Abstract
In a randomised, controlled study, we compared the efficacy of Grafix®, a human viable wound matrix (hVWM) (N = 50), to standard wound care (n = 47) to heal diabetic foot ulcers (DFUs). The primary endpoint was the proportion of patients with complete wound closure by 12 weeks. Secondary endpoints included the time to wound closure, adverse events and wound closure in the crossover phase. The proportion of patients who achieved complete wound closure was significantly higher in patients who received Grafix (62%) compared with controls (21%, P = 0·0001). The median time to healing was 42 days in Grafix patients compared with 69·5 days in controls (P = 0·019). There were fewer Grafix patients with adverse events (44% versus 66%, P = 0·031) and fewer Grafix patients with wound‐related infections (18% versus 36·2%, P = 0·044). Among the study subjects that healed, ulcers remained closed in 82·1% of patients (23 of 28 patients) in the Grafix group versus 70% (7 of 10 patients) in the control group (P = 0·419). Treatment with Grafix significantly improved DFU healing compared with standard wound therapy. Importantly, Grafix also reduced DFU‐related complications. The results of this well‐controlled study showed that Grafix is a safe and more effective therapy for treating DFUs than standard wound therapy.
Keywords: Diabetes, Infection, Stem cells, Ulcer
Introduction
There is a worldwide epidemic of diabetes. According to data from the World Health Organization, the global prevalence of diabetes among adults was 6·4% in 2010, affecting 285 million people worldwide. The prevalence of diabetes is expected to increase to 7·7% by 2030 and affect 439 million adults 1, 2. In the USA, the rates are higher where 8·2% of the US population, 26 million people, has diabetes, according to the Centers for Disease Control and Prevention. In the past 5 years, the prevalence of diabetes increased by 26%, and the cost increased by 40% 3.
One of the most frequent underlying causes of hospitalisation and amputation among persons with diabetes is a non‐healing foot ulceration 2, 4. It is estimated that 25% of persons with diabetes will experience a foot ulcer in their lifetime 4. In the USA, approximately one‐quarter of the overall cost of diabetes treatment is spent on lower extremity complications, totaling $43·5 billion 5 a year. In addition to the costs to society, foot ulcers negatively affect quality of life, productivity, employment, depression and mortality 6, and when a diabetic foot ulcer (DFU) ends in amputation the impact and costs are even greater. Unfortunately, the rate of wound healing with the current standard of care for DFUs is poor. Only about 24% of wounds heal after 12 weeks of therapy 7, 8. Wound chronicity is associated with increased risk of soft tissue and bone infection and amputation. Therefore, treatments to improve and accelerate DFU healing should reduce morbidity and costs of complications.
Despite recent advances in wound healing with the use of advanced skin substitutes, large, multi‐centre, randomised trials have demonstrated varying efficacy rates with the best relative effect of only 64% 9 to date. Superior therapies that can restart the healing process of stalled wounds to allow progression through the three phases of normal wound healing are still needed 10, 11. Placental membranes, described in the literature as a treatment for wounds for over 100 years, contain a combination of growth factors, collagen‐rich extracellular matrix and cells including mesenchymal stem cells (MSCs), neonatal fibroblasts and epithelial cells that provide the necessary mechanisms for coordinated wound healing. Multiple growth factors and proteins including anti‐scarring proteins (TGF‐β3 and human growth factor), anti‐microbial proteins (neutrophil gelatinase‐associated lipocalin and defensins) and angiogenic factors (vascular endothelial growth factor, platelet‐derived growth factor and basic fibroblast growth factor) are present in the matrix 12. Grafix® (Osiris Therapeutics, Inc., Columbia, MD), a human viable wound matrix (hVWM), is designed to preserve the native components of the human placental membrane in a cryopreserved product that can be used on demand at point‐of‐care. The objective of this study was to evaluate the efficacy and safety of Grafix compared with standard wound care to treat chronic DFUs.
Methods
The study was a prospective, multi‐centre, randomised, single‐blinded clinical trial to evaluate the safety and efficacy of Grafix for the treatment of chronic DFUs. The primary hypothesis was that Grafix was superior to standard wound care for the primary endpoint of complete wound closure. Patients were enrolled from May 2012 through April of 2013. Key inclusion criteria included confirmed type I or type II diabetes, patients age between 18 and 80 years, index wound present between 4 and 52 weeks, wound located below the malleoli on plantar or dorsal surface of the foot and ulcer between 1 and 15 cm2. Main exclusion criteria included haemoglobin A1c above 12%, evidence of active infection including osteomyelitis or cellulitis, inadequate circulation to the affected foot defined by an ankle brachial index <0·70 or >1·30, or toe brachial index ≤0·50 or Doppler study with inadequate arterial pulsation, exposed muscle, tendon, bone or joint capsule and reduction of wound area by ≥30% during the screening period.
Following a 1‐week screening period, patients were randomised to the Grafix or control treatment arm in a 1:1 ratio. Patients randomised to Grafix treatment received an application of Grafix once a week (±3 days) for up to 84 days (blinded treatment phase). Patients in the control group received standard wound therapy once a week (±3 days) for up to 84 days. All wounds were appropriately cleaned and surgically debrided to remove all non‐viable soft tissue from the wound by scalpel, tissue nippers and/or curettes at each weekly visit. For patients randomised to the Grafix group, the hVWM was placed to come in full contact with the wound and edges. Wounds in both groups received standard wound care that included surgical debridement, off‐loading and non‐adherent dressings. All patients received a non‐adherent dressing: Adaptic® (Systagenix, Gatwick, UK) and either saline moistened gauze or Allevyn® (Smith & Nephew, London, UK) for moderately draining wounds. An outer dressing was then applied. Patients were provided walking boots for wounds on the sole of the foot or a post‐op shoe if the wound was on the dorsum of the foot or at the ankle. Custom off‐loading boots could be prescribed at the discretion of the site investigator. In addition, the off‐loading device used could be changed as needed to accommodate changes in wound size or position.
Patients were evaluated weekly at the clinical site. Patients who achieved complete wound closure then continued to be evaluated during the follow‐up phase, twice during the first month and then monthly for two additional visits). Control patients whose wounds were not closed by the end of the blinded treatment phase were able to receive Grafix in the open‐label treatment phase, in which Grafix was applied weekly for up to 84 days. Outcome and safety assessments occurred at each visit during the blinded treatment phase, follow‐up visits, as well as during the open‐label treatment phase.
The primary endpoint of the study was evaluation of complete wound closure of the index wound. Complete wound closure was defined as 100% re‐epithelialisation with no wound drainage as determined by the site investigator. Confirmation of wound closure was confirmed at an initial follow‐up visit 2 weeks later. Wound closure was independently confirmed via a central wound core laboratory with two blinded wound care experts who reviewed all wounds via digitised acetate tracing and photography. The secondary objectives included the time to initial wound closure among patients who received Grafix versus those who received control as measured by Kaplan–Meier analysis. The proportion of patients who achieved 50% or greater reduction in wound size by 28 days, the number of applications needed for closure and wound recurrence after initial wound healing were also determined. In the open‐label treatment phase, wound closure with Grafix for patients who were in the control group in the blinded treatment phase was assessed. Safety assessments included the number, type and severity of adverse events as outlined in National Cancer Institute's (NCI) common terminology criteria for adverse events (CTCAE) version 3.
Sample size and statistical analysis
The study sample size was based on an assumed closure rate of 30% in the control arm and 50% in the Grafix group with a 30% drop‐out rate. Under these assumptions, 94 patients, who completed the treatment, in each treatment arm were required to meet the two‐sided type 1 error rate of 0·05 with 80% power. A pre‐specified interim analysis was planned at 50% enrollment. The interim analysis used a one‐sided superiority design based on an Emerson–Fleming symmetric group sequential design using an O'Brien‐Fleming boundary shape [Emerson and Fleming 13]. The analysis was performed by an unblinded statistician and reported to the blinded review committee. Following the interim analysis, the blinded review committee recommended to terminate study enrollment because of overwhelming superiority of the Grafix arm versus the control arm.
The statistical analyses were performed using SAS version 9.2 on an intent‐to‐treat basis. Baseline demographic and clinical variables were summarised for each treatment arm of the study. Descriptive summaries of the distribution of continuous variables included the mean, standard deviation, median and subject counts; categorical variables were summarised in terms of frequencies and percentages. Treatment group summaries were constructed across all study sites. Statistical comparisons between treatment groups were performed using χ 2 testing for categorical variables and analysis of variance (ANOVA) techniques for continuous measures. A Cox proportional hazard regression analysis was performed on time‐to‐event (wound closure) data.
Results
Patient demographics and baseline characteristics are presented in Table 1. During screening, 139 patients were evaluated. There were 42 patients who failed screening, of which 6 were disqualified after the 1 week run‐in period because there was a 30% or greater wound area reduction. Ninety‐seven patients were subsequently randomised: 50 received Grafix and 47 received standard wound therapy. Among the 97 patients evaluated, there were 85 plantar foot wounds and 12 dorsal foot wounds. There were eight dorsal wounds in the Grafix treatment group and four dorsal wounds in the control arm of the study. There were no significant differences in baseline characteristics among the two treatment groups. The planned interim analysis showed overwhelming efficacy among patients who received Grafix for the primary and secondary endpoints when compared with the control group (Table 2). Following the interim analysis, the blinded review committee recommended to terminate study enrollment because of overwhelming superiority.
Table 1.
Patient demographics and baseline characteristics
| Grafix (n = 50) | Control (n = 47) | P‐value | 95% confidence interval | ||
|---|---|---|---|---|---|
| Mean age, in years (SD) | 55·5 (11·5) | 55·1 (12·0) | 0·849 | −5·2 | 4·3 |
| Age ≥65 years (N, %) | 11 (22%) | 13 (27·7%) | 0·521 | 0·292 | 1·861 |
| Male (N, %) | 33 (66·0%) | 35 (74·5%) | 0·365 | 0·276 | 1·603 |
| Mean years DM (SD) | 15·4 (11·1) | 14·0 (11·0) | 0·549 | −5·80 | 3·10 |
| Mean BMI (SD) | 33·5 (7·7) | 32·2 (7·9) | 0·419 | −4·40 | 1·90 |
| BMI ≥30 (N, %) | 36 (72%) | 25 (53·2%) | 0·057 | 0·975 | 5·253 |
| Race (N, %) | |||||
| White or Caucasian | 35 (70%) | 32 (68·1%) | 0·581 | −1·847 | 2·073 |
| Black or African American | 13 (26%) | 12 (25·5%) | 0·521 | −1·866 | 2·054 |
| American Indian or Alaska Native | 1 (2%) | 1 (2·1%) | 0·482 | −1·932 | 1·988 |
| Other | 1 (2%) | 2 (4·3%) | 0·263 | −1·947 | 1·973 |
| Mean wound size at baseline (cm2, SD) | 3·41 (3·23) | 3·93 (3·22) | 0·433 | −0·80 | 1·80 |
| Wound duration (days, SD) | 115·0 (72·6) | 122·9 (83·9) | 0·621 | −23·7 | 39·5 |
| Mean glycated haemoglobin (SD) | 8·0 (1·6) | 7·8 (1·5) | 0·511 | −0·90 | 0·4 |
| Glycated haemoglobin >9% (N, %) | 14 (28%) | 13 (27·7%) | 0·970 | 0·418 | 2·473 |
| Mean albumin (g/dl) (SD) | 4·0 (0·4) | 4·0 (0·3) | 0·418 | −0·20 | 0·10 |
| Albumin >3·5 g/dl (N, %) | 44 (88%) | 42 (89·4%) | 0·263 | −1·947 | 1·973 |
| Ankle bachial index (ABI) | |||||
| ABI 0·07–0·90 (N, %) | 10 (21·7%) | 10 (22·2%) | 0·44 | −1·89 | 2·03 |
| ABI >0·90 | 36 (78·3%) | 35 (77·8%) | 0·39 | −1·89 | 2·00 |
DM, diabetes mellitus.
Table 2.
Wound healing and safety clinical outcomes
| Grafix (n = 50) | Control (n = 47) | P‐value | |
|---|---|---|---|
| Wound healing | |||
| Healed wounds, (N, %) | 31 (62%) | 10 (21%) | <0·001 |
| Median time to wound closure (days) | 42·0 | 69·5 | 0·019 |
| 50% wound area reduction at day 28 (N, %) | 31 (62%) | 19 (40·4%) | 0·035 |
| Median study visits (single blind phase) | 6 | 12 | <0·001 |
| Adverse events | |||
| Subjects experiencing at least one adverse event* (N, %) | 22 (44%) | 31 (66%) | 0·031 |
| Subjects with an infection (N, %) | 13 (26%) | 21 (44·7%) | 0·055 |
| Subjects with a skin or subcutaneous tissue disorder (N, %) | 7 (14%) | 8 (17%) | NS |
| Subjects with injury, poisoning and procedural complications (N, %) | 5 (10%) | 7 (14·9%) | NS |
| Subjects with general disorders (N, %) | 4 (8%) | 3 (6·4%) | NS |
| Subjects with musculoskeletal and connective tissue disorders (N, %) | 4 (8%) | 2 (4·3%) | NS |
| Subjects with a wound‐related infection (N, %) | 9 (18%) | 17 (36·2%) | 0·044 |
| Subjects with a serious adverse event due to wound infection (N, %) | 4 (8%) | 10 (21·3%) | 0·084 |
| Subjects having an amputation due to an adverse event (N, %) | 0 (0%) | 1 (2·1%) | NS |
NS, non‐significant.
Included overall number of subjects experiencing at least one adverse event and those with at least 5% adverse events.
Efficacy evaluation
Blinded treatment phase
The proportion of patients who achieved complete wound closure was significantly higher in patients who received Grafix (31 of 50, 62·0%) compared with controls (10 of 47, 21·3%, P = 0·0001). The odds ratio for complete healing for a Grafix patient compared with control was 6·037 (95% CI 2·449–14·882). The Grafix group had significantly faster median time to complete wound closure compared with controls (42·0 versus 69·5 days, P = 0·019), among the wounds that closed in both groups. The Kaplan–Meier analysis illustrated a statistically greater probability of complete wound healing during the 12‐week evaluation period for Grafix (Figure 1). The probability of closure for the Grafix group was 67·1% compared with 27·1% for the standard care group (Log‐Rank, P < 0·0001). Grafix patients also required fewer study visits (i.e. applications) to achieve closure compared with patients in the control arm (6 versus 12, P < 0·001). Comparison of patients with at least a 50% reduction in wound size by day 28 showed that 31 of 50 patients (62·0%) in the Grafix group achieved this reduction versus 19 of 47 (40·4%) in the control group (P = 0·035). There were 8 (16%) patients who withdrew from the study prior to completion in the Grafix group versus 11 (23·4%) patients who withdrew from the control group.
Figure 1.
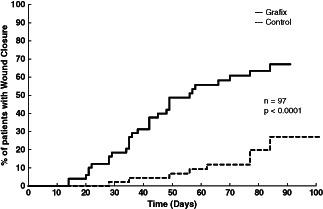
Kaplan–Meier analysis of probability of 100% closure for Grafix versus control.
Wound recurrence of DFUs closed during the initial 12‐week study period was assessed. Follow‐up every 4 weeks for an additional 12 weeks showed that ulcers remained closed in 82·1% of patients (23 of 28 patients) in the Grafix group versus 70·0% (7 of 10 patients) in the control group (P = 0·42).
Open‐label phase
Patients in the control arm who failed to heal during the initial 12‐week treatment period could cross over to receive up to 12 weeks of Grafix therapy (n = 26). After receiving treatment with Grafix, the probability of closure was 67·8% with a mean time to closure for these patients of 42 days.
Regression analysis
Cox proportional hazard regression analysis was performed with treatment group, duration of ulcer, baseline ulcer area, glucose control (glycated haemoglobin), ulcer location and BMI as covariates. Following adjustment for these variables, Grafix was found to have a significant effect on time to closure (P < 0·0001). The hazard ratio was 4·77 (95% CI 2·279, 9·971), indicating superior odds of closure with Grafix relative to standard wound therapy.
Safety evaluation
Overall, fewer Grafix patients experienced at least one adverse events compared with control patients (44·0% versus 66·0%, P = 0·031). Among the patients randomised to Grafix, there were significantly fewer patients with wound‐related infections (Grafix, 9 of 50, 18·0%, versus control, 17 of 47, 36·2%, P = 0·044) and fewer hospitalisations related to infections in the Grafix group than control (6% versus 15%, P = 0·15).
Discussion
The results of this study demonstrate that weekly application of Grafix led to improved healing rates of DFUs and reduced diabetic foot complications compared with standard wound therapy. In this study, all primary and secondary endpoints showed clinical benefit of Grafix, in the only multi‐centre DFU trial to date to meet statistically significant pre‐specified interim analyses. This is also the first report of a multi‐centre randomised controlled trial (RCT) to investigate the use of human amniotic membrane for the treatment of DFUs. In addition, to the authors' knowledge, this is the first large, multi‐centre RCT for advanced skin substitutes in which the primary endpoint, 100% re‐epithelialisation, was confirmed by third‐party blinded wound care experts, further removing potential bias and increasing reliability of the endpoint results.
Despite the introduction of numerous advanced wound care products including bioengineered skin substitutes for the treatment of DFUs over the last 15 years, only a few have demonstrated significant clinical efficacy compared with control in multi‐centre RCTs 9, 10, 14, which are considered the best level I evidence in trial conduct. Of these studies to date, this study has shown the best relative effect (191% compared with standard of care), among these multi‐centre RCTs. In addition, the primary outcome for this trial, which showed a healing rate of 62% at 12 weeks, compares favourably to the RCTs of a bilayered, cell‐based product (56%) and human fibroblast‐derived dermal substitute (30%) 10. The healing rate in the control arm of this study was 21·3%, which is similar to the average rate of DFU healing in phase 3 randomised clinical trials with a 12‐week study duration (24%) 8. Grafix is also available in multiple sizes to allow the product to decrease as the wound size decreases, thereby reducing waste and cost. The healing rates of 62–68% in this trial are consistent with previous published reports of healing of 85% and 68% by 12 weeks among DFU and venous leg ulcer patients, respectively, in an open‐label, retrospective study using Grafix 15.
The standard of care treatments selected for this study included surgical wound debridement, high‐quality off‐loading devices and non‐adherent dressings that were provided uniformly to all patients in both treatment groups making the differences in the treatment effect from these interventions alone unlikely. Off‐loading is one of the most important elements in DFU treatment 16, 17. Several studies have reported significant differences in pressure reduction 18 and wound healing based on the off‐loading strategy 17, 19, 20, 21. In this study, better quality and more effective off‐loading devices were provided for all patients than what is commonly provided to patients with DFUs 22. For wounds on the sole of the foot, removable cast walkers were provided; for wounds on the dorsum of the foot and ankle, a post‐op shoe was used. Several studies have shown that a higher proportion of wounds heal with removable cast walkers 16 compared with healing sandals or shoes 17, 23; however, in community practice only about 15% of patients with DFUs receive this quality of pressure reduction therapy 22. The majority of patients receive less effective off‐loading with healing sandals, post‐op shoes or therapeutic shoes and insoles as was done in previous randomised trials 21, 24. Debridement is another important element in wound therapy. In this study, surgical debridement was done for every patient at every study visit. Other studies have reported that a minority of DFUs received surgical debridement in phase 3 DFU trials 25, 26, and that the frequency of wound debridement was associated with differences in wound healing 25 (Table 3). Despite the high‐quality standard of care that patients in both groups received, there were overwhelming differences in outcomes between the Grafix‐treated group and the control group. In addition, the probability of closure was 67·8% among patients who crossed over from the control group to Grafix after failing to heal. These outcomes, therefore, can only be explained by the effect that Grafix had on these chronic wounds as the significant variable factor between groups. Grafix would be an ideal product to combine with more rigorous off‐loading strategies such as total contact casts because it is applied once a week and does not require additional dressing changes 17, 19, 20, 21. Furthermore, off‐loading in a cast would guarantee compliance with off‐loading.
Table 3.
Comparison of standard of care among multi‐centre, controlled wound care trials
| Grafix® | Dermagraft® | Apligraf® | |
|---|---|---|---|
| Mean wound size (cm2) | 3·7 | 2·3 | 3·0 |
| Healed % treatment versus control | 62% versus 21%* | 30% versus 18%* | 56% versus 38%* |
| Time to closure | 42 versus 70 days* | Not stated | 65 versus 90 days* |
| All adverse events | 44% versus 66%* | 67% versus 73% | Not stated |
| Wound‐related infection | 18% versus 36·2%* | 19% versus 32%* | 22% versus 32% |
| Off‐loading | Walking boot or Post‐op shoe | Therapeutic shoes and custom insoles or healing sandals | Custom sandal |
| Debridement | Every visit | ad lib | ad lib |
P < 0·05.
There was a significant reduction in wound‐related adverse events in patients treated with Grafix. The lower incidence of wound‐related infections was likely related to the faster rate of healing and the higher proportion of wounds that healed. The longer the duration of the wound, the greater is the risk of developing a soft tissue or bone infection 27, 28. Use of Grafix reduced the median time to wound healing by 4 weeks compared with standard DFU care. Reducing the risk of infection is a key factor in reducing amputations and the cost of DFUs. DFU patients who develop infection are about 56 times more likely to require hospitalisation and 155 times more likely to require amputation 28. Once a patient has an amputation of the foot or leg, the risk of repeated ulcers, infections and amputations increases dramatically 29, as do the associated health care costs.
Placental membranes, first reported as a treatment for wounds in 1910, have a combination of growth factors, collagen‐rich extracellular matrix and viable cells including MSCs, neonatal fibroblasts and epithelial cells 12, 30, 31, 32. However, preservation of cellular viability has previously limited widespread use 12, 15. Grafix, which maintains the native structure of the human tissue, contains living components, which in in vitro studies and wound repair models have demonstrated advantages in angiogenic, anti‐inflammatory and anti‐oxidant effects over dehydrated amniotic membrane (dHAM) products, further support the use of this cellular wound matrix. Arnold et al. reported a 7·5‐fold increase in the angiogenic growth factor vascular endothelial growth factor (VEGF) compared to dHAM, important for blood vessel formation. Additional studies performed by Arnold et al. identified reductions in pro‐inflammatory cytokines and enhanced anti‐oxidant activity as measured by a reduction in oxidant‐induced apoptosis of human dermal fibroblasts with cellular wound matrices versus dHAMs 33, 34, 35.
Despite advances in advanced wound therapy, chronic DFUs continue to increase in frequency causing significant associated morbidity and rising health care costs. The results of this randomised clinical study demonstrated that weekly application of Grafix increases the proportion of DFUs that heal, accelerates the time to heal, decreases the number of treatments and reduces infections and infection‐related hospitalisations when compared with high‐quality standard wound care. This is the first clinical product with viable stem cells studied in a randomised clinical trial to successfully show statistically greater closure rates of chronic DFUs. Based on these findings, Grafix is an important treatment option for health care providers and their patients for the safe and effective treatment of chronic DFUs.
Acknowledgements
This study was funded by Osiris Therapeutics, Inc. All the authors received research funding from Osiris Therapeutics, Inc. for their participation in the study. LAL researched data and wrote and edited the manuscript. JF, KAS, MR, DV, DF, HK, TO and JN researched data and reviewed and edited the manuscript. The authors wish to thank William Hiatt, MD, and Ron McWilliams of CPC Clinical Research and William Namen, DPM of River City Clinical Research for their assistance with the trial and Douglas Jacobstein, MD and Sharron McCulloch of Osiris Therapeutics, Inc. for their assistance with the trial and review of the manuscript.
The Grafix Diabetic Foot Ulcer Study Group
Duncan Grant, DPM, Ohio Health Research Institute, Columbus, OH; Michael Lowhorn, DPM, St. Louis Center for Clinical Research, St. Louis, MO; Thomas Hendrick, MD, Nature Coast Clinical Research, Iverness, FL; Dan Streja, MD, Infosphere Clinical Research, Inc., West Hills, CA; Gary Friedlander, DPM, Clinical Trials of Arizona, Inc., Glendale, AZ; Daniel Goldman, MD, Vassar Brothers Medical Center Wound Care and Hyperbaric Therapy Center, Poughkeepsie, NY; Adam Budny, DPM, Blair Orthopedic Associates, Inc., Altoona, PA; Terry Treadwell, MD, The Institute for Advanced Wound Care at Baptist Medical Center South, Montgomery, AL; David Ware, MD, Horizon Research Group, Eunice, LA; Michael Kerzner DPM, Duke University Medical Center, Durham NC; Ian Gordon, MD, Long Beach VA Healthcare System, Long Beach, CA.
References
- 1. Guariguata L, Whiting D, Weil C, Unwin N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract 2011;94:322–32. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC) . 2007 National Diabetes Fact Sheet. Atlanta: CDC, 2008. [Google Scholar]
- 3. American Diabetes A. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 5. Kantor J, Margolis DJ. Treatment options for diabetic neuropathic foot ulcers: a cost‐effectiveness analysis. Dermatol Surg 2001;27:347–51. [DOI] [PubMed] [Google Scholar]
- 6. Morbach S, Furchert H, Groblinghoff U, Hoffmeier H, Kersten K, Klauke GT, Klemp U, Roden T, Icks A, Haastert B, Rumenapf G, Abbas ZG, Brarara M, Armstrong DG. Long‐term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care 2012;35:2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Markowitz JS, Gutterman EM, Magee G, Margolis DJ. Risk of amputation in patients with diabetic foot ulcers: a claims‐based study. Wound Repair Regen 2006;14:11–7. [DOI] [PubMed] [Google Scholar]
- 8. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta‐analysis. Diabetes Care 1999;22:692–5. [DOI] [PubMed] [Google Scholar]
- 9. Marston WA, Hanft J, Norwood P, Pollak R, Dermagraft Diabetic Foot Ulcer Study Group . The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–5. [DOI] [PubMed] [Google Scholar]
- 10. Veves A, Falanga V, Armstrong DG, Sabolinski ML, Apilgraft Diabetic Foot Ulcer Study . Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 11. Hanson SE, Bentz ML, Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg 2010;125:510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maxson S, Lopez EA, Yoo D, Danilkovitch‐Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emerson SS, Fleming TR. Symmetric group sequential test designs. Biometrics 1989;45:905–23. [PubMed] [Google Scholar]
- 14. Reyzelman A, Crews RT, Moore JC, Moore L, Mukker JS, Offutt S, Tallis A, Turner WB, Vayser D, Winters C, Armstrong DG. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J 2009;6:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Regulski M, Jacobstein DA, Petranto RD, Migliori VJ, Nair G, Pfeiffer D. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage 2013;59:38–43. [PubMed] [Google Scholar]
- 16. Armstrong DG, Lavery LA, Bushman TR. Peak foot pressures influence the healing time of diabetic foot ulcers treated with total contact casts. J Rehabil Res Dev 1998;35:1–5. [PubMed] [Google Scholar]
- 17. Cavanagh PR, Bus SA. Off‐loading the diabetic foot for ulcer prevention and healing. Plast Reconstr Surg 2011;127(Suppl 1):248S–56S. [DOI] [PubMed] [Google Scholar]
- 18. Lavery LA, Vela SA, Lavery DC, Quebedeaux TL. Reducing dynamic foot pressures in high‐risk diabetic subjects with foot ulcerations. A comparison of treatments. Diabetes Care 1996;19:818–21. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong DG, Lavery LA, Wu S, Boulton AJ. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care 2005;28:551–4. [DOI] [PubMed] [Google Scholar]
- 20. Katz IA, Harlan A, Miranda‐Palma B, Prieto‐Sanchez L, Armstrong DG, Bowker JH, Mizel MS, Boulton AJ. A randomized trial of two irremovable off‐loading devices in the management of plantar neuropathic diabetic foot ulcers. Diabetes Care 2005;28:555–9. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off‐loading the diabetic foot wound: a randomized clinical trial. Diabetes Care 2001;24:1019–22. [DOI] [PubMed] [Google Scholar]
- 22. Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care 2008;31:2118–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morona JK, Buckley ES, Jones S, Reddin EA, Merlin TL. Comparison of the clinical effectiveness of different off‐loading devices for the treatment of neuropathic foot ulcers in patients with diabetes: a systematic review and meta‐analysis. Diabetes Metab Res Rev 2013;29:183–93. [DOI] [PubMed] [Google Scholar]
- 24. Mueller MJ, Diamond JE, Sinacore DR, Delitto A, Blair VP 3rd, Drury DA, Rose SJ. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care 1989;12:384–8. [DOI] [PubMed] [Google Scholar]
- 25. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg 1996;183:61–4. [PubMed] [Google Scholar]
- 26. Saap LJ, Falanga V. Debridement performance index and its correlation with complete closure of diabetic foot ulcers. Wound Repair Regen 2002;10:354–9. [DOI] [PubMed] [Google Scholar]
- 27. Lavery LA, Peters EJ, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Res Clin Pract 2009;83:347–52. [DOI] [PubMed] [Google Scholar]
- 28. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–93. [DOI] [PubMed] [Google Scholar]
- 29. Lavery LA, Peters EJ, Williams JR, Murdoch DP, Hudson A, Lavery DC, International Working Group on the Diabetic Foot . Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care 2008;31:154–6. [DOI] [PubMed] [Google Scholar]
- 30. Davis J. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. John Hopkins Med J 1910;15:307–96. [Google Scholar]
- 31. Sabella N. Use of fetal membranes in skin grafting. Med Records NY 1913;83:478–80. [Google Scholar]
- 32. Stern M. The grafting of unpreserved amniotic membrane to burned and ulcerated skin surfaces substituting skin graft. JAMA 1913;60:973–4. [Google Scholar]
- 33. Arnold Y, Leroux JD, Williams M, Danilkovitch A. A comparison study of the anti‐oxidant effects of cellular versus acellular human repair matrices. In: Symposium on Advanced Wound Care; 2013 May 2–5; Las Vegas (NV).
- 34. Arnold Y, Leroux JD, Williams M, Danilkovitch A. A comparison study of the angiogenic effect of cellular versus acellular human repair matrices. In: Symposium on Advanced Wound Care; 2013 May 2–5; Las Vegas (NV).
- 35. Arnold Y, Leroux JD, Williams M, Danilkovitch A. A comparison study of the anti‐inflammatory effects of cellular versus acellular human repair matrices. In: Symposium on Advanced Wound Care; 2013 May 2–5; Las Vegas (NV).


