Abstract
Proteases play a critical role in the ordered remodelling of extracellular matrix (ECM) components during wound healing and tissue regeneration. However, the usually ordered proteolysis is compromised in chronic wounds due to over‐expression and high concentrations of matrix metalloproteinase's (MMPs) and neutrophil elastase (NE). Ovine forestomach matrix (OFM) is a decellularised extracellular matrix‐based biomaterial developed for tissue regeneration applications, including the treatment of chronic wounds, and is a heterogeneous mixture of ECM proteins and proteoglycans that retains the native structural and functional characteristics of tissue ECM. Given the diverse molecular species present in OFM, we hypothesised that OFM may contain components or fragments that inhibit MMP and NE activity. An extract of OFM was shown to be a potent inhibitor of a range of tissue MMPs (IC50s = 23 ± 5 to 115 ± 14 µg/ml) and NE (IC50 = 157 ± 37 µg/ml), and was more potent than extracts prepared from a known protease modulating wound dressing. The broad spectrum activity of OFM against different classes of MMPs (i.e. collagenases, gelatinases and stromelysins) may provide a clinical advantage by more effectively addressing the protease imbalance seen in chronic wounds.
Keywords: Enzyme inhibitor, Inhibition, Matrix metalloproteinase, Neutrophil elastase, Ovine forestomach matrix, Wound healing
Introduction
Tissue proteases, for example, matrix metalloproteinases (MMPs) and neutrophil elastase (NE) play a critical role in normal tissue turn over and during ordered tissue regeneration and repair (1). Protease digestion of extracellular matrix (ECM) components facilitates cell migration and proliferation, and plays a role in the regulation of inflammatory processes 2, 3. Conversely, over‐expression of tissue proteases and increased protease concentrations has been associated with a number of pathologies, including chronic wounds 4, 5, cancer (6) and vascular disease (7). MMPs are a large family of 23 closely related zinc‐fingered proteases that digest ECM components including collagens I, III and IV, fibronectin, laminin and proteoglycans (5). The serine protease NE targets structural elements of the ECM, but has additionally been shown to degrade fibronectin (8) and peptide growth factors (9). In typically ordered tissue regeneration, the proteolytic activity of MMPs and NE are maintained by endogenous protease inhibitors, including tissue inhibitors of MMPs (TIMPs), α2‐macroglobin and α1‐proteinase inhibitor (4).
A recognised pathology of chronic wounds is elevated concentrations of various tissue proteases and increased protease expression relative to acute wounds (10). The increased concentration of proteases contributes to the stalled healing observed in chronic wounds, with rampant digestion of the ECM leading to a prolonged inflammatory phase. In addition, there is a known imbalance between proteases and native protease inhibitors (e.g. TIMPs), such that chronic wounds become overrun by degrading enzymes in the absence of any native regulatory mechanism 8, 9, 11, 12, 13. The resultant ‘hyper‐proteolytic’ state leads to the degradation of structural and adhesion proteins, as well as, growth factors and growth factor receptors that would otherwise stimulate repair 9, 12.
Given the role of elevated protease activity in the status of chronic wounds, there is a demand for technologies to modulate proteolytic activity as a treatment intervention (10). Modulation of proteases by wound dressings comprising collagen 14, 15, 16, 17, 18, functionalised gauze (19) and rationally designed hydrogels 20, 21, 22 has been reported. In clinical studies, the application of protease‐modulating dressings has been shown to reduce wound protease concentrations with concomitant decreases in wound area (23).
Decellularised extracellular matrix (dECM)‐based biomaterials play an important role in the treatment of chronic wounds by providing a native provisional matrix and signalling molecules to kick‐start the healing process (24). Ovine forestomach matrix (OFM; Endoform™ Dermal Template; Mesynthes Ltd, Lower Hutt, New Zealand) is a dECM‐based biomaterial that mimics normal tissue ECM. OFM undergoes cell infiltration and remodelling, promotes cell proliferation and migration, and is angioinductive 25, 26. While primarily composed of collagens I and III, OFM additionally contains a diverse array of biological macromolecules, including fibronectin, elastin, laminin and glycosaminoglycans (25). Given the molecular heterogeneity of OFM, studies were undertaken to assess whether components or fragments of OFM could inhibit wound proteases and, therefore, mediate protease activity in chronic wounds.
Materials and methods
MMP solid state assay
Inhibition of MMPs by intact OFM was quantified according to Cullen et al.(14) with modifications. Samples of OFM (Mesynthes Ltd, Lower Hutt, New Zealand) and oxidised regenerated cellulose/collagen (ORC/C; Promogran™; Systagenix, Gatwick, UK) were cut into 5‐mm‐diameter discs and rehydrated in phosphate‐buffered saline (500 µl, PBS) for approximately 1 minute. Test materials were blotted to remove excess PBS and then added to 70 µl of either recombinant human MMP9 (0·4 µg/ml; Enzo Life Sciences, Farmingdale, NY) or recombinant human MMP8 (0·83 µg/ml; Enzo Life Sciences), both prepared in 50 mM Hepes, 10 mM CaCl2, 0·05% Tween 20, pH 7·5. Samples were incubated for 15, 30, 60, 120, 240 and 360 minutes at 37°C with shaking. Samples were removed from solution, discarded and the residual enzymatic activity of the solution quantified using a fluorogenic substrate Mca‐Pro‐Leu‐Gly‐Leu‐Dpn‐Ala‐Arg‐NH2 (27) according to the manufacturer's instructions. In a 96‐well plate, 20 µl of the protease solution was mixed with in 60 µl of MMP assay buffer (50 mM Hepes, 10 mM CaCl2, 0·05% Tween 20, pH 7·5) and fluorogenic substrate (20 µl, 20 µM). Relative florescence units (RFU) were quantified on a plate reader (SpectraMax®; M4; Molecular Devices, Sunnyvale, CA) every 1 minute over a 10‐minute period at 37°C, using excitation and emission wavelengths of 328 and 420 nM, respectively. RFU was plotted versus time to derive the rate of formation of the fluorophore. Residual MMP activity was determined by comparing the rate of the test sample, relative to an untreated control, and expressed as percentage activity. P‐values were determined by an unpaired t‐test (Excel 2007; Microsoft Corporation, Redmond, WA) with a P‐value of <0·05 considered statistically significant.
MMP inhibition assay
Aqueous extracts of OFM and ORC/C were prepared by incubating approximately 30–100 mg of sample in PBS (1 ml) for 1 hour at 37°C with shaking. The samples were centrifuged at 9500 g for 10 minutes and the supernatant was transferred. Protein concentrations of the extracts were determined using bicinchoninic acid (BCA) protein assay, according to the manufacturer's instructions (Pierce, Thermo Fisher Scientific, Waltham, MA). Extracts were serially diluted over a final concentration range of 3–600 µg/ml protein using MMP assay buffer (50 mM Hepes, 10 mM CaCl2, 0·05% Tween 20, pH 7·5). Test solution (20 µl) was added to recombinant human MMPs (20 µl) at the final concentration specified in Table 1. MMP assay buffer (40 µl) was added and samples were incubated for 60 minutes at 37°C, prior to the initiation of enzymatic activity by addition of fluorogenic substrate Mca‐Pro‐Leu‐Gly‐Leu‐Dpn‐Ala‐Arg‐NH2 (20 µl, 20 µM). Production of the fluorophore was quantified every 1 minute over a 10‐minute period at 37°C, using excitation and emission wavelengths of 328 and 420 nm, respectively. RFU was plotted versus time to derive the rate of formation of the fluorophore (RFU/minute) for each concentration of the extract (Figure 2A). Percent activity was determined from the observed rate relative to the rate of an untreated control. Sigmoidal dose–response curves were plotted (SigmaPlot v11.0; Systat Software, San Jose, CA) of percent activity versus extract concentration and the half‐maximal inhibitory concentration (IC50) derived for test extracts against each of the MMPs tested (Figure 2B). Extracts were tested in triplicate against each MMP.
Table 1.
Inhibitory activity of OFM and ORC/C extracts against tissue proteases *
| Protease | OFM IC50 (µg/ml) | ORC/C IC50 (µg/ml) |
|---|---|---|
| MMP1 (Collagenase 1) (0·153 U/µl) | 115 ± 14 | ∼600 |
| MMP8 (Collagenase 2) (0·018 U/µl) | 86 ± 2 | ∼600 |
| MMP13 (Collagenase 3) (0·014 U/µl) | 38 ± 2 | ∼600 |
| MMP3 (Stromelysin‐1) (0·029 U/µl) | 96 ± 8 | >600 |
| MMP10 (Stromelysin‐2) (0·01 U/µl) | 45 ± 1 | 355 ± 51 |
| MMP2 (Gelatinase‐A) (0·009 U/µl) | 23 ± 5 | 31 ± 6 |
| MMP9 (Gelatinase‐B) (0·009 U/µl) | 50 ± 14 | 55 ± 7 |
| MMP12 (Macrophage metalloelastase) (0·007 U/µl) | 24 ± 1 | ∼600 |
| MMP14 (Membrane type 1 MMP) (0·024 U/µl) | 45 ± 3 | ∼600 |
| Neutrophil elastase (0·002 mU/µl) † | 157 ± 37 | ∼1740 |
MMP, matrix metalloproteinase; OFM, ovine forestomach matrix; ORC/C, oxidised regenerated cellulose/collagen.
*Errors represent standard errors from triplicate experiments. Concentration in brackets indicates the enzyme concentration used in the respective assay.
†Errors represent standard errors from duplicate experiments.
Neutrophil elastase inhibition assay
Aqueous extracts of OFM and ORC/C were prepared by incubating approximately 30–100 mg of sample per ml PBS for 1 hour at 37°C with shaking. Extracts were concentrated on a 10 kDa MW centrifugal concentrator (Amicon; Merck Millipore, Billerica, MA). The OFM and ORC/C extracts were serially diluted in NE assay buffer (100 mM Hepes, 500 mM NaCl, 0·05% Tween 20, pH 7·2) over a final concentration range of 8–1740 µg/ml and 12 to 1800 µg/ml, respectively. Inhibition of the NE was quantified using a colorimetric NE kit (Enzo Life Sciences), according to the manufacturer's instructions. Diluted solutions of the extracts (20 µl), purified human NE (0·02 mU/µl, 10 µl) and NE assay buffer (60 µl) were pre‐incubated for 30 minutes at 37°C, and then colorimetric substrate MeOSuc‐Ala‐Ala‐Pro‐Val‐pNA (10 µl, 1 mM) (28) was added. Production of the colorimetric cleavage product was quantified at 405 nm every 1 minute for 10 minutes at 37°C. The rate of the reaction (Abs/min) was determined from the slope of a plot of absorbance versus time. Percent activity was determined from the observed rate relative to the rate of an untreated control. Sigmoidal dose–response curves were plotted (SigmaPlot, v11.0) of percent activity versus extract concentration and the half‐maximal IC50 derived. Extracts were tested in duplicate.
Results
MMP solid state assay
Protease inhibition of intact OFM was assessed according to the approach of Cullen et al.(14) using MMP8 and MMP9 as a representative collagenase and gelatinase, respectively. In the presence of either intact OFM or ORC/C, the residual activity of MMP8 was reduced relative to the untreated control at all time points sampled (P < 0·05), but only OFM displayed a time‐dependent decrease in activity (Figure 1A). There was no statistical difference between the activity of intact OFM and ORC/C against MMP8 (P > 0·05), except following a 360‐minute incubation (P = 0·02). The activity of intact ORC/C was increased relative to OFM when assayed against the gelatinase, MMP9 (P < 0·001) (Figure 1B), and both intact OFM and ORC/C demonstrated significant activity relative to the untreated control at all time points (P < 0·05). Like MMP8, the inhibitory activity of OFM against MMP9 was time‐dependent.
Figure 1.
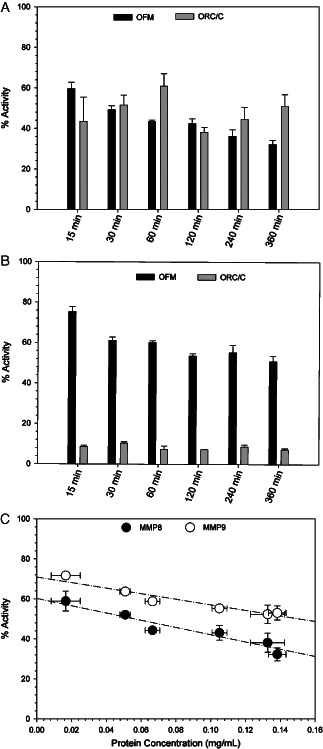
Residual protease activity of solutions of MMP8 (A) and MMP9 (B) after exposure to either intact ovine forestomach matrix (OFM) or oxidised regenerated cellulose/collagen (ORC/C). Error represents standard error from triplicate experiments. (C) Correlation between protein concentration of the solution following incubation with intact OFM and percent residual protease activity. Dotted line indicates linear regression. Error bars represent standard error between triplicate experiments.
The time‐dependent decrease in the activity of MMP8 and MMP9 in the presence of OFM suggested aqueous soluble components were responsible for the observed effect, rather than direct adsorption of proteases to the surface of the intact biomaterial. This was supported by the observed correlation between the residual activity of the OFM‐treated enzyme and the corresponding protein concentration following incubation of the biomaterial in solution (Figure 1C).
Protease inhibition
Dose–response curves of percentage enzyme activity versus protein concentration (Figure 2B) were determined using aqueous extracts of both OFM and ORC/C. Inhibitory activity against a range of proteases (Table 1 and Figure 2C) were expressed as an IC50, where lower concentrations represent more potent enzyme inhibition. The IC50s were estimated when a complete sigmoidal dose–response could not be calculated due the concentration limits (e.g. ORC/C, Figure 3). Extracts were prepared by incubating either OFM or ORC/C in PBS at 37°C for 1 hour. The incubation time used to prepare the extract (e.g. 1 or 16 hours) was shown to have little effect on the bioactivity of the extracts (data not shown) suggesting that the composition and diversity of solubilised components were not significantly altered by extending the incubation period. OFM extracts inhibited all MMPs tested in this study, including collagenases, stromelysins and gelatinases. Inhibitory IC50s ranged from 23 ± 5 to 115 ± 14 µg/ml, with the most potent inhibition being determined against MMP2 (IC50 = 23 ± 5 µg/ml) and MMP12 (IC50 = 24 ± 1 µg/ml). The weakest inhibition was recorded against MMP1 (IC50 = 115 ± 14 µg/ml). The bioactivity of ORC/C extracts were more variable across the MMPs surveyed, with similar inhibitory potency to OFM determined only for the gelatinases, MMP2 and MMP9 (IC50 = 31 ± 6 and 55 ± 7 µg/ml, respectively). ORC/C extracts were only modest inhibitors of MMP1, MMP3, MMP8, MMP12, MMP13 and MMP14 with IC50s estimated to be approximately 600 µg/ml or greater. The OFM and ORC/C extracts were concentrated using a 10‐kDa MW concentrator prior to assaying against NE to derive full does response curves. The OFM extracts inhibited NE in a dose‐dependent manner and were approximately 10‐fold more potent than the ORC/C extracts (IC50s = 157 ± 37 and ∼1740 µg/ml, respectively).
Figure 2.
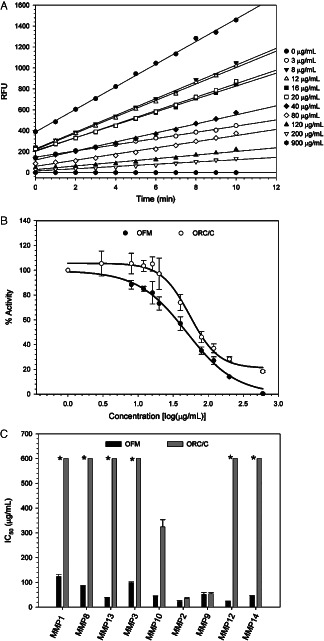
Bioactivity (IC50) of ovine forestomach matrix (OFM) and oxidised regenerated cellulose/collagen (ORC/C) extracts against various wound proteases. (A) Representative plots of MMP9 activity in the presence of an OFM extract at various concentrations. The slope of the line was used to determine the rate (RFU/min). (B) Representative dose–response curve (RFU/min versus concentration) of OFM and ORC/C extracts against MMP9 and determination of the half‐maximal inhibition (IC50). Error bars represent standard deviation from triplicate experiments. (C) Bioactivity of OFM and ORC/C extracts against a range of matrix metalloproteinases (MMPs). Errors represent standard error from triplicate experiments (Table 1). ‘*’ indicate samples where the IC50 was estimated to be approximately 600 µg/ml or greater.
Figure 3.
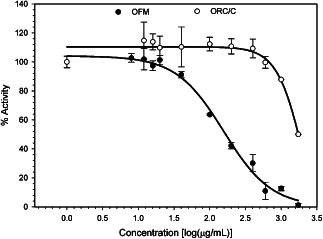
Inhibition of neutrophil elastase (NE) by ovine forestomach matrix (OFM) and oxidised regenerated cellulose/collagen (ORC/C) extracts. Error represents standard error from duplicate experiments.
Discussion
Inhibition of tissue proteases by biomaterials has traditionally been demonstrated using solid‐state assays, whereby the intact samples are incubated in enzyme solutions and then the residual enzyme activity quantified following removal of the sample 14, 15, 16, 17, 18, 19, 22, 29. In the case of soluble or partially soluble biomaterials, such as OFM and ORC/C, reductions in enzyme activity using this approach can be accounted for either by (i) surface adsorption of the enzyme to the biomaterial; or (ii) dissolution of soluble inhibitory components. The correlation between the concentration of soluble proteinaceous OFM components and reduction in enzyme activity (Figure 1C) suggests that solubilised matrix components contribute to the inhibitory effect of OFM. By determining the IC50‐value of these soluble components, a direct comparison between the bioactivity of OFM and ORC/C extracts could be made against a panel of proteases. In addition, this approach is more consistent with clinical practice as both OFM and ORC/C are absorbed into the wound bed over time, releasing soluble components through hydrolysis and proteolysis. The IC50 values were based on the protein concentration of the extracts, and therefore did not take into account any soluble ORC present in solution. Therefore, the IC50s determined for ORC/C may be significantly higher if total solubilised material (i.e. collagen and ORC) was taken into account.
Extracts of OFM had broad spectrum activity against a range of tissue proteases, whereas the activity of ORC/C was limited to the gelatinases, MMP2 and MMP9 (Table 1 and Figure 2C). The greater relative bioactivity of OFM versus ORC/C is most likely encoded in the composition of the biomaterials. OFM is relatively heterogeneous, containing a range of structural, adhesion and signalling molecules that constitute native ECM in tissues (25). In addition to the collagenous major components of OFM, there is the potential for a variety of secondary macromolecules to contribute to the broad spectrum activity of OFM. In contrast, ORC/C comprises only acid solubilised and denatured bovine collagen I and ORC (15). It has been proposed that ORC/C acts as a sacrificial substrate in the presence of proteases (15) and, therefore, given the composition of ORC/C, the gelatinases (MMP2 and MMP9) would be most susceptible to inhibition, through sacrificial means. The results of this study support this notion as ORC/C only inhibited the gelatinases (Table 1 and Figure 2.C). Interestingly, it has been shown in previous studies that unlike OFM, extracts of the dECM‐based biomaterial small intestinal submucosa (SIS) do not inhibit MMP1, MMP2 or MMP9, but rather SIS attenuates protease activity through the non selective mechanism of surface adsorption (17).
While it is recognised that tissue proteases play an important role in the state of chronic wounds there is still no literature consensus as to which proteases are most important in contributing to chronicity and, therefore, should be targeted for therapeutic intervention 30, 31. Various reports have identified elevated concentrations or expression of MMP1 (11), MMP2 16, 32, MMP3 (33), MMP8 34, 35, MMP9 32, 36, MMP13 16, 33, 37 and elastase (16) in chronic wound fluid and/or wound tissues. In this study, target proteases were selected based on those known to be in abundance in chronic wounds, as well as MMP12 and MMP14 that are known to play a role in cutaneous wound repair (30). OFM was shown to inhibit all classes of proteases included in the panel and we propose that this broad spectrum activity potentially provides a significant clinical advantage over inhibitory activity against a single protease class. For example, ECM collagens type I, II and III are known to be sequentially degraded, first by the action of collagenases MMP‐8, MMP‐1 and MMP‐13, respectively, which cleave the collagen fibrils to initiate degradation and second, by the gelatinases (MMP2 and MMP8) that act exclusively on the cleaved fibril (38). Initiation of the proteolytic cascade additionally requires activation of the collagenases themselves, either through auto‐proteolysis of the pro‐enzymes or via MMP‐mediated proteolysis. For example, stromelysin‐1 (MMP‐3) is known to activate both MMP13 and the gelatinases leading to collagen III degradation (38). Therefore, while the gelatinases may be in higher abundance in chronic wounds, relative to the collagenases (31), biomaterials that are capable of inhibiting both the upstream (i.e. stromelysins, collagenases) and downstream (i.e. gelatinases) proteases are more likely to halt collagen proteolysis relative to the agents that exclusively inhibit the downstream gelatinases. The notion of broad spectrum activity against a variety of protease classes can be likened to the combinatorial therapies that have been successfully used in the treatment of HIV infection and cancer. This is the first report of soluble components of a dECM‐based biomaterial being inhibitory towards a range of tissue proteases and suggests that OFM has the potential to modulate the hyper‐proteolytic environment of chronic wounds. Additional clinical studies are warranted to validate our understanding of the inhibitory activity of OFM and how this effect translates into the resolution of chronicity and subsequent healing outcomes.
Acknowledgements
All authors are shareholders of Mesynthes Limited.
References
- 1. Toriseva M , Kahari VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci 2009. ; 66 : 203 – 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gill SE , Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008. ; 40 : 1334 – 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mott JD , Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 2004. ; 16 : 558 – 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yager DR , Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen 1999. ; 7 : 433 – 41. [DOI] [PubMed] [Google Scholar]
- 5. Herouy Y , Trefzer D , Zimpfer U , Schopf E , Wanscheidt W , Norgauer J. Matrix metalloproteinases and venous leg ulceration. Eur J Dermatol 2000. ; 10 : 173 – 80. [PubMed] [Google Scholar]
- 6. Egeblad M , Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002. ; 2 : 161 – 74. [DOI] [PubMed] [Google Scholar]
- 7. Galis ZS , Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 2002. ; 90 : 251 – 62. [PubMed] [Google Scholar]
- 8. Grinnell F , Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1‐proteinase inhibitor, and alpha2‐macroglobulin. J Invest Dermatol 1996. ; 106 : 335 – 41. [DOI] [PubMed] [Google Scholar]
- 9. Yager DR , Chen SM , Ward SI , Olutoye OO , Diegelmann RF , Kelman Cohen I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen 1997. ; 5 : 23 – 32. [DOI] [PubMed] [Google Scholar]
- 10. Xue M , Le NT , Jackson CJ. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin Ther Targets 2006. ; 10 : 143 – 55. [DOI] [PubMed] [Google Scholar]
- 11. Saito S , Trovato MJ , You R , Lal BK , Fasehun F , Padberg FT Jr , Hobson RW 2nd , Duran WN , Pappas PJ. Role of matrix metalloproteinases 1, 2, and 9 and tissue inhibitor of matrix metalloproteinase‐1 in chronic venous insufficiency. J Vasc Surg 2001. ; 34 : 930 – 8. [DOI] [PubMed] [Google Scholar]
- 12. Trengove NJ , Stacey MC , Macauley S , Bennett N , Gibson J , Burslem F , Murphy G , Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999. ; 7 : 442 – 52. [DOI] [PubMed] [Google Scholar]
- 13. Lobmann R , Ambrosch A , Schultz G , Waldmann K , Schiweck S , Lehnert H. Expression of matrix‐metalloproteinases and their inhibitors in the wounds of diabetic and non‐diabetic patients. Diabetologia . 2002. ; 45 : 1011 – 6. [DOI] [PubMed] [Google Scholar]
- 14. Cullen B , Smith R , McCulloch E , Silcock D , Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 2002. ; 10 : 16 – 25. [DOI] [PubMed] [Google Scholar]
- 15. Cullen B , Watt PW , Lundqvist C , Silcock D , Schmidt RJ , Bogan D , Light ND. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol 2002. ; 34 : 1544 – 56. [DOI] [PubMed] [Google Scholar]
- 16. Wiegand C , Schonfelder U , Abel M , Ruth P , Kaatz M , Hipler UC. Protease and pro‐inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res 2010. ; 302 : 419 – 28. [DOI] [PubMed] [Google Scholar]
- 17. Shi L , Ramsay S , Ermis R , Carson D. In vitro and in vivo studies on matrix metalloproteinases interacting with small intestine submucosa wound matrix. Int Wound J 2011. ; 9 : 44 – 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schonfelder U , Abel M , Wiegand C , Klemm D , Elsner P , Hipler UC. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials . 2005. ; 26 : 6664 – 73. [DOI] [PubMed] [Google Scholar]
- 19. Edwards JV , Yager DR , Cohen IK , Diegelmann RF , Montante S , Bertoniere N , Bopp AF. Modified cotton gauze dressings that selectively absorb neutrophil elastase activity in solution. Wound Repair Regen 2001. ; 9 : 50 – 8. [DOI] [PubMed] [Google Scholar]
- 20. Rayment EA , Dargaville TR , Shooter GK , George GA , Upton Z. Attenuation of protease activity in chronic wound fluid with bisphosphonate‐functionalised hydrogels. Biomaterials 2008. ; 29 : 1785 – 95. [DOI] [PubMed] [Google Scholar]
- 21. Cao Y , Croll TI , Rizzi SC , Shooter GK , Edwards H , Finlayson K , Upton Z , Dargaville TR. A peptidomimetic inhibitor of matrix metalloproteinases containing a tetherable linker group. J Biomed Mater Res A 2011. ; 96 : 663 – 72. [DOI] [PubMed] [Google Scholar]
- 22. Vachon DJ , Yager DR. Novel sulfonated hydrogel composite with the ability to inhibit proteases and bacterial growth. J Biomed Mater Res A 2006. ; 76 : 35 – 43. [DOI] [PubMed] [Google Scholar]
- 23. Smeets R , Ulrich D , Unglaub F , Woltje M , Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J 2008. ; 5 : 195 – 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calne S. International consensus. Acellular matrices for the treatment of wounds. An expert working group review. Wounds International. London; 2010.
- 25. Lun S , Irvine SM , Johnson KD , Fisher NJ , Floden EW , Negron L , Dempsey SG , McLaughlin RJ , Vasudevamurthy M , Ward BR , May BCH. A functional extracellular matrix biomaterial derived from ovine forestomach. Biomaterials 2010. ; 31 : 4517 – 29. [DOI] [PubMed] [Google Scholar]
- 26. Irvine SM , Cayzer J , Todd EM , Lun S , Floden EW , Negron L , Fisher JN , Dempsey SG , Alexander A , Hill MC , O'Rouke A , Gunningham SP , Knight C , Davis PF , Ward BR , May BCH. Quantification of in vitro and in vivo angiogenesis stimulated by ovine forestomach matrix biomaterial. Biomaterials 2011. ; 32 : 6351 – 61. [DOI] [PubMed] [Google Scholar]
- 27. Knight CG , Willenbrock F , Murphy G. A novel coumarin‐labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Letters 1992. ; 296 : 263 – 6. [DOI] [PubMed] [Google Scholar]
- 28. Nakajima K , Powers JC , Ashe BM , Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1‐protease inhibitor reactive site. J Biol Chem 1979. ; 254 : 4027 – 32. [PubMed] [Google Scholar]
- 29. Edwards JV , Howley PS. Human neutrophil elastase and collagenase sequestration with phosphorylated cotton wound dressings. J Biomed Mater Res A 2007. ; 83 : 446 – 54. [DOI] [PubMed] [Google Scholar]
- 30. Rayment EA , Upton Z. Finding the culprit: a review of the influences of proteases on the chronic wound environment. Int J Low Extrem Wounds 2009. ; 8 : 19 – 27. [DOI] [PubMed] [Google Scholar]
- 31. Beidler SK , Douillet CD , Berndt DF , Keagy BA , Rich PB , Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen 2008. ; 16 : 642 – 8. [DOI] [PubMed] [Google Scholar]
- 32. Wysocki AB , Staiano‐Coico L , Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol 1993. ; 101 : 64 – 8. [DOI] [PubMed] [Google Scholar]
- 33. Fray MJ , Dickinson RP , Huggins JP , Occleston NL. A potent, selective inhibitor of matrix metalloproteinase‐3 for the topical treatment of chronic dermal ulcers. J Med Chem 2003. ; 46 : 3514 – 25. [DOI] [PubMed] [Google Scholar]
- 34. Yager DR , Zhang LY , Liang HX , Diegelmann RF , Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996. ; 107 : 743 – 8. [DOI] [PubMed] [Google Scholar]
- 35. Nwomeh BC , Liang HX , Cohen IK , Yager DR. MMP‐8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res 1999. ; 81 : 189 – 95. [DOI] [PubMed] [Google Scholar]
- 36. Rayment EA , Upton Z , Shooter GK. Increased matrix metalloproteinase‐9 (MMP‐9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol 2008. ; 158 : 951 – 61. [DOI] [PubMed] [Google Scholar]
- 37. Vaalamo M , Weckroth M , Puolakkainen P , Kere J , Saarinen P , Lauharanta J , Saarialho‐Kere UK. Patterns of matrix metalloproteinase and TIMP‐1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 1996. ; 135 : 52 – 9. [PubMed] [Google Scholar]
- 38. Klein T , Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41 : 271 – 90. [DOI] [PMC free article] [PubMed] [Google Scholar]


