Abstract
Fractionated irradiation (IR) before or after surgery of malignant tumours causes a high frequency of wound healing complications. Our aim was to investigate the effect of curcumin (CUM) on the healing of deep excision wound of mice exposed to fractionated IR by mimicking clinical conditions. A full‐thickness dermal excision wound was created on the shaved dorsum of mice that were orally administered or not with 100 mg of CUM per kilogram body weight before partial body exposure to 10, 20 or 40 Gy given as 2 Gy/day for 5, 10 or 20 days. The wound contraction was determined periodically by capturing video images of the wound from day 1 until complete healing of wounds. Fractionated IR caused a dose‐dependent delay in the wound contraction and prolonged wound healing time, whereas CUM administration before fractionated IR caused a significant elevation in the wound contraction and reduced mean wound healing time. Fractionated IR reduced the synthesis of collagen, deoxyribonucleic acid (DNA) and nitric oxide (NO) at different post‐IR times and treatment of mice with CUM before IR elevated the synthesis of collagen, DNA and NO significantly. Histological examination showed a reduction in the collagen deposition, fibroblast and vascular densities after fractionated IR, whereas CUM pre‐treatment inhibited this decline significantly. Our study shows that CUM pre‐treatment accelerated healing of irradiated wound and could be a substantial therapeutic strategy in the management of irradiated wounds.
Keywords: Collagen, Curcumin, Irradiation, Mice, Wound
INTRODUCTION
Ionising radiations cause multiple negative effects on healing of irradiated wounds leading to high incidence of complications in patients receiving radiotherapy before or after surgery 1, 2, 3. The skin irradiation (IR) inhibits inflammatory reactions, connective tissue proliferation, formation and maturation of granulation tissue, transcription of collagen mRNAs, secretion of collagen and neovascularisation that are fundamental to wound healing 4, 5. IR reduces replicative capacity of fibroblasts leading to retardation in the formation of collagen, delayed appearance of mature collagen and reduction in the tensile strength of the newly regenerated skin wound 6, 7, 8, 9, 10. Several endeavours have been made to delineate the new potential therapeutic options to augment healing in this setting. Hydrogel and hydrocolloid gel dressings have been reported to abate wound discomfort and delayed wound healing after IR (11). Phenytoin sodium has been reported to accelerate fibroblast proliferation and synthesis of collagen in irradiated wounds (12). Supplemental vitamin A has been found to prevent radiation‐induced defects in wound healing by enhancing the early inflammatory reaction during wound healing and by increasing the recruitment of monocytes and macrophages at the wound site (13). Our earlier reports have shown that ascorbic acid pre‐treatment enhanced the healing of wounds after IR in mice 8, 9, 10, 14. Certain radioprotective compounds such as mercaptoethylamine, serotonin and WR2721 have also been reported to be useful in combined injuries (15). Several growth factors and antimicrobial agents have been explored in animal models as potential options to improve wound healing in radiation‐damaged skin 16, 17, 18.
Many potential therapies and prophylaxes have been used for the management of irradiated wounds. However, little attention has been paid to delineate regenerative response of herbal products/dietary ingredients in the irradiated wound, which emphasises a need for continued research in the area of medical management of radiation casualties. The wounds and subsequent wound healing abnormalities cause a great physical and psychological discomfort and trauma to affected patients. Their treatment is extremely expensive and also leave scars after complete healing of wounds. Therefore, newer paradigms are required, which are non toxic, economic and not only useful for wound healing but also can augment general immunity during radiotherapy. The use of dietary ingredients in the reconstruction of irradiated wounds is an attractive proposition, because they are in daily use, have wide acceptability, better tolerance, do not have reported side effects, economic and can be safely manipulated for human use (19).
Curcumin (CUM) or diferuloylmethane [1,7‐bis (4‐hydroxy‐3‐methoxy‐phenyl) hepta‐1,6‐diene‐3,5‐dione] (Figure 1) is one of the most active principles derived from the turmeric rhizome (family: Zingiberaceae), which imparts a characteristic yellow colour to curry powder, the turmeric (20). CUM is widely used as a dietary pigment, and as an Indian folk medicine for the treatment of various illnesses including cough, common cold and sore throat. Traditionally, turmeric has been used to treat rheumatism, body pain, skin diseases, intestinal worms, diarrhoea, intermittent, fevers, hepatic disorders, biliousness, urinary discharges, dyspepsia, inflammations, constipation, leucoderma, amenorrhoea and colic. Turmeric has been used as an emmenagogue, diuretic, carminative and applied externally against bruises, pains, sprains, boils, swellings and skin diseases 21, 22. In China, it is used to treat angina pectoris, stomachache, postpartum abdominal pain and gallstones (23). This traditional Indian medicine uses turmeric to treat biliary disorders, anorexia, cough, diabetic wounds, hepatic disorders, rheumatism and sinusitis. This non nutritive phytochemical is pharmacologically safe, considering that it has been consumed as a dietary spice, at doses up to 100 mg/day, for centuries (24). The recent reports indicate that humans can tolerate a dose as high as 12 g/day of CUM without toxic side effects (25), which reaffirm its non toxic nature.
Figure 1.
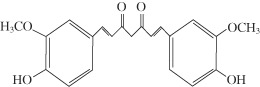
Chemical structure of curcumin (CUM).
The oral and topical administration of CUM has been reported to be effective in wound repair of normal and diabetic wounds 20, 26. CUM has been reported to inhibit hydrogen peroxide‐induced oxidative damage in human keratinocytes and fibroblasts (27). It has also been reported to possess potent antiinflammatory, antioxidant, immunomodulatory and anticancer activities (21). Recently, CUM treatment has been reported to accelerate healing of excision wounds in mice exposed to different doses of γ radiation 28, 29, 30. However, its effect after fractionated IR has not been evaluated. Fractionated IR before or after surgery retards the regenerative responses and impede healing of surgical wounds indicating a need for the pharmacological agents that can counteract the multiple negative effects of radiotherapy on the wound‐healing process. This implies that studies need to be designed in suitable preclinical models, which mimic the clinical conditions. Therefore, this study was designed to study the effect of CUM on the healing of deep dermal excision wounds in mice partially exposed to different doses of fractionated γ radiation delivered as 2 Gy/fraction.
MATERIALS AND METHODS
The animal care and handling were carried out according to the guidelines issued by the World Health Organization (Geneva, Switzerland) and the Indian National Science Academy (New Delhi, India). Eight‐ to ten‐week‐old Swiss albino mice of both sexes (1:1 ratio) weighing 30–36 g were selected from an inbred colony maintained under the controlled conditions of temperature (23 ± 2°C), humidity (50 ± 5%) and light (14 and 10 hours of light and dark, respectively). The animals had free access to sterile food and water. The food consisted of 50% cracked wheat, 40% Bengal gram, 4% milk powder, 4% yeast powder, 0·75% sesame oil, 0·25% cod liver oil and 1% salt. Four animals were housed in a sterile polypropylene cage containing sterile paddy husk (procured locally) as bedding throughout the experiment. This study was approved by the institutional animal ethical committee of Kasturba Medical College, Manipal.
Chemicals
CUM (catalogue no: C1386), hydroxyproline (catalogue no: H5534), chloramine‐T (catalogue no: C9887), deoxyribonucleic acid (DNA; catalogue no: D4522), diphenylamine (catalogue no: D2385), N‐(1‐naphthyl)ethylenediamine dihydrochloride (NEDD, catalogue no: N5889) and sulphanilamide (catalogue no: S9251) were procured from Sigma Chemical Co. (St Louis, MO), whereas trichloroacetic acid (TCA, catalogue no: 15213‐5000) and ρ‐dimethylaminobenzaldehyde (catalogue no: 42363‐0250) were requisitioned from Across Organics (Geel, Belgium). Ethanol (catalogue no: SIN 1170) was purchased from Hayman Ltd (Witham, Essex, UK) and sterillium disinfectant solution was procured from Bode Chemie (Hamburg, Germany). Perchloric acid, diethyl ether, formalin, sodium hydroxide, sodium nitrite, phosphoric acid, hydrochloric acid and sodium chloride were purchased from Ranbaxy Fine Chemical (New Delhi, India).
Preparation of drug and mode of administration
Because CUM is insoluble in water, its required amount was suspended in 0·5% carboxymethylcellulose (CMC) immediately before use. The animals were administered with 0·01 ml of CMC per kilogram body weight or 100 mg of CUM per kilogram body weight orally through an oral gavage.
Experimental protocol
The wound‐healing potential of CUM was studied by dividing the animals into the following groups.
Carboxymethylcellulose + irradiation
The animals of this group received 0·01 ml 0·5% CMC per kilogram body weight daily, consecutively for 5, 10 and 20 days, respectively, before exposure to 0, 10, 20 or 40 Gy of γ radiation (8).
Curcumin + irradiation
This group of animals was administered with a single dose of 100 mg of CUM per kilogram body weight once daily, consecutively for 5, 10 and 20 days, respectively, before exposure to 0, 10, 20 or 40 Gy of γ radiation (8).
A gap of 2 days was allowed for 20 and 40 Gy IR before 6th or 12th administration of CMC or CUM as the case may be.
Irradiation
One hour after each administration of CMC or CUM, each animal was placed into a specially designed well‐ventilated acrylic restrainer and the lower half of the animals (below the rib cage) was exposed to 0 [sham irradiation (SIR)] or 2 Gy once daily, delivered at a dose rate of 1·35 Gy/min (10) from a 60Co Teletherapy source (Theratron, Atomic Energy Agency, Ottawa, ON, Canada). Treatments were given once daily for 5, 10 or 20 days to a total dose of 10 Gy (a total of 5 fractions of 2 Gy each), 20 Gy (a total of 10 fractions of 2 Gy each) or 40 Gy (a total of 20 fractions of 2 Gy each), respectively. A gap of two days was allowed between the delivery of fifth and sixth fractions of radiation for mice receiving a total dose of 20 and 40 Gy.
Production of full‐thickness skin wound
The fur of the dorsum of each animal was removed with a cordless electric mouse clipper (Wahl Clipper Corporation, Sterling, IL) before exposure to last fraction of γ radiation and a full‐thickness skin wound was created on the dorsum (below the rib cage) as described earlier within 1 hour after IR 8, 9, 10. Briefly, the animals were anaesthetised using diethyl ether and the entire body was thoroughly cleaned and decontaminated by wiping with sterillium (Bode Chemie) disinfectant solution. The cleared dorsal surface of skin was marked with a sterile circular (15‐mm diameter) stainless steel stencil. A full‐thickness wound was created by excising the full‐thickness skin flap in an aseptic environment using sterile scissors and forceps. Each wounded animal was housed in a separate sterile polypropylene cage until the termination of experiments.
Wound contraction
Wound contraction was monitored by capturing the video images of each full‐thickness wound with a charge‐coupled device camera connected to a computer 28, 29, 30. The first image of each wound from different groups was obtained on day 1 post‐wounding, and that day was considered as day 1. The subsequent images were captured on days 3, 6, 9, 12 and 15 post‐wounding. The wound area was calculated using Auto CAD R14 (Autodesk Inc, San Rafael, CA) software. Ten animals were used in each concurrent group for each radiation dose, except 40 Gy, where a minimum of 14 animals was used to account for radiation‐related mortality, if any. A total number of 88 mice were used for this experiment.
Mean wound healing time
A separate experiment was performed to determine the mean wound healing time in mice receiving CUM or not before exposure to different doses of fractionated γ radiation, where grouping and other conditions were essentially similar to that described above, except that all the animals in each group were monitored regularly until complete healing of wounds and the day by which each wound healed completely was recorded. Mean of all healed wounds was calculated and expressed as mean wound healing time in days. Ten animals were used in each concurrent group for each radiation dose, except 40 Gy radiation, where a minimum of 14 animals was used to account for radiation‐related mortality, if any. A total number of 88 mice were used in this experiment.
Biochemical estimations
A separate set of experiments was carried out to study the effect of CUM on various biochemical profiles of irradiated wounds after exposure to 0, 10, 20 or 40 Gy of fractionated γ radiation. Grouping of animals and production of wounds were essentially similar to that described in the experimental protocol section. Granulation tissues were collected from regenerating excision wound/s on days 4, 8 or 12 post‐IR and stored at – 70°C until analysis. Six animals were used for each radiation dose in each concurrent group at each interval and a minimum of 144 animals was used for various estimations listed in the following sections.
Collagen
As an indication of total collagen content, hydroxyproline concentration was determined as described earlier (31). The weighed granulation tissues were hydrolysed in 6 N HCl for 3 hours at 130°C, neutralised to pH 7 with 2·5 N NaOH and diluted with Milli‐Q water. The diluted solution was mixed with chloramine‐T reagent and incubated for 20 minutes at room temperature. Thereafter, freshly prepared ρ‐dimethylaminobenzaldehyde (Ehrlich's reagent) solution was added and incubated for 15 minutes at 60°C. The absorbance of each sample was measured at 550 nm using a double beam ultraviolet (UV)‐visible spectrophotometer (Shimadzu UV‐260, Shimadzu Corporation, Tokyo, Japan). The amount of hydroxyproline was determined by comparing with the standard curve. Total collagen from hydroxyproline analysis was determined by multiplying with a factor of 6·94 (32). Collagen content of granulation tissues has been expressed as milligram per gram dry tissue weight.
Deoxyribonucleic acid
Estimation of DNA gives an indication of cell proliferation and was measured by homogenising the dry granulation tissues in 5% TCA followed by centrifugation. The pellets were washed with 10% TCA, resuspended in 5% TCA and incubated at 90°C for 15 minutes. The contents were centrifuged again and the resultant supernatant was used for the estimation of DNA by the method of Burton (33). The DNA was hydrolysed with 60% perchloric acid at 80°C for 20 minutes followed by the addition of Burton's diphenylamine reagent and overnight incubation at room temperature. Thereafter, 95% ethanol was added and absorbance was read at 600 nm using a double beam UV‐visible spectrophotometer. The amount of DNA was determined by comparing with the standard curve and has been expressed as milligram/gram dry tissue weight.
Nitric oxide
The stable end products of nitric oxide (NO) biosynthesis were measured by estimating nitrite levels in the granulation tissue of wounds. The granulation tissues were homogenised in hypotonic saline and centrifuged. Nitrite concentrations were determined with Griess reagent (34). Briefly, the supernatant was mixed with freshly prepared Griess reagent (0·1% NEDD, 1% sulphanilamide and 5% phosphoric acid in a 1:1:1 ratio), incubated at 37°C for 30 minutes and the absorbance was recorded at 543 nm using a double beam UV‐visible spectrophotometer. Sodium nitrite was used as standard. Nitrite levels have been expressed in terms of µM/100 mg dry tissue weight.
Histological studies
A separate experiment was conducted to evaluate the histological alterations during wound healing after exposure to 0, 10, 20 or 40 Gy fractionated γ radiation. Grouping of animals and production of wounds were carried out as already described above. The cross‐sectional full‐thickness skin biopsies from each group were collected on days 4, 8 and 12 post‐IR. The samples were fixed in 10% buffered formalin, passed through different grades of alcohol to ensure complete dehydration and were embedded in paraffin wax. Medial sections were cut (5 µm) perpendicular to the surface, starting from the centre of the wound and stained with haematoxylin and eosin. Tissue sections were assessed in a blinded fashion under transmitted light microscope (Photomicroscope III; Carl Zeiss, Oberkochen, Germany) using a planimeter for fibroblast proliferation, neovascularisation and collagen deposition. For collagen deposition studies, faintest traces of staining reaction, hyalinisation and irregular arrangement of collagen bundles were considered as +, whereas the most intense reaction and compactly arranged collagen bundles were considered as +++. Two areas in each section were counted for neovascularisation and fibroblast proliferation. The elongated spindle‐shaped cells with purple nuclei and pink cytoplasm were identified as fibroblasts and scored. Blood vessels that were conspicuous with haematoxylin and eosin stains were scored for vascular repopulation studies. Four animals were used in each concurrent group at each interval for each radiation dose and a minimum of 128 animals was used for histological investigations.
ANALYSIS OF DATA
Statistical significance between the treatments was determined using one‐way analysis of variance and Bonferroni's post hoc test was applied for multiple comparisons wherever necessary. The Solo 4 Statistical Package (BMDP Statistical Software Inc, Los Angeles, CA) was used for data analysis. All data are expressed as mean ± standard error of the mean.
RESULTS
Wound contraction
Progression of healing of excision wounds can be assessed by periodic computation of wound contraction. The area of each wound at a specific time has been expressed as the percentage of its original size on day 1. The mean corresponding area of wound for each group was plotted as a function of days after wounding. Wound contraction accelerated with time and a steady contraction of excision wound was observed in both the CMC + SIR and CUM + SIR (0 Gy) groups (Figure 2). A maximum wound contraction was observed during days 6–12 post‐IR in both CMC and CUM + SIR groups. CUM treatment resulted in a significant but a number of administration‐dependent enhancements of wound contraction at all post‐IR times when compared with CMC + SIR group (Figure 2). Furthermore, CMC + SIR group showed formation of scab, whereas scab formation was relatively less in CUM + SIR group.
Figure 2.
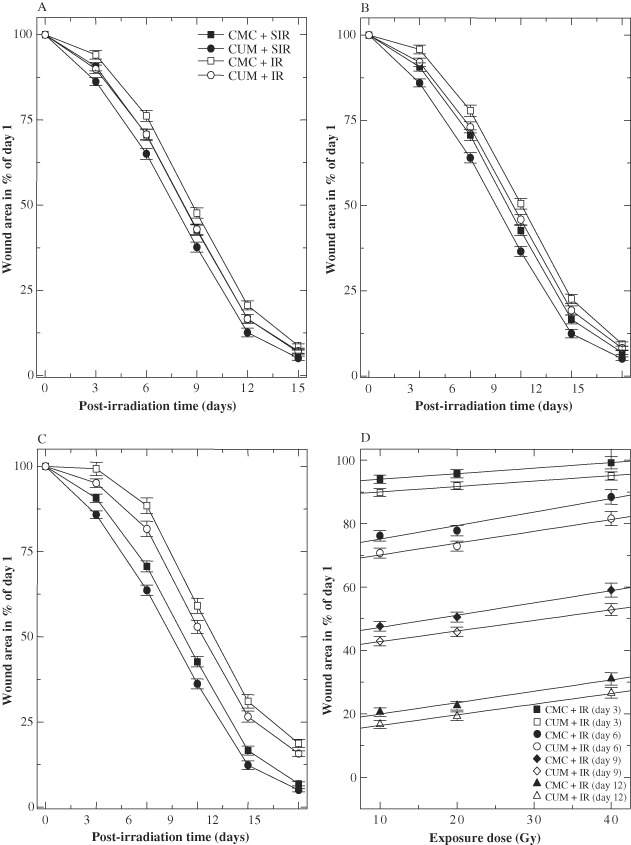
Effect of curcumin (CUM) treatment on contraction of excision wound in the skin of mice exposed to different doses of fractionated γ radiation: (A) 10 Gy; (B) 20 Gy; (C) 40 Gy; and (D) dose–response curve.
The exposure of mice to various doses of fractionated γ radiation resulted in a dose‐dependent delay in wound contraction (Figure 2D). Exposure of animals to 10 Gy, delayed the wound contraction at days 6 (P < 0·025) and 9 (P < 0·032) post‐IR (Figure 2A) accompanied by an early formation of scab in wound when compared with CMC+ SIR group. The wound contraction between CMC + IR (20 Gy) and CMC + SIR group was significantly greater on days 3 (P < 0·01), 6 (P < 0·006), 9 (P < 0·002), 12 (P < 0·006) and 15 (P < 0·013) post‐IR (Figure 2B). Exposure of animals to 40 Gy significantly delayed wound contraction at all the post‐IR days (Figure 2C) and the scab formation was quite thick when compared with the CMC + SIR group.
CUM treatment before exposure to 10 Gy IR resulted in a significant reduction in the radiation‐induced delay in contraction of wounds at days 3 (P < 0·03), 6 (P < 0·03), 9 (P < 0·04) and 12 (P < 0·05) post‐IR (Figure 2A) and reduced the scab formation as a result scab was thin when compared with CMC + IR group. Administration of CUM before 20 Gy caused a significant rise in wound contraction on days 3 (P < 0·05), 6 (P < 0·05) and 9 (P < 0·05) post‐IR when compared with the concurrent CMC + IR group (Figure 2B). A further increase in IR dose up to 40 Gy after CUM treatment resulted in a significant elevation in the wound contraction in CUM + IR group only at days 6 (P < 0·05) and 9 (P < 0·05) post‐IR when compared with CMC + IR group (Figure 2C).
Mean wound healing time
The excision wounds completely healed by days 18 ± 0·45 post‐IR in CMC + SIR group, whereas treatment of mice with CUM for 5, 10 or 20 days before SIR resulted in a significant and number of CUM administration‐dependent reduction in the mean wound healing time in the CUM + SIR group (Figure 3). Exposure of mice to different doses of fractionated γ radiation resulted in a dose‐dependent prolongation in the complete closure of wounds and a mean wound healing time of 20 ± 0·55, 23 ± 0·7 and 29·4 ± 1·5 days post‐IR was recorded for 10, 20 and 40 Gy, respectively, in CMC + IR group (Figure 3). Treatment of mice with 100 mg/kg of CUM before exposure to various doses of fractionated γ radiation inhibited the radiation‐induced delay in healing of excision wounds as a result there was a significant (P < 0·05) reduction in mean wound healing time in the animals exposed to 10 and 20 Gy. A mean wound healing time of 18·6 ± 0·46, 21 ± 0·53 and 28·8 ± 1·03 days was observed for 10, 20 and 40 Gy, respectively, in CUM + IR group (Figure 3).
Figure 3.
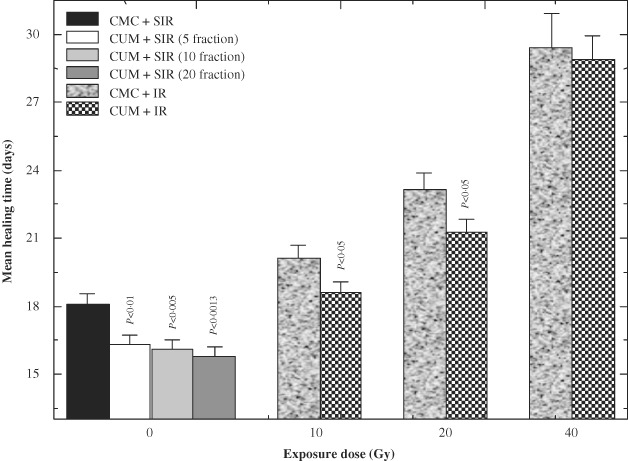
Effect of curcumin (CUM) treatment on the progression of wound closure with time in mice exposed to various doses of fractionated γ radiation. P values when CUM groups are compared with carboxymethylcellulose (CMC) groups.
Biochemical estimations
Collagen
Hydroxyproline content is an index of collagen and is a measure of synthesis of newly formed collagen. Estimation of hydroxyproline content showed the greatest synthesis of collagen in CMC or CUM + SIR groups on day 8 post‐IR; thereafter, the status of neocollagen synthesis remained unaltered in these groups for 5, 10 and 20 administrations of CMC or CUM. IR of animals to 10, 20 and 40 Gy resulted in a significant but dose‐dependent decline in the collagen synthesis at all post‐IR times (Figure 4). The collagen synthesis decreased significantly on days 4 (P < 0·002), 8 (P < 0·0003) and 12 (P < 0·004) post‐IR in the granulation tissues of wounds produced after 40 Gy in CMC + IR group when compared with the CMC + SIR group. Despite this reduction in collagen synthesis, a maximum synthesis of collagen was observed on day 8 post‐IR in the CMC + IR group; thereafter, collagen synthesis declined and a nadir was reached on day 12 post‐IR. A similar pattern of collagen synthesis was seen in CUM + IR group, except that the treatment of mice with 100 mg/kg CUM before exposure to various doses of fractionated γ radiation caused a significant rise in the collagen synthesis when compared with the concurrent CMC + IR group (Figure 4). Pre‐treatment of mice with CUM before exposure to various doses of fractionated radiation could not restore the level of collagen to normal even by day 12 post‐IR for 20 and 40 Gy IR in CUM + IR group (Figure 4).
Figure 4.
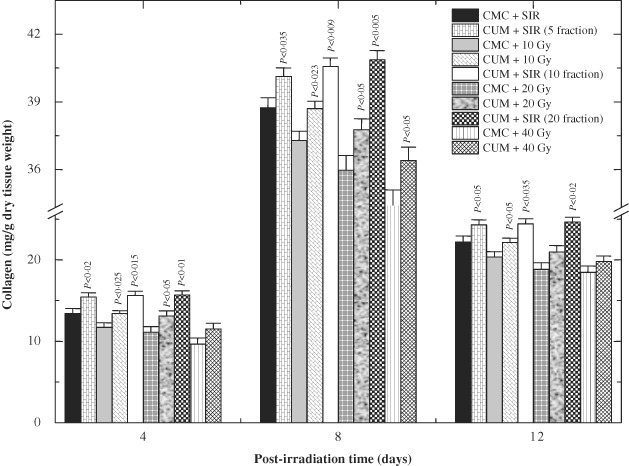
Effect of curcumin (CUM) treatment on biosynthesis of collagen in the excision wound of mice exposed to various doses of fractionated γ radiation. P values when CUM groups are compared with carboxymethylcellulose (CMC) groups.
Deoxyribonucleic acid
The increase in DNA contents of treated wounds indicates hyperplasia of cells. There was a rapid rise in DNA content up to day 8 post‐IR in both SIR groups (Figure 5). Exposure of animals to various doses of fractionated γ radiation significantly reduced the DNA contents at all post‐IR times in CMC + IR group, whereas CUM treatment before IR resulted in a significant escalation in the DNA contents at days 4, 8 and 12 post‐IR in CUM + IR group, except for 40 Gy, where this rise was significant on 8 days post‐IR only (Figure 5).
Figure 5.
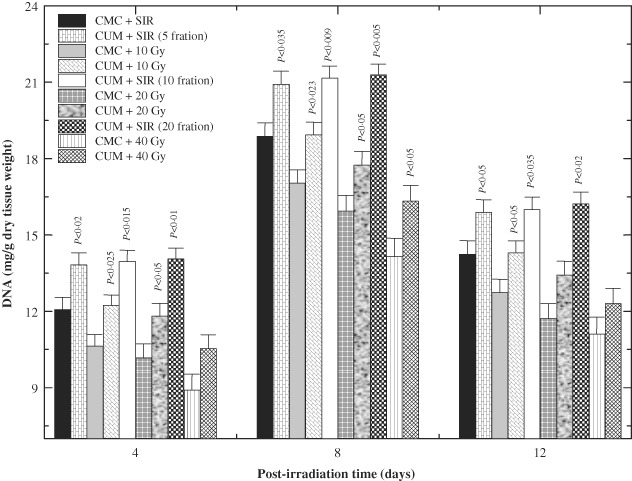
Effect of curcumin (CUM) treatment on biosynthesis of deoxyribonucleic acid (DNA) in the excision wound of mice exposed to various doses of fractionated γ radiation. P values when CUM groups are compared with carboxymethylcellulose (CMC) groups.
Nitric oxide
End product of NO synthesis, the nitrite was elevated at day 4 post‐IR in the granulation tissue and the level of nitrite decreased substantially in both SIR groups (Figure 6). IR of animals to various doses of fractionated γ radiation considerably decreased nitrite contents in the granulation tissue at all post‐IR times (Figure 6), which was significantly (P < 0·05) less on all post‐IR days in CMC + IR group when compared with the CMC + SIR group. Treatment of mice with CUM before exposure to different doses of γ radiation resulted in a significant rise in the nitrite synthesis at all post‐IR times for 10 and 20 Gy IR, whereas this rise in nitrite content was significant only on eight days post‐IR for 40 Gy exposure in CUM + IR group (Figure 6).
Figure 6.
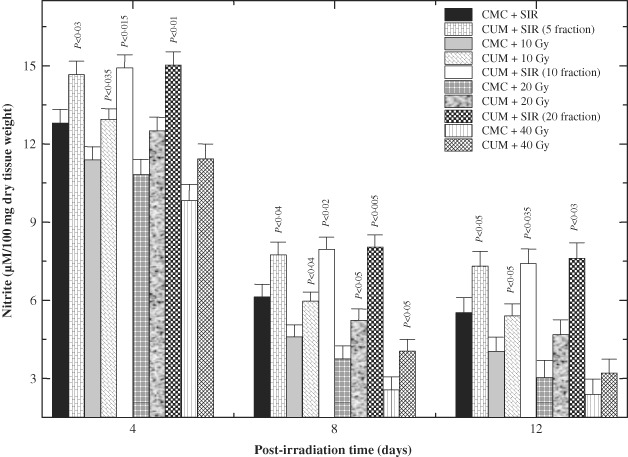
Effect of curcumin (CUM) treatment on biosynthesis of nitrite in the excision wound of mice exposed to various doses of fractionated γ radiation. P values when CUM groups are compared with carboxymethylcellulose (CMC) groups.
Histological studies
Histological evaluation of wound biopsies at various post‐IR times showed that CUM treatment alone did not alter the histology picture of regenerating wound except that it increased the collagen deposition, fibroblast and vasculature densities when compared with the SIR non drug‐treated controls (7, 8, 9). Exposure of mice to different doses of fractionated γ radiation caused degeneration and hyalinisation of collagen bundles that were more prominent at 20 and 40 Gy IR. Isolated ‘fragments’ of collagen surrounded by empty spaces that did not bind the stain could be seen at higher radiation doses. Pre‐treatment of irradiated mice with CUM caused an increase and restoration of collagen bundles. Fractionated IR decreased the density of fibroblasts (Figure 7) and blood vessels (Figure 8) in a dose‐dependent manner when compared with CMC + SIR group. Large, unusual and stellate cells or ‘radiation fibroblast' were discernible in the dermis at higher radiation doses, especially at 20 and 40 Gy. Variation in the epidermal thickness was also evident with increasing radiation dose. Likewise, irregularly shaped blood vessels were seen after exposure to different doses of radiation in the CMC + IR group. Treatment of mice with CUM protected against the radiation‐induced damage to fibroblasts and vasculature as showed by an increase in the density of fibroblasts and vasculature (7, 8, 9). However, normal histological picture was not restored.
Figure 7.
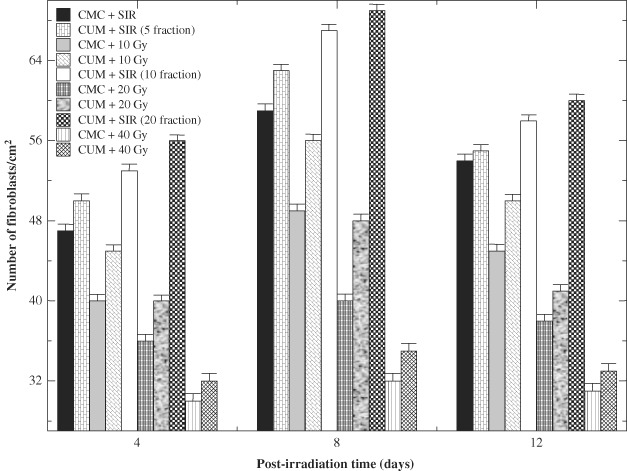
Effect of curcumin (CUM) on fibroblast density of wounded mice exposed to various doses of fractionated γ radiation.
Figure 8.
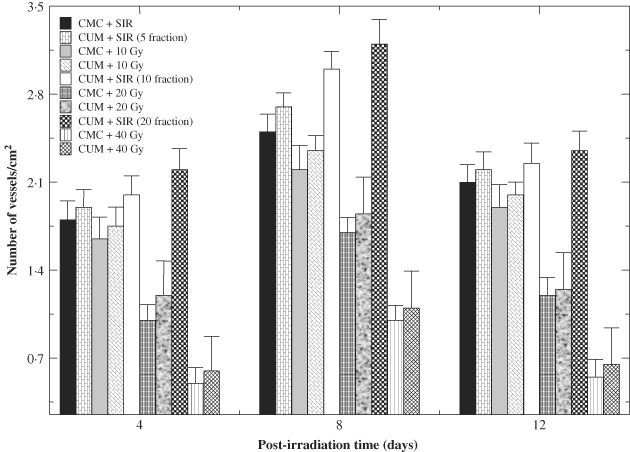
Effect of curcumin (CUM) on vasculature density of wounded mice exposed to various doses of fractionated γ radiation.
Figure 9.
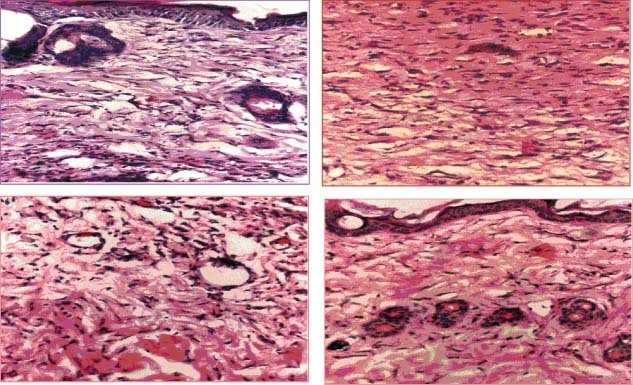
Histological picture of wound biopsy on day 6 post‐irradiation (IR) of mice treated with 100 mg/kg body weight of curcumin (CUM) before exposure to 10 Gy fractionated γ radiation delivered as 2 Gy/fraction for five days. Upper left: carboxymethylcellulose (CMC) + sham‐irradiation (SIR) group; upper right: CUM + SIR; lower left: CMC + IR; and lower right: CUM + IR group.
DISCUSSION
The process by which wounds heal has been the subject of research for several centuries and various factors have been implicated in retarding an organism's ability to heal. Introduction of pre‐ or postoperative radiotherapy as an adjuvant treatment to surgery has been reported to increase wound healing complications 1, 2. However, investigations on the modulation of radiation‐induced delay in wound repair by means of conventional wound care therapies are so infrequent that management policies must be derived in part from different but analogous clinical situations and/or from studies in the experimental animals. Moreover, to combat against increasing health care costs, it is important that the health care professional has an adequate understanding of the role of natural products or their active principles in the healing of irradiated wounds. There is also a need for a multifunctional pharmacological agent that conforms to all criteria of an optimal radioprotector including effectiveness, toxicity, availability, wider biological functions and tolerance. The use of herbal radioprotectors/antioxidants to overcome negative effects of ionising radiation by targeting reactive oxygen species could be a prudent therapeutic strategy to improve healing of irradiated wounds. CUM, an important active principle of turmeric and a widely used spice has been reported to promote wound healing in various wound models 26, 35, 36. Furthermore, it has been found to be a good antioxidant (37) and radioprotective agent (38). Therefore, an attempt has been made to explore the usefulness of CUM treatment in deep dermal excision wound of mice exposed to various doses of fractionated γ radiation.
Wound contraction can be defined as the centripetal movement of the edges of a full‐thickness wound to facilitate closure of the defect (39). Wound contraction is the culmination of various processes that set in immediately after wounding including inflammation, proliferation and migration of fibroblasts into the wound bed (40). The progression of wound healing can be judged by the periodic assessment of contraction of excision wounds. The exposure of mice to different doses of fractionated γ radiation delayed wound contraction in a dose‐dependent manner and the longest delay was observed for 40 Gy IR. A similar delay in wound contraction after exposure to γ radiation has been observed earlier, indicating that IR alters the local conditions of wound, which hampers wound repair 2, 8, 10, 14, 41. Treatment of mice with 100 mg/kg body weight of CUM before exposure to different doses of fractionated radiation resulted in an enhancement of wound healing as is evident by a greater degree of wound contraction and reduction in mean wound healing time. As far as authors are aware, CUM has not been used to treat wound injury after fractionated IR. However, CUM has been reported to accelerate the healing of wounds after acute whole‐ or hemi‐body IR 30, 31, 32. Ascorbic acid has been shown to enhance the healing of wounds after fractionated IR (8) as well as after whole‐ or hemi‐body exposure in mice earlier 9, 10, 14. Similarly, vitamin A supplementation has been reported to reduce the radiation‐induced delay in wound healing (13). Vitamin E treatment has also been reported to normalise the wound breaking strength after preoperative IR (42).
Collagen plays a pivotal role in the healing of wounds, as it is a principal component of connective tissues, which provides a structural framework, strength and milieu for the regenerating tissue. Collagen is produced by fibroblasts and it assists the wound in gaining tensile strength during wound repair (43). IR of animals caused a significant reduction in collagen synthesis, as is evident from the estimation of hydroxyproline contents in the granulation tissue. A progressive destruction of the native collagen fibrils has been reported after exposure to different doses of γ rays (44). A similar effect has been observed earlier in mice exposed to whole‐body, hemi‐body and fractionated IR 8, 14, 29, 30. CUM inhibited the radiation‐induced decrease in collagen synthesis at all post‐IR days. Likewise, CUM has been reported to elevate collagen synthesis in mice after acute whole‐ and hemi‐body IR 29, 30. Similarly, ascorbic acid has been reported to increase the collagen synthesis in the granulation tissue of wounded mice exposed to different doses of γ radiation including fractionated IR earlier 8, 9, 10. The increase in collagen on day 4 post‐IR may be due to the migration of fibroblasts on day 3 in the extracellular matrix (ECM) in the wound bed and their rapid proliferation leading to a maximum collagen synthesis by day 8 post‐IR (40). By the end of seven days, there will be enough deposition of ECM and the fibroblasts get transformed into the myofibroblasts that are responsible for contraction of the wound that may be the reason of decline in collagen contents at 12 day post‐IR and increased wound contraction. The reduced collagen contents in the CMC + IR group may be due to the killing of fibroblasts by ionising radiations that led to alleviation in collagen synthesis. This is supported by histological evaluation, where IR reduced fibroblast density significantly.
The increase in DNA contents in the wounds indicates a rise in the cell proliferation. The fractionated IR decreased DNA contents, whereas CUM treatment significantly increased DNA contents at days 4 and 8 post‐IR. Similarly, CUM has been reported to abate the radiation‐induced decline in the DNA synthesis after whole‐ or hemi‐body IR 29, 30. Likewise, ascorbic acid has been reported to increase the collagen synthesis in the granulation tissue of wounded mice exposed to whole‐ or hemi‐body IR 10, 14. Wound repair results from a series of well‐orchestrated cellular and biochemical events, including increased synthesis of the bioregulatory molecule NO. Most evidences suggest that adequate rate of NO production promotes processes central to wound healing such as inflammation, angiogenesis, fibroblast synthetic function, epithelial cell proliferation, regulation of collagen formation and wound contraction in distinct ways 45, 46. Inflammation is an early event during wound repair and regeneration and levels of nitrite, a stable end product of NO synthesis, are elevated early and transiently in fluid obtained from sponges implanted in subcutaneous wounds (47). A similar trend has been observed in the granulation tissues of unirradiated mice. However, exposure of mice to different doses of fractionated γ radiation reduced NO synthesis in a dose‐dependent manner, which explains the delay in the repair of irradiated wound because of inhibition of inflammatory response. Earlier, IR has been reported to reduce the NO synthesis in the granulation tissue of wounded mice 10, 14, 29, 30. The NO increase is essential to initiate the inflammatory reaction in wound microenvironment as it helps in the repair and regeneration of wounds. The decrease in NO expression has been correlated with radiation‐induced impairment in wound healing 48, 49. CUM treatment caused a significant rise in NO synthesis after fractionated IR, which may have stimulated the inflammatory phase and thus helped in the early repair and regeneration of wounds after fractionated IR. Earlier studies have shown that CUM increases the NO production in wounded mice after whole‐ and hemi‐body IR 29, 30. Likewise, ascorbic acid has been found to abate the radiation‐induced decline in the NO synthesis in wounded mice after whole‐ or hemi‐body IR resulting in the early repair and regeneration of irradiated wound 10, 14.
Histological observations support the decrease in collagen content in irradiated wounds, where a decline in collagen deposition has been observed. This is further supported by a decrease in the proliferation of fibroblasts, which are responsible for collagen synthesis. Ionising radiation has been reported to impede wound fibroblast proliferation, neovascularisation and collagen deposition 8, 14, 29, 30, 50. Similarly, total‐ and hemi‐body IR decreased cellular influx, vascularisation and collagen deposition in mice 7, 29, 30. The decrease in fibroblast proliferation and vascularisation is in accord with earlier reports, where a similar decrease in fibroblast proliferation, retardation of collagen maturation and overall delay in wound repair has been reported 7, 8, 15, 29, 30, 50, 51. Cell culture studies of fibroblasts exposed to ionising radiation have also shown that irradiated fibroblasts have a significantly prolonged generation time when compared with normal fibroblasts (6). Pre‐treatment of mice with CUM improved collagen deposition, reduced hyalinisation and increased vascularity and fibroblast density in the CUM + IR group. CUM has been reported to increase collagen deposition and to improve overall histological picture in artificially wounded animals 26, 35, 36 and also in irradiated wounds 29, 30.
The response of normal tissues to radiation can be viewed as two concurrent ongoing and interacting processes. The first has many features in common with the healing of traumatic wounds, perturbed by the radiation treatment. The second is a set of transient or permanent alterations of cellular and extracellular components within the irradiated volume, which may be responsible for the progression of injury over a period of time. In contrast to physical trauma, fractionated radiation therapy produces a series of repeated insults to tissues with the delivery of each fraction of radiation dose. Moreover, normal tissue responses are also influenced by rate of dose accumulation and with repetitive radiation exposure many cellular and molecular responses will be substantially exacerbated, suppressed or substantially altered when compared with single radiation exposure or traumatic injury. The detrimental consequences of ionising radiation on wound healing are multifaceted as it causes direct cytotoxic effects on various cellular/molecular components of wound repair and indirectly through the production of a burst of free radicals, which rearranges tissue components immediately, causing DNA damage and also altering proteins, lipids, carbohydrates and other complex molecules involved in tissue repair and regeneration. Injuries that are benign by themselves may become lethal when combined even with relatively small doses of radiation. Ionising radiation has been reported to cause severe damage to vital tissues, especially those with a high rate of cell division such as the haematopoietic system 13, 52. The loss of significant numbers of bone marrow cells can lead to an immunosuppressed state in which the individuals become highly susceptible to bacterial infections leading to complications in the healing of wounds. Shielding of bone marrow during acute whole‐body X‐IR has been reported to lower mortality and increase the closure of open‐dorsal skin wounds of rats (53). These studies suggest a requirement of radiation‐sensitive bone marrow‐derived cells in tissue repair and regeneration. Other possibility includes a delay in fixation of the wound edges to underlying tissues, which may be due to lack of fibroblast proliferation and decrease in fibroblast synthetic function in the granulation bed. The contraction of open‐excision wounds has been found to be a function of contractile fibroblasts, known as myofibroblasts (54). Recently, importance of bone marrow in wound healing has been reaffirmed as it has been found to contribute non inflammatory cells including fibroblast‐like cells and keratinocytes apart from the inflammatory cells, which play a pivotal role in the repair and regeneration of wound (55). IR is known to impair wound healing in skin through its cytotoxic effect on fibroblasts. This impairment in wound repair may be due to the delay in the progression of cells through the cell cycle induced by radiation 6, 56. Radiation may have adverse effect on fibroblasts through bone marrow depression, because some fibroblasts of the normal subcutaneous connective tissue, participating in wound healing have been shown to originate from the bone marrow 57, 58. The recent studies suggest that vascular cell progenitors are resident in bone marrow and contribute to blood vessel formation during tissue repair and in other pathological conditions 59, 60. A similar effect cannot be ruled out in this study because long bones of hind limbs were irradiated during the fractionated IR. Furthermore, radiation‐induced cytotoxicity is mediated through the production of oxygen‐derived free radicals. The overproduction of reactive oxygen species is the main cause of oxidative stress that induces detrimental cytotoxic effects, causing delayed wound healing (61). The peroxisome proliferator‐activated receptor (PPAR) is upregulated during wound repair and regeneration. The retardation in wound healing after IR may be due to suppression of PPAR expression. The IR has been reported to reduce the expression of PPAR (62).
The accelerated wound healing in this investigation by CUM may not be due to a single mechanism but could be due to the interplay of several putative mechanisms. CUM has a polyphenolic and beta‐diketone functional groups and therefore acts as strong antioxidant and inhibits lipid peroxidation 37, 63. The potent oxidative stress and lipid peroxidation induced by wound injury and IR may be neutralised by CUM resulting in the early healing of irradiated wounds in this study. CUM has been reported to scavenge‐free radicals, increase antioxidant status, inhibit lipid peroxidation, elevate glutathione‐s‐transferase (GST), glutathione peroxidase (GSHpx), superoxide dismutase (SOD), glutathione (GSH) and sulphydryl groups and heal the wounds faster 37, 63, 64, 65. A similar increase in antioxidants and inhibition of lipid peroxidation has been observed in this study after fractionated IR of mouse skin (data not shown). Numerous studies indicate the beneficial effects of CUM on wound healing through changes in cell regeneration and collagen synthesis 26, 35, 36. An increased collagen synthesis in this study also supports this contention. Similarly, increased synthesis of DNA and NO contents may also have been responsible for the accelerated healing of irradiated wounds in CUM pre‐treated groups. Degradation of fibrin deposited immediately after injury is essential for migration, cell proliferation and adhesion of various cells into the wound bed for proper wound repair. CUM treatment may have activated fibrinolyis by upregulating urokinase plasminogen activator mRNA and subsequently augmented healing of irradiated wound (66). The molecular mechanisms of action of CUM are fairly worked out, which may have also played a key role in enhancing the healing of irradiated wounds. Ionising radiation has been reported to induce COX‐II protein and upregulate COX‐II mRNA and inhibition of radiation‐induced COX‐II and COX‐II mRNA by CUM may have contributed in enhancing the repair and regeneration of irradiated wounds 67, 68, 69. The wounding and IR elicit proinflammatory response by transcriptional activation of NF‐κB 70, 71. Therefore, CUM pre‐treatment may have blocked the radiation and wound injury‐induced transcriptional activation of NF‐κB resulting in the accelerated healing of wounds without adversely affecting the required inflammatory process for wound healing. In fact, CUM has been reported to abrogate the transcriptional activation of NF‐κB in a number of studies 68, 72. The fact that treatment of keratinocytes with pyrrolidine dithiocarbamate before HeNe IR was found to abrogate laser and wounding‐induced NF‐κB activation (73) supports this contention. IR adversely affects the expression of PPAR (62) and upregluation of PPAR by CUM may have also contributed to early closure of irradiated wounds as PPAR is essential for cell proliferation and wound repair 74, 75.
Most of the animal models of radiation‐impaired wound healing involve IR of the whole animal or a localised flap of skin with single large doses of radiation, making it difficult to apply data to human beings, where the treatment is usually given with multiple smaller doses of radiation over an extended period of time. This study has used deep excision wound model, which is of great clinical relevance, because regimen similar to clinical radiotherapeutic treatment has been used to study the effect of fractionated radiation on wound repair and explore the application of CUM in this setting. This study shows that treatment of CUM once daily before exposure to fractionated γ radiation accelerates wound healing in mice, as is evident from acceleration in the contraction of wound, reduction in mean wound healing time. The early closure of wound by CUM may be due to increased synthesis of collagen, DNA and NO, enhanced proliferation of fibroblasts, increased vasculature into the wound bed and reduction in oxidative stress. The accelerated wound repair by CUM may be due to reduction in radiation‐induced oxidative stress, upregulation of urokinase plasminogen activator mRNA, downregulation of nuclear transcription factor NF‐κB, COX‐II and upregulation of another nuclear factor PPAR. Observations suggest that strategies to manipulate pathophysiological environment of irradiated wounds by CUM are likely to be of an outstanding significance in radiation‐impaired healing of wounds. Additional studies will be purposeful towards better understanding of the molecular mechanism of action of CUM in initiating and supporting the cascade of tissue repair and regenerative processes in irradiated wounds.
ACKNOWLEDGEMENTS
We are thankful to Dr MS Vidyasagar, Prof. and Head and Dr JGR Solomon, Department of Radiotherapy and Oncology, Kasturba Medical College Hospital, Manipal, for providing the necessary IR facilities and for dosimetric calculations, respectively. The financial assistance in the form of Senior Research Fellowship to Dr GK Rajanikant by the Indian Council of Medical Research (ICMR), Government of India, and New Delhi, India, to carry out the above study is gratefully acknowledged.
REFERENCES
- 1. Bell RS, O’Sullivan B, Langer F, Mahoney JL, Lichtenstein SV, Moffat FL, Cummings BJ, Hawkins NV, Fornasier VL. Complications and functional results after limb‐salvage surgery and radiotherapy for difficult mesenchymal neoplasms: a prospective analysis. Can J Surg 1989;32:69–73. [PubMed] [Google Scholar]
- 2. Kumar P, Jagetia GC. Modulation of wound healing in Swiss albino mice by different doses of gamma radiation. Burns 1995;21:163–5. [DOI] [PubMed] [Google Scholar]
- 3. Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol 1997;42:99–106. [DOI] [PubMed] [Google Scholar]
- 4. Bernstein EF, Sullivan FJ, Mitchell JB, Salomon GD, Glatstein E. Biology of chronic radiation effect on tissues and wound healing. Clin Plast Surg 1993;20:435–53. [PubMed] [Google Scholar]
- 5. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol 1998;17:117–23. [PubMed] [Google Scholar]
- 6. Rudolph R, Vande Berg J, Schneider JA, Fischer JC, Poolman WL. Slowed growth of cultured fibroblasts from human radiation wounds. Plast Reconstructr Surg 1988;82:669–77. [DOI] [PubMed] [Google Scholar]
- 7. Vegesna V, Withers HR, Holly FE, McBride WH. The effect of local and systemic irradiation on impairment of wound healing in mice. Radiat Res 1993;135:431–3. [PubMed] [Google Scholar]
- 8. Jagetia GC, Rajanikant GK, Rao SK. Evaluation of the effect of ascorbic acid treatment in the artificially wounded mouse exposed to different doses of fractionated gamma radiation. Radiat Res 2003;159:371–80. [DOI] [PubMed] [Google Scholar]
- 9. Jagetia GC, Rajanikant GK, Baliga MS, Rao KVNM, Kumar P. Augmentation of wound healing by ascorbic acid treatment in mice exposed to γ‐radiation. Int J Radiat Biol 2004;80:347–54. [DOI] [PubMed] [Google Scholar]
- 10. Jagetia GC, Rajanikant GK, Rao KVNM. Ascorbic acid increases healing of excision wounds of mice whole body exposed to different doses of γ‐radiation. Burns 2007;33:484–94. [DOI] [PubMed] [Google Scholar]
- 11. Orsted H. Radiation skin reaction. Can Nurse 1989;85:30–1. [PubMed] [Google Scholar]
- 12. Song S, Cheng T. The effect of systemic and local irradiation on wound macrophage and repair promoting action of phenytion sodium. Zhonghma Yi Xue Za Zhi 1997;77:54–7. [PubMed] [Google Scholar]
- 13. Levenson SM, Gruber CA, Rettura G, Gruber DK, Demetriou AA, Seifter A. Supplemental vitamin A prevents the acute radiation‐induced defect in wound healing. Ann Surg 1984;200:494–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jagetia GC, Rajanikant GK, Rao KVNM. Modulation of radiation‐induced delay in the wound healing by ascorbic acid in mice hemi‐body exposed to different doses of γ‐radiation. Wounds 2003;15:324–38. [Google Scholar]
- 15. Stromberg LR, McLaughlin MM, Donati RM. Combined surgical and radiation injury‐3. The effect of pre‐irradiation radioprotective drug treatment. Proc Soc Exp Biol Med 1968; 129:140–3. [DOI] [PubMed] [Google Scholar]
- 16. Donati RM. Combined surgical and radiation injury IV: effect of anti‐microbials on the wound healing pattern of the X‐irradiated rat. Arch Surg 1971;102:132–5. [DOI] [PubMed] [Google Scholar]
- 17. Mustoe TA, Purdy J, Gramates P, Deuel TF, Thomason A, Pierce GF. Reversal of impaired wound healing in irradiated rats by platelet‐derived growth factor‐BB. Am J Surg 1989;158: 345–50. [DOI] [PubMed] [Google Scholar]
- 18. Vegesna V, McBride WH, Taylor JM, Withers HR. The effect of interleukin‐1 beta or transforming growth factor‐beta on radiation‐impaired murine skin wound healing. J Surg Res 1995;59:699–704. [DOI] [PubMed] [Google Scholar]
- 19. Jagetia GC, Venkatesha VA. Effect of mangiferin on radiation‐induced micronucleus formation in cultured human peripheral blood lymphocytes. Environ Mol Mutagen 2005;46:12–21. [DOI] [PubMed] [Google Scholar]
- 20. Leung A. Encyclopedia of common natural ingredients used in food, drugs, and cosmetics. New York, NY: John Wiley, 1980:313–4. [Google Scholar]
- 21. Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol 2007;27:19–35. [DOI] [PubMed] [Google Scholar]
- 22. Nadkarni KM. Editor, Indian Materia Medica. 1st ed. Bombay, India: Popular Prakashan Private Ltd, 1976. [Google Scholar]
- 23. Chang H, But PP. Pharmacology and applications of Chinese materia medica. 2nd edn. Singapore: World Scientific Publishing Company, 1987. [Google Scholar]
- 24. Ammon HP, Wahl MA. Pharmacology of curcuma longa. Planta Medica 1991; 57:1–7. [DOI] [PubMed] [Google Scholar]
- 25. Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complem Altern Med 2006;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen 1999;7:362–74. [DOI] [PubMed] [Google Scholar]
- 27. Phan TT, See P, Lee ST, Chan S‐Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma 2001;51:927–31. [DOI] [PubMed] [Google Scholar]
- 28. Jagetia GC, Rajanikant GK. Effect of various doses of curcumin on the radiation‐impaired healing of excision wound in mice: a preliminary study. J Wound Care 2004;13:107–9. [DOI] [PubMed] [Google Scholar]
- 29. Jagetia GC, Rajanikant GK. Role of curcumin, a naturally occurring phenolic compound of turmeric in accelerating the repair of excision wounds in mice whole‐body exposed to various doses of γ‐radiation. J Surg Res 2004b;120:127–38. [DOI] [PubMed] [Google Scholar]
- 30. Jagetia GC, Rajanikant GK. Curcumin treatment enhances the repair and regeneration of wounds in mice hemi‐body exposed to γradiation. Plast Reconstructr Surg 2005;115:515–28. [DOI] [PubMed] [Google Scholar]
- 31. Woessner JF. Jr The determination of hydroxyproline in tissues and protein samples containing small proportions of this amino acid. Arch Biochem Biophys 1961;93:440–7. [DOI] [PubMed] [Google Scholar]
- 32. Jackob DS, Cleary EG. The determination of collagen and elastin. In: Glick D, editor. Methods of biochemical analysis, vol 15. New York: Interscience publishers, John Wiley and Sons, 1967:25–76. [DOI] [PubMed] [Google Scholar]
- 33. Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochemical J 1956;62:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hevel JM, Marletta MA. Nitric‐oxide synthase assays. Methods Enzymol 1994;233:250–8. [DOI] [PubMed] [Google Scholar]
- 35. Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, Maheshwari RK. Enhancement of wound healing by curcumin in animals. Wound Repair Regen 1998;6:167–77. [DOI] [PubMed] [Google Scholar]
- 36. Mani H, Sidhu GS, Kumari R, Gaddipati JP, Seth P, Maheshwari RK. Curcumin differentially regulates TGF‐beta‐1, its receptors and nitric oxide synthase during impaired wound healing. Biofactors 2002;16:29–43. [DOI] [PubMed] [Google Scholar]
- 37. Kunchandy E, Rao MNA. Oxygen radical scavenging activity of curcumin. Int J Pharm 1990;38:239–40. [Google Scholar]
- 38. Abraham SK, Sara L, Caravan PC. Protective effects of chlorogenic acid, curcumin and beta‐carotene against gamma‐radiation‐induced in vivo chromosomal damage. Mutat Res 1993;303:109–12. [DOI] [PubMed] [Google Scholar]
- 39. Peacock EE. Contraction. In: Peacock EE, editor. Wound repair, 2nd edn. Philadelphia: Saunders, 1984:39–55. [Google Scholar]
- 40. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 2009;37:1528–42. [DOI] [PubMed] [Google Scholar]
- 41. Grillo HC, Potsaid MS. Studies on wound healing – IV, retardation of contraction by local X‐irradiation and observations relating to the origin of fibroblast in repair. Ann Surg 1961;154:741–50. [PMC free article] [PubMed] [Google Scholar]
- 42. Taren DL, Chvapil M, Weber CW. Increasing the breaking strength of wounds exposed to preoperative irradiation using vitamin E supplementation. Int J Vit Nutr Res 1987;57:133–7. [PubMed] [Google Scholar]
- 43. Martin P. Wound healing–aiming for perfect skin regeneration. Science 1997;276:75–81. [DOI] [PubMed] [Google Scholar]
- 44. Grant RA, Cox RW, Kent CM. The effects of gamma irradiation on the structure and reactivity of native and cross‐linked collagen fibrils. J Anat 1973;115:29–43. [PMC free article] [PubMed] [Google Scholar]
- 45. Schaffer MR, Tantry U, Gross SS, Wasserburg HL, Barbul A. Nitric oxide regulates wound healing. J Surg Res 1996;63:237–40. [DOI] [PubMed] [Google Scholar]
- 46. Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg 2002;183:406–12. [DOI] [PubMed] [Google Scholar]
- 47. Albina JE, Mills CD, Henry WL Jr, Caldwell MD. Temporal expression of different pathways of L‐arginine metabolism in healing wounds. J Immunol 1991;1990;144:3877–80. [PubMed] [Google Scholar]
- 48. Eroğlu A, Kurtman C, Ayylidiz A, Karadayi K, Demicri S. Effect of granulocyte‐macrophage colony‐stimulating factor on wound nitrite level in normal and irradiated rats. Med Sci Res 1999;27:685–8. [Google Scholar]
- 49. Schaffer M, Weimer W, Wider S, Stulten C, Bongartz M, Budach W, Becker HD. Differential expression of inflammatory mediators in radiation‐impaired wound healing. J Surg Res 2002;107:93–100. [PubMed] [Google Scholar]
- 50. Stajic J, Jovanovic M. Radiation and wound healing: histological changes in the damaged skin of irradiated rats. Strahlentherapie 1971;141:244–9. [PubMed] [Google Scholar]
- 51. Doyle JW, Li YQ, Salloum A, FitzGerald TJ, Walton RL. The effects of radiation on neovascularization in a rat model. Plast Reconstr Surg 1996; 98:129–35. [DOI] [PubMed] [Google Scholar]
- 52. Zelman D, Song IC, Porteous DD, Bromberg BE. The effect of total body irradiation on wound healing and hematopoietic system. Bull NY Acad Med 1969;45:293–300. [PMC free article] [PubMed] [Google Scholar]
- 53. Stromberg LR, Woodward KT, Mahin DT, Donati RM. Altered wound healing in X‐irradiated rats: the effect of bone marrow shielding. Experientia 1967;23:1064–5. [DOI] [PubMed] [Google Scholar]
- 54. Gabbiani G, Hirschal BJ, Ryan GB, Statkov PR, Majno G. Granulation tissue as a contractile organism: a study of structure and function. J Exp Med 1972;135:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow‐derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 2010;28:905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bumann J, Santo‐Holtje L, Loffler H, Bamberg M, Rodemann HP. Radiation‐induced alterations of the proliferation dynamics of human skin fibroblasts after repeated irradiation in the subtherapeutic dose range. Strahlenther Onkol 1995;171:35–41. [PubMed] [Google Scholar]
- 57. Vasil’eva TV, Michurina TV, Khrushchov NG. Immunofluorescent study of the origin of connective tissue cells in xenogenic radiation chimeras under normal conditions and in aseptic inflammation. Ontogenez 1978;9:288–90. [PubMed] [Google Scholar]
- 58. Langev MA, Vasil’eva TV, Michurina TV. Origin of fibroblasts and macrophages of skin wound granulation tissue. Arkhiv Anat Gistol Embriol 1979;77:22–8. [PubMed] [Google Scholar]
- 59. Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001;107:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone‐marrow‐derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430–6. [DOI] [PubMed] [Google Scholar]
- 61. Trabucchi E, Pallotta S, Morini M, Corsi F, Franceschini R, Casiraghi A, Pravettoni A, Foschi D, Minghetti P. Low molecular weight hyaluronic acid prevents free radical damage to granulation tissue during wound healing. Int J Tissue React 2002;24:65–71. [PubMed] [Google Scholar]
- 62. Linard C, Grémy O, Benderitter M. Reduction of peroxisome proliferation‐activated receptor γ expression by γ‐irradiation as a mechanism contributing to inflammatory response in rat colon: modulation by the 5‐aminosalicylic acid agonist. J Pharmacol Exp Ther 2008;324:911–20. [DOI] [PubMed] [Google Scholar]
- 63. Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem 2006;290:87–96. [DOI] [PubMed] [Google Scholar]
- 64. Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary n‐3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim Biophys Acta 1994;1224:255–63. [DOI] [PubMed] [Google Scholar]
- 65. Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF‐kappaB activation and interleukin‐8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal 2005;7:32–41. [DOI] [PubMed] [Google Scholar]
- 66. Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. Curcumin facilitates fibrinolysis and cellular migration during wound healing by modulating urokinase plasminogen activator expression. Pathophysiol Haemost Thromb 2009;37:59–66. [DOI] [PubMed] [Google Scholar]
- 67. Tessner TG, Muhale F, Schloemann S, Cohn SM, Morrison AR, Stenson WF. Ionizing radiation up‐regulates cyclooxygenase‐2 in I407 cells through p38 mitogen‐activated protein kinase. Carcinogenesis 2004;25:37–45. [DOI] [PubMed] [Google Scholar]
- 68. Park K. Curcumin inhibits interferon‐alpha induced NF‐kappaB and COX‐2 in human A549 non‐small cell lung cancer cells. Biochem Biophys Res Commun 2005;334:313–8. [DOI] [PubMed] [Google Scholar]
- 69. Atsumi T, Murakami Y, Shibuya K, Tonosaki K, Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase‐2 gene expression, by curcumin and its analog, alpha‐diisoeugenol. Anticancer Res 2005;25:4029–36. [PubMed] [Google Scholar]
- 70. Rödel F, Hantschel M, Hildebrandt G, Schultze‐Mosgau S, Rödel C, Herrmann M, Sauer R, Voll RE. Dose‐dependent biphasic induction and transcriptional activity of nuclear factor kappa B (NF‐κB) in EA.hy.926 endothelial cells after low‐dose X‐irradiation. Int J Radiat Biol 2004;80:115–23. [DOI] [PubMed] [Google Scholar]
- 71. Adams S, Pankow S, Werner S, Munz B. Regulation of NF‐kappaB activity and keratinocyte differentiation by the RIP4 protein: implications for cutaneous wound repair. J Invest Dermatol 2007;127:538–44. [DOI] [PubMed] [Google Scholar]
- 72. Singh S, Aggarwal BB. Activation of transcription factor NF‐κB is suppressed by curcumin (diferulolylmethane). J Biol Chem 1995;270:24995–5000. [DOI] [PubMed] [Google Scholar]
- 73. Haas AF, Wong JW, Iwahashi CK, Halliwell B, Cross CE, Davis PA. Redox regulation of wound healing? NF‐κB activation in cultured human keratinocytes upon wounding and the effect of low energy HeNe irradiation. Free Rad Biol Med 1998;25:998–1005. [DOI] [PubMed] [Google Scholar]
- 74. Siddiqui AM, Cui X, Wu R, Dong W, Zhou M, Hu M, Simms HH, Wang P. The anti‐inflammatory effect of curcumin in an experimental model of sepsis is mediated by up‐regulation of peroxisome proliferator‐activated receptor‐γ. Crit Care Med 2006;34:1874–82. [DOI] [PubMed] [Google Scholar]
- 75. Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest 2006;116:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]


