Engl J Med 1985;312: 159–63. [DOI] [PubMed] [Google Scholar]
- 14. Kuracka L, Kalnovicova T, Liska B, Turcani P. HPLC method for measurement of purine nucleotide degradation products in cerebrospinal fluid. Clin Chem 1996;42:756–60. [PubMed] [Google Scholar]
- 15. Stover JF, Lowitzsch K, Kempski OS. Cerebrospinal fluid hypoxanthine, xanthine and uric acid levels may reflect glutamate‐mediated excitotoxicity in different neurological diseases. Neurosci Lett 1997;238:25–8. [DOI] [PubMed] [Google Scholar]
- 16. Gizzi F, Papponetti M, Palka GD, Ruffini I, Di Ilio C, Odorisio M, Spoto G. Hypoxanthine and xanthine as markers in early diagnosis of foetal diseases. Adv Exp Med Biol 1998;431:777–80. [DOI] [PubMed] [Google Scholar]
- 17. Amorini AM, Petzold A, Tavazzi B, Eikelenboom J, Keir G, Belli A, Giovannoni G, Di Pietro V, Polman C, D’Urso S, Vagnozzi R, Uitdehaag B, Lazzarino G. Increase of uric acid and purine compounds in biological fluids of multiple sclerosis patients. Clin Biochem 2009;42:1001–6. [DOI] [PubMed] [Google Scholar]
- 18. Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 2002;33:774–97. [DOI] [PubMed] [Google Scholar]
- 19. Pritsos CA. Cellular distribution, metabolism and regulation of the xanthine oxidoreductase enzyme system. Chem Biol Interact 2000;129: 195–208. [DOI] [PubMed] [Google Scholar]
- 20. Vorbach C, Harrison R, Capecchi MR. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol 2003;24:512–7. [DOI] [PubMed] [Google Scholar]
- 21. Darr D, Fridovich I. Free radicals in cutaneous biology. J Invest Dermatol 1994;102:671–5. [DOI] [PubMed] [Google Scholar]
- 22. Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Med 2006; 36:327–58. [DOI] [PubMed] [Google Scholar]
- 23. Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem 2005;12: 1161–208. [DOI] [PubMed] [Google Scholar]
- 24. Wlaschek M, Scharffetter‐Kochanek K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen 2005;13:452–61. [DOI] [PubMed] [Google Scholar]
- 25. Stotts NA, Rodeheaver GT, Thomas DR, Frantz RA, Bartolucci AA, Sussman C, Ferrell BA, Cuddigan J, Maklebust J. An instrument to measure healing in pressure ulcers: development and validation of the pressure ulcer scale for healing (PUSH). J Gerontol A Biol Sci Med Sci 2001;56:M795–9. [DOI] [PubMed] [Google Scholar]
- 26. Fernandez ML, Broadbent JA, Shooter GK, Malda J, Upton Z. Development of an enhanced proteomic method to detect prognostic and diagnostic markers of healing in chronic wound fluid. Br J Dermatol 2008;158:281–90. [DOI] [PubMed] [Google Scholar]
- 27. Hoke SH, Morand KL, Greis KD, Baker TR, Harbol KL, Dobson RLM. Transformations in pharmaceutical research and development, driven by innovations in multidimensional mass spectrometry‐based technologies. Int J Mass Spectr 2001;212:135–96 [DOI: 10.1016/S1387-3806(01)00499-7]. [DOI] [Google Scholar]
- 28. Hon J, Lagden K, McLaren A‐M, O’sullivan D, Orr L, Houghton PE, Woodbury MG. A prospective, multicentre study to validate use of the pressure ulcer scale of healing [PUSH] in patients with diabetic, venous, and pressure ulcers. Ostomy Wound Manage 2010;56:26–36. [PubMed] [Google Scholar]
- 29. Sarnesto A, Linder N, Raivio KO. Organ distribution and molecular forms of human xanthine dehydrogenase/xanthine oxidase protein. Lab Invest 1996;74:48–56. [PubMed] [Google Scholar]
- 30. Parker RB, McCollam PL. Adenosine in the episodic treatment of paroxysmal supraventricular tachycardia. Clin Pharm 1990;9:261–71. [PubMed] [Google Scholar]
- 31. Hayashida M, Fukunaga A, Fukuda K, Sakurai S, Mamiya H, Ichinohe T, Kaneko Y, Hanaoka K. The characteristics of intravenous adenosine‐induced antinociception in a rabbit model of acute nociceptive pain: a comparative study with remifentanil. Anesth Analg 2006;103:1004–10. [DOI] [PubMed] [Google Scholar]
- 32. Granger DN, Hollwarth ME, Parks DA. Ischemia‐reperfusion injury: role of oxygen‐derived free radicals. Acta Physiol Scand Suppl 1986;548: 47–63. [PubMed] [Google Scholar]
- 33. Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN. Wound healing is accelerated by agonists of adenosine A2 (G alpha s‐linked) receptors. J Exp Med 1997;186:1615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol 2002;160:2009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang S, Zavitz CC, Wang J, Saraf A, Zielinski R, Ramsbottom JD, Ballerini P, D’Alimonte I, Romano S, Fischione G, Traversa U, Werstiuk ES, Rathbone MP. Non‐adenine based purines accelerate wound healing. Purinergic Signal 2006;2:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol 2005;67:1385–7. [DOI] [PubMed] [Google Scholar]
- 37. Valls MD, Cronstein BN, Montesinos MC. Adenosine receptor agonists for promotion of dermal wound healing. Biochem Pharmacol 2009;77: 1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest 2010;120: 1939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCarty DJ, Hollander JL. Identification of urate crystals in gouty synovial fluid. Ann Intern Med 1961;54:452–60. [DOI] [PubMed] [Google Scholar]
- 40. Shi Y, Mucsi AD, Ng G. Monosodium urate crystals in inflammation and immunity. Immunol Rev 2010;233:203–17. [DOI] [PubMed] [Google Scholar]
- 41. Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003;189:41–54. [DOI] [PubMed] [Google Scholar]
- 42. Schreck R, Baeuerle PA. A role for oxygen radicals as second messengers. Trends Cell Biol 1991;1:39–42. [DOI] [PubMed] [Google Scholar]
- 43. Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF‐kappa B and AP‐1 in intact cells: AP‐1 as secondary antioxidant‐responsive factor. Embo J 1993;12:2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition 1996;12:274–7. [DOI] [PubMed] [Google Scholar]
- 45. Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med 1996;21: 335–48. [DOI] [PubMed] [Google Scholar]
- 46. Halliwell B, Gutteridge J. Free radicals in biology and medicine, 3rd edn. New York: Oxford University Press; Inc, 1999. [Google Scholar]
- 47. Wenk J, Brenneisen P, Wlaschek M, Poswig A, Briviba K, Oberley TD, Scharffetter‐Kochanek K. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP‐1‐mediated induction of matrix‐degrading metalloprotease‐1. J Biol Chem 1999;274:25869–76. [DOI] [PubMed] [Google Scholar]
- 48. Yeoh‐Ellerton S, Stacey MC. Iron and 8‐isoprostane levels in acute and chronic wounds. J Invest Dermatol 2003;121:918–25. [DOI] [PubMed] [Google Scholar]
- 49. Moseley R, Hilton JR, Waddington RJ, Harding KG, Stephens P, Thomas DW. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen 2004;12:419–29. [DOI] [PubMed] [Google Scholar]
- 50. Moran LK, Gutteridge JM, Quinlan GJ. Thiols in cellular redox signalling and control. Curr Med Chem 2001;8:763–72. [DOI] [PubMed] [Google Scholar]


