Abstract
This study compared moisture vapour transmission rate (MVTR) and wear time or fluid‐handling capacities of six adhesive foam dressings to a reformulated control dressing. Standardised in vitro MVTR methodology and a previously published in vivo artificial wound model (AWM) were used. Mean inverted MVTR for the reformulated dressing was 12 750 g/m2/24 hours and was significantly higher than four of the six comparator dressings (P < 0·0001), which ranged from 830 to 11 360 g/m2/24 hours. Mean upright MVTR for the reformulated dressing was 980 g/m2/24 hours and was significantly different than all of the comparator dressings (P < 0·0001), which ranged from 80 to 1620 g/m2/24 hours (three higher/three lower). The reformulated dressing median wear time ranged from 6·1 to >7·0 days, compared with 1·0 to 3·5 days for the comparator dressings (P = 0·0012 to P < 0·0001). The median fluid volume handled ranged from 78·0 to >87 ml compared with 13·0 to 44·5 ml for the comparator dressings (P = 0·0007 to P < 0·001). Interestingly, inverted MVTR did not correspond well to the AWM. These results suggest that marked differences exist between the dressings in terms of both MVTR and wear time or fluid‐handling capacity. Furthermore, high inverted MVTR does not necessarily predict longer wear time or fluid‐handling capacities of absorbent dressings.
Keywords: Foam dressing, Moisture vapour transmission, Performance, Wear time, Wound exudate
Introduction
Over the past two decades, the wound care clinician has seen tremendous growth in the introduction of new varieties, shapes and sizes of absorbent wound dressings. One category of dressing that has seen particular growth is adhesive foam dressings. In spite of the plethora of adhesive foam dressings available to the clinician, there still appears to be a gap between clinician expectations and product performance. In a survey study of 307 wound care clinicians conducted in the USA, Canada, UK and Germany (Table 1), clinicians expressed particular dissatisfaction in the performance of current adhesive foam dressings for attributes associated with exudate‐handling capabilities. In particular, absorbency, wear time and prevention of maceration were often cited as dressing deficiencies.
Table 1.
Summary of foam dressing clinician survey (n = 307)*
| Country | Sample size (confidence level) | Top two most commonly used adhesive foam dressings | Primary adhesive foam used most often | Average no. of adhesive foam dressings used per week | Attributes not being fulfilled† |
|---|---|---|---|---|---|
| USA/Canada‡ | 156 (90% ± 6·6%) | Mepilex (78%), Allevyn (63%) | Mepilex (44%) | 19 | Absorbency, length of wear time and prevention of maceration |
| UK | 61 (90% ± 10·6%) | Allevyn (92%), Mepilex (80%) | Allevyn (52%) | 20 | Absorbency, ease of removing, patient comfort and prevention of maceration |
| Germany | 90 (90% ± 8·7%) | Allevyn (67%), Tegaderm§ (47%) | Allevyn (39%) | 15 | Prevention of maceration |
Unpublished data, 3M Health Care, 2009.
Revealed by gap analysis: higher importance with lower satisfaction.
Includes 146 responders in the USA and 10 in Canada.
Predecessor formulation of the Tegaderm HP Adhesive Foam dressing tested in this study.
Wound exudate contains numerous growth factors and enzymes that can be both beneficial and inhibitory to the wound‐healing process depending on the nature of the wound, stage of healing and amount of exudate present 1, 2, 3, 4, 5, 6. For wounds that are highly exudative, adhesive foam dressings are popular among clinicians because of the ability of this class of dressing to rapidly manage large amounts of fluid. However, given the results of the recent clinician survey study, further improvements in exudate‐handling capabilities of adhesive foam dressings are needed. Furthermore, it is expected that as wounds heal they will become smaller and produce less exudate over time. Ideally, the dressing should adapt with the wound, handling more fluid under heavily exudating conditions while retaining required moisture under dry conditions. Such ‘smart’ or ‘moisture‐reactive’ dressings have been previously described in the literature 7, 8, 9.
The importance of a dressing adapting to changing wound exudate production was demonstrated in a retrospective study of 400 patients with a variety of chronic wound aetiologies. In this study, wounds that were treated with inappropriate exudate management dressings were found to be less likely to heal in 3 months compared with wounds receiving appropriate exudate management dressings 10. The authors pointed out that dressing materials differ in their properties of permeability and wound protection, and that the many available dressing choices may confuse clinicians. Therefore, moisture‐reactive dressings have the potential to not only simplify wound care by eliminating the guesswork in dressing selection but may also improve cost‐effectiveness and quality of care through improved wound healing by minimising the possibility of inappropriate dressing selection.
Recently, an adhesive foam dressing that has been commercially available for many years was redesigned to allow it to be more responsive to the degree of moisture present in the wound. This newly reformulated dressing differs from its predecessor in that it incorporates a new ‘moisture control layer’ that modulates moisture vapour permeability through the film backing under varying wound conditions. Under low exudating conditions, the dressing is designed to retain moisture within the wound, whereas under high exudating conditions, the dressing is designed to absorb moisture quickly and release the excess moisture from the dressing through evaporation. The absorbent layers in the dressing are also designed to prevent backward migration of wound fluid to help prevent periwound skin maceration and to facilitate lateral distribution of the moisture across the film backing to allow more effective use of dressing surface area for evaporation 11.
Given the high level of clinician dissatisfaction for exudate‐handling capabilities of currently marketed adhesive foam dressings, this series of studies were undertaken. The objectives of these studies were threefold, to compare the newly redesigned dressing with six commonly used adhesive foam dressings for: (i) in vitro moisture vapour transmission rate (MVTR) under simulated ‘wet’ and ‘dry’ wound conditions, (ii) in vivo wear time using an artificial wound model (AWM) procedure and (iii) in vivo fluid‐handling capability using an AWM procedure. The redesigned dressing and the six comparator dressings included in these studies are listed in Table 2 along with manufacturer names, product numbers and product study names used throughout this study.
Table 2.
Adhesive foam dressings under evaluation
| Study name | Dressing trade name | Product number | Manufacturer |
|---|---|---|---|
| Redesigned dressing | |||
| Tegaderm HP Foam | 3M™ Tegaderm™ High Performance Foam Adhesive Dressing | 90612 | 3M Company, St. Paul, MN |
| Comparator dressings | |||
| Allevyn | Allevyn™ Adhesive Hydrocellular Adhesive Dressing | 66020044 | Smith & Nephew, Largo, FL |
| Allevyn GB | Allevyn™ Gentle Border Gel Adhesive Hydrocellular Foam Dressing with Border | 66800279 | Smith & Nephew, Largo, FL |
| Mepilex Border | Mepilex® Border Dressing | 295400 | Mölnlycke Health Care US, Norcross, GA |
| Biatain | Biatain® Adhesive Foam Dressing | 3421 | Coloplast Corp., Minneapolis, MN |
| Optifoam | Optifoam® Adhesive Foam Wound Dressing | MSC1066 | Medline Industries, Mundelein, IL |
| Versiva | Versiva® XC® Gelling Foam Dressing | 410610 | ConvaTec, Skillman, NJ |
Methods
Study overview and test products
The fluid‐handling capacity of six adhesive foam dressings was determined and compared with a newly reformulated adhesive foam dressing (see Table 2 for full dressing names, study code names and manufacturer information). Fluid‐handling capabilities were compared by two test methods: a standardised in vitro MVTR test method 12 and a previously published in vivo AWM test method, which tested for both wear time and volume of infused artificial wound fluid (AWF) to dressing failure on ambulatory human volunteers 13. All seven test dressings were of comparable size, and all experiments were conducted between November 2009 and March 2010.
In vitro fluid‐handling capacity
The in vitro fluid‐handling capacity of all seven test dressings was determined using the procedures outlined in the European Standard EN 13726‐2:2002 12, which measured the MVTR of the seven test dressings using the standardised laboratory methodology. Briefly, the test method used a Paddington Cup apparatus, which consisted of a chamber with one open end and a clamping ring, each with a circular opening of 10 cm2. A sample of the test dressing was cut to fit the apparatus and placed between the two chambers that were clamped together with a moisture‐tight seal. The closed chamber contained a known quantity of deionised water. The fluid was in contact with the wound contact portion of the dressing and the upper surface of the dressing was exposed to ambient air. The test was conducted per EN 13726‐2:2002 in a controlled environmental chamber with a circulating fan that maintained a temperature of 37°C ± 1°C and 19% relative humidity (RH). The amount of moisture lost through the dressing was determined by gravimetric analysis.
This in vitro method determined both the ‘wet’ and ‘dry’ MVTR of the dressings. During the ‘wet’ method, the apparatus was inverted and the dressings were in contact with water, thus determining the theoretical MVTR of the dressing when placed over a highly exudating wound. During the ‘dry’ method, the apparatus was left upright with the dressing in contact with only the water vapour, thus determining the theoretical MVTR of the dressing on a dry or low‐exudating wound. For all seven test dressings, three lots of each dressing with a minimum of five replicates per lot were tested. Additional replicates of Tegaderm HP Foam and Allevyn were performed as internal controls within the various tests.
In vivo wear time and fluid‐handling capacity
The in vivo wear time and the fluid‐handling capacity of the seven test dressings were determined using a previously published AWM procedure on healthy adult volunteers 13. The enrolled subjects were selected such that they had no history of skin allergies or sensitivities to any of the products or components used in the study, had no pre‐existing skin conditions on the lower backs and no history of diabetes. Subjects were asked to refrain from using moisturisers or other skin products in the lower back area during the study and for 24 hours prior to starting the study. Additionally, subjects were asked to refrain from supplemental exercise and from getting the dressings excessively wet during the study (e.g. no tub bathing or showering directly on dressings).
Artificial wounds were constructed on the lower backs of the enrolled subjects and dressings of comparable sizes were placed over the artificial wounds (Figure 1). Twelve 1·0‐ml injections of AWF were intermittently infused into the models at intervals no less than 1 hour apart for a total daily dose of 12·0 ml of fluid. This dose of AWF was chosen to model a highly exudating wound (as determined from clinical observations by Mulder) 14, which is likely to be encountered in a clinical environment with adhesive foam dressings. Also, this total daily dose of fluid was consistent on an equivalent wound size basis with the highest observation of exudate made by Thomas et al. 15 from ten venous ulcers (1·2 g/cm2/24 hours). Composition of the AWF and general study procedures has been previously published 13.
Figure 1.
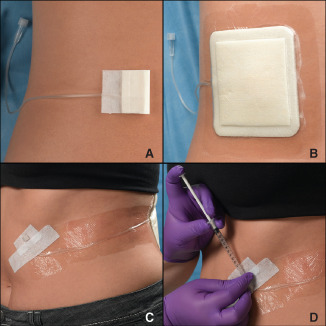
Photo sequence showing: (A) an artificial wound model (AWM) placed on the lower left back of a volunteer; (B) placement of a foam dressing over the AWM; (C) securement of the catheter and hub to the front of the volunteer for easy access and (D) injection of artificial wound fluid into the wound model. Injection of the model can be done by the study coordinator (as pictured) or by the subject in between assessment visits.
Briefly, the study used a paired design with each subject receiving two test dressings alternately placed on the left and right sides of the lower back region. Six individual studies were performed with Tegaderm HP Foam tested in each of the studies. Thus, Tegaderm HP Foam served as a control and comparator dressing across the six studies. The test dressings were worn continuously for up to approximately 7·25 days (7 complete days plus three fluid injections on the morning of day 8) or until failure of the test dressings. Failure was predefined as dressing fall‐off, leakage, edge lift sufficient to cause leakage or migration of the dressing from the application site. On each weekday of the study (Monday to Friday), dressings were assessed by a study coordinator for signs of dressing failure. The subjects kept an AWF infusion log and noted if and when dressings leaked between assessments. Both the time (in hours) and cumulative dose (in millilitres) of AWF to dressing failure were recorded.
An adaptive sequential design was used for each of the six individual studies. This design called for the enrolment of 24 subjects using an interim analysis conducted on the first 12 subjects enrolled in the study to ascertain whether pre‐determined efficacy stopping criteria were met.
Ethical considerations
Each of the six studies that used human volunteers conformed to the ethical guidelines of the 1975 Declaration of Helsinki (as amended, October 2008) and was conducted in compliance with the Good Clinical Practice guidelines and the Health Insurance Portability and Accountability Act regulations 16, 17, 18. An ethic review was conducted before the start of each of the studies, and all participants signed an informed consent before study enrolment.
Outcome measures
The main outcome of interest for the in vitro studies was a comparison of the mean inverted (wet) and upright (dry) MVTR values for each of the seven test dressings, as well as the mean difference in the inverted and upright MVTR measurements, which is defined as ‘moisture reactivity’ of the dressings. The main outcomes of interest for the in vivo studies were a comparison of the median wear time with volume of AWF infused until dressing failure.
Statistical methods
In vitro MVTR
Mean inverted and upright MVTR values, as well as the mean difference in inverted and upright MVTR values (moisture reactivity) for each of the six comparator dressings were compared with Tegaderm HP Foam using a mixed effects linear model with MVTR measures as the response and dressing lots treated as a random variable.
In vivo wear time and fluid‐handling capacity
Both the time (hours) and volume of AWF (millilitres) until dressing failure were compared among dressing types using a Cox proportional hazard model that accounted for the clustered data using a robust sandwich covariance matrix estimate. If the dressing remained intact at the end of the study, or if the dressing was removed for reasons not associated with dressing performance (e.g. caught on clothing), then the time to those events was treated as censored. The primary test of difference was a one‐sided Wald test, with a null hypothesis that the hazard ratio is equal to 1·0. The estimate of the proportion of dressings remaining intact after a specific time or fluid exposure (the survival curve) was computed using the Actuarial method with 24‐hour (12 dose) intervals. The median survival time and the median dose to failure were estimated using the Kaplan–Meier method.
Stopping criteria at the 50% point (first 12 enrolled subjects) were predefined as having achieved statistical significance with the efficacy endpoint of wear time to dressing failure. The study was stopped only if the P‐value crossed the pre‐defined O'Brien–Fleming stopping boundary, which was 0·00557, with a one‐sided Wald test P‐value and the null hypothesis that the hazard ratio equal to 1·0.
Results
In vitro MVTR
The mean (SD) values for both the inverted (wet) and the upright (dry) MVTR measurements and the mean difference in the upright and inverted MVTR values for each of the test dressings are provided in Figure 2. The mean inverted (wet) MVTR value was statistically higher for Tegaderm HP Foam compared with the Mepilex Border, Biatain, Optifoam and Versiva (all P < 0·001), but not for the Allevyn (P = 0·4642) or Allevyn GB dressings (P = 0·8433). The mean upright (dry) MVTR value for Tegaderm HP Foam was statistically different than all of the other test dressings (P < 0·0001). Compared with the Allevyn, Allevyn GB and Mepilex Border, Tegaderm HP Foam exhibited significantly lower upright (dry) MVTR values (all P < 0·0001); however, when compared with Biatain, Optifoam and Versiva, Tegaderm HP Foam exhibited significantly higher upright (dry) MVTR values (all P < 0·0001). Moisture reactivity was the highest for Tegaderm Foam, Allevyn and Allevyn GB, and was statistically different than that of Mepilex Border, Biatain, Optifoam and Versiva (P < 0·0001).
Figure 2.
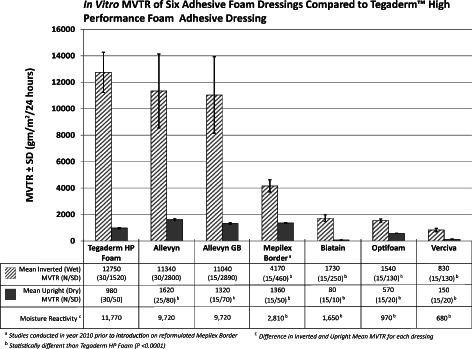
Mean inverted (wet) and upright (dry) moisture vapour transmission rate (MVTR) for the seven adhesive foam dressings under study. All data were collected using the procedures outlined in European Standard EN 13726‐2:2002 12.
In vivo wear time and fluid‐handling capacity
For five of the six in vivo wound model studies, the P‐value for the time to dressing failure crossed the pre‐planned O'Brien–Fleming stopping boundary at the 50% enrolment point, and the studies were ended after enrolment of 12 subjects because of overwhelming differences in the primary efficacy endpoint (dressing wear time). This included the studies involving the Allevyn GB, Optifoam, Biatain, Mepilex Border and Versiva. For one study (Tegaderm HP Foam versus Allevyn), the P‐value did not reach the stopping criteria and the study continued with all 24 subjects enrolled.
Data for the in vivo fluid‐handling studies are summarised in Table 3 and Figure 3. For all six studies, Tegaderm HP Foam stayed functional significantly longer and handled significantly more AWF prior to failure than any of the other test dressings. The median wear time for Tegaderm HP Foam ranged from 6·1 to >7·0 days, whereas the comparator dressings ranged from 1·0 to 3·5 days. Actuarial analysis of the pooled data for Tegaderm HP Foam across all six studies showed an estimated 59% survival rate at 7 days of wear (Figure 3) and an estimated median fluid‐handling capacity of >87 ml (Figure 4). Four of the six comparator dressings had an estimated 0% survival rate at 7 days of wear, and only Allevyn (8%) and Biatain (<42%) dressings exhibited some survival at 7 days of testing (Table 3). In all cases, the survival curves differed significantly from Tegaderm HP Foam based on the Cox regression model (Table 3 and Figure 3).
Table 3.
Dressing performance of six adhesive foam dressings compared with reformulated Tegaderm™ High Performance Foam Adhesive Dressing using an in vivo artificial wound model simulating a highly exudating wound
| Dressing | N | Kaplan–Meier estimated survival rate at 7 days (SE) | Median days to dressing failure | P‐value* (time to failure) | Median fluid volume to dressing failure (ml) | P‐value* (volume to failure) |
|---|---|---|---|---|---|---|
| Tegaderm HP Foam | 24 | 39% (10) | 6·1 | 0·0006 | 78·0 | 0·0007 |
| Allevyn | 24 | 8% (6) | 3·5 | 44·5 | ||
| Tegaderm HP Foam | 12 | 67% (14) | >7·0 | <0·001 | >87 | <0·001 |
| Allevyn GB | 12 | 0% (—) | 1·0 | 13·0 | ||
| Tegaderm HP Foam | 12 | 57% (15) | >7·0 | <0·001 | >87 | <0·001 |
| Mepilex Border† | 12 | 0% (—) | 2·5 | 32·5 | ||
| Tegaderm HP Foam | 12 | 57% (15) | 7·0 | <0·001 | >87 | <0·001 |
| Versiva XC | 12 | 0% (1) | 1·2 | 18·0 | ||
| Tegaderm HP Foam | 12 | 43% (15) | 7·0 | <0·001 | 87·0 | <0·001 |
| Optifoam | 12 | 0% (—) | 3·0 | 36·0 | ||
| Tegaderm HP Foam | 12 | 73% (14) | 7·0 | 0·0012 | >87 | 0·0005 |
| Biatain | 12 | <42% (14) | 3·2 | 43·5 |
Wald test with null hypothesis that the hazard ratio < 1·0.
Studies conducted in the year 2010 prior to introduction of reformulated Mepilex Border Dressing.
Figure 3.
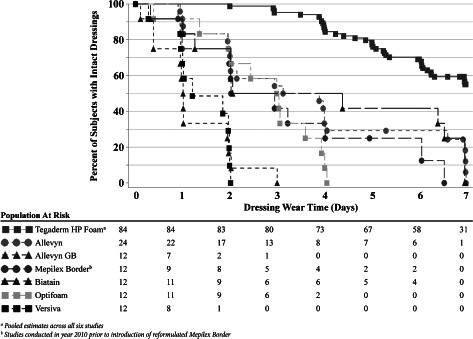
Kaplan–Meier estimated survival rates for six adhesive foam dressings compared with Tegaderm™ High Performance Foam Adhesive Dressing using an in vivo artificial wound model.
Figure 4.
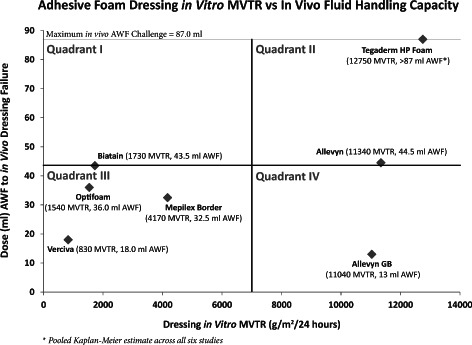
Mean in vitro moisture vapour transmission rate (MVTR) values of the seven test dressings compared to the median in vivo fluid‐handling capacity of the same dressings derived from Kaplan–Meier analysis of artificial wound model data. Data are segregated into four quadrants for easier viewing. Dressings with lower MVTR values tended to have lower in vivo fluid‐handling capacity; however, higher in vitro MVTR values did not necessarily result in higher in vivo fluid‐handling capabilities.
Figure 4 presents a plot of the in vitro inverted (wet) MVTR results versus the in vivo fluid‐handling results. For ease of viewing, the graph is divided into four quadrants set at the midpoints of both the in vitro and the in vivo fluid‐handling axes.
Reasons for dressing failure
A summary of the reasons for dressing failure is presented in Figure 5. With the exception of a few non product‐related early dressing removals, all of the dressings that were removed prior to the end of the study were removed because of leakage of the dressing resulting from adhesive failure at one or more edges.
Figure 5.
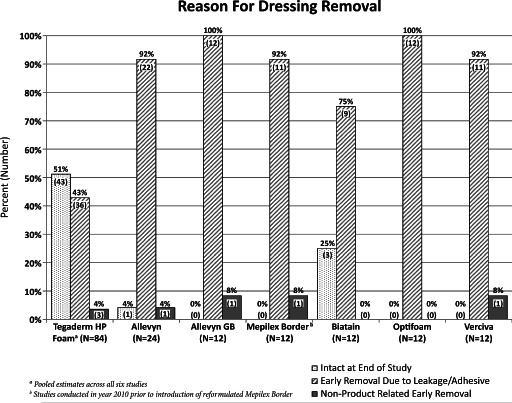
Dressings were removed before reaching the end of this 7‐day study primarily because of fluid leakage due to adhesive border failure. Approximately half (51%) of the Tegaderm™ High Performance Foam Adhesive Dressing samples survived to the end of the study, which is in stark contrast to the comparator dressings.
Discussion
The inverted (wet) in vitro MVTR data from this study agree remarkably well with the data previously published in the literature. Thomas and Young compared Allevyn with another foam dressing not tested in this study 8, and in another study White et al. 7 compared 12 adhesive foam dressings with each other, three of which (Mepilex Border, Biatain and Allevyn) were included in this study. The calculated mean 24‐hour MVTR for the Mepilex Border, Biatain and Allevyn dressings published in these prior studies was generally similar to the results in this study. In this study, the mean MVTR for Allevyn was 11 400 g/m2/24 hours compared with approximately 12 000 g/m2/24 hours in both the studies by Thomas and Young, and by White et al. In the study by White et al., Mepilex Border exhibited a mean MVTR of about 5500 g/m2/24 hours compared with 4170 g/m2/24 hours in this study, and Biatain exhibited a mean MVTR of about 1100 g/m2/24 hours compared with 1730 g/m2/24 hours in this study. This high level of agreement across the studies performed in different laboratories is undoubtedly due to the high degree of standardisation in the conditions of the test across these studies when they are conducted according to EN 13726‐2:2002 12. Thomas et al. demonstrated the importance of such standardisation when comparing MVTR values across dressings, particularly with regard to the control of temperature and humidity 19. Similar comparisons for in vitro upright (dry) MVTR values of the foam dressings are not available in the literature.
In this study, Tegaderm HP foam provided the highest mean inverted MVTR value of all the test dressings and was statistically higher than that of Mepilex Border, Biatain, Optifoam and Versiva; however, the clinical relevance of the MVTR data is unclear with regard to predicting wear time and fluid‐handling capacity of dressings. Thomas and Young, and Thomas 8, 9 used dressing MVTR values in combination with dressing absorbency data to project a theoretical range of wound exudate volumes that several foam dressings could handle over time and then compared the range to that of published exudate values for a variety of chronic wounds. However, as the authors point out, the usefulness of such projections is limited by a number of factors, the most important being that the test is conducted under static and environmentally controlled conditions that may or may not approximate real‐world conditions. In particular, the in vitro MVTR apparatus simply cannot model clinically relevant adhesive–skin interactions. That is, the relevance of high inverted MVTR data is questionable if the dressing cannot maintain a moisture‐tight seal over the wound; exudate may simply leak out the side of the dressing. At a minimum, this would reduce the clinical wear time (hence cost‐effectiveness) of the dressing to less than that predicted by the MVTR + absorbency data. More importantly, leakage and pooling of exudate around the wound could potentially cause enlargement of the wound through maceration or excoriation of the periwound skin.
It is even more difficult to interpret the upright in vitro MVTR values, as little is known about optimal MVTR values under dry wound conditions. Furthermore, MVTR data are collected at temperatures and humidity levels that are not clinically relevant. Typically, these data are collected at 37°C and <20% RH, but as Thomas et al. demonstrated 19, MVTR values are inversely related to changes in RH and directly related to changes in temperature. Therefore, at more clinically relevant temperatures of 20–25°C and humidity levels closer to 40–50%, one would expect much lower MVTR values than that predicted by the standardised conditions of the EN 13726‐2:2002 test method 12. Adding even more complexity to the interpretation of MVTR results is that body temperature will likely affect the MVTR of the dressings in the opposite direction.
Therefore, rather than attaching a particular relevance to either the inverted or the upright in vitro MVTR values, it is probably more important that the dressing simply be ‘adaptive’ in nature, that is, to be moisture reactive by exhibiting higher MVTR under wet wound conditions and lower MVTR under dry wound conditions and that comparisons across dressings be done under similar conditions. In this study, using the standardised conditions of the EN 13726‐2:2002 test method 12, Tegaderm HP Foam provided the highest degree of moisture reactivity of all the dressings tested (Figure 2), and was statistically more moisture responsive than Mepilex Border, Biatain, Optifoam and Versiva in this regard (P < 0·0001).
Because of the limitations of the in vitro method, the six test dressings were additionally compared Tegaderm HP Foam under simulated artificial wound test conditions using a previously published in vivo AWM test method 13. This in vivo test method overcomes many of the limitations of the in vitro method 12, 13, and is conducted on the lower backs of ambulatory volunteers so it incorporates significant adhesive–skin interactions that are difficult to model in vitro. In this series of studies, the in vivo model was designed to mimic a highly exudating wound 14; therefore, the studies should be interpreted as modelling performance of the dressings in a highly exudating wound environment.
In this series of in vivo comparisons, Tegaderm HP Foam provided the highest fluid‐handling capacity of the six test dressings to which it was compared (Table 3 and Figure 3). The median fluid‐handling capacity of Tegaderm HP Foam across the six studies was >87 ml compared with 44·5 ml for the nearest comparator dressing (Allevyn). In all comparisons, Tegaderm HP Foam absorbed significantly more fluid and provided significantly longer wear time than any of the six test dressings (Table 3).
Figure 4 illustrates the relationship between the in vitro and in vivo data. It is clear from this graph that lower in vitro inverted MVTR measurements correspond reasonably well with lower in vivo fluid‐handling capacity (dressings in quadrant III). These dressings appear to have limited ability to handle moisture through evaporation (lower MVTR), thus allowing a build‐up of unabsorbed and non transpired fluid beneath the dressings that eventually undermines the adhesive border. Figure 5 presents reasons for dressing removals, and in most cases the dressings were removed prematurely because of leakage as a result of adhesive border failure.
An alternative explanation for premature dressing failure could be that the adhesive simply could not maintain a moisture seal under the ambulatory test conditions modelled in these studies regardless of the absorptive or MVTR capabilities of the dressings. Data for Allevyn, Allevyn Gentle Border and Tegaderm HP Foam tend to favour this latter explanation. These three dressings exhibited very high in vitro inverted MVTR measurements that did not differ significantly from each other, and they have very similar absorptive capacities (unpublished data); however, the three dressings differed markedly in their in vivo fluid‐handling capacities. In particular, the Allevyn Gentle Border exhibited one of the highest mean in vitro MVTR measurements of all the dressings tested; yet it exhibited the lowest in vivo fluid‐handling capability, indicating that the probable mode of failure was corruption of the adhesive seal surrounding the dressing rather than saturation of the dressing itself.
Regardless of the exact reason for the differences between the in vitro and in vivo data, it is clear that the two methods only moderately correspond with each other and that use of in vitro MVTR and absorptive data to predict in vivo fluid‐handling performance of absorbent dressings could lead to false conclusions. At best, in vitro MVTR and absorption measurements provide insight into only two of several important dressing attributes needed to predict and compare in vivo fluid‐handling properties of absorbent dressings.
Limitations of the studies
As previously discussed, the in vitro MVTR test method has limitations that must be considered when comparing dressings with each other and for projecting the results to clinical performance of the dressings. First, a relatively small sample of dressing is tested (10 cm2) and the entire surface of this sample is exposed to the test fluid. In a clinical setting, the clinician would likely to choose a dressing much larger than the actual wound, thus significantly changing the dynamics of fluid absorption, wicking and transpiration. Second, the dressing is cut to size and rigidly fixed within the test apparatus. Modern foam dressings are complex in design with numerous interacting layers, and there is no way to predict how cutting the dressings might affect the measured MVTR. Thomas 9 noted separation of the structure of two foam dressings tested with this methodology, and a similar artefact with one of those dressings (Biatain) was noted in this study as well. Third, the static nature of the test does not model movement of the patient or clinically relevant adhesive–skin interactions that may contribute to dressing failure prior to reaching limitations imposed by fluid‐handling capacity. Fourth, the in vitro method is conducted under tightly controlled temperature (37°C/99°F) and RH (19%) conditions that probably do not reflect actual clinical conditions to which dressings are routinely exposed. Finally, the in vitro method as standardised in EN 13726‐2:2002 is conducted with either a simple isotonic salt solution or water that differ in many important ways from chronic wound fluid.
The in vivo test method was designed to address these limitations and has been shown to be useful in modelling differences between the relative performance of a hydrocolloid and transparent absorbent dressing in a clinical environment 20, 21. The main limitation of the in vivo method is that conditions to which a dressing is exposed in a clinical environment differ from setting to setting and from patient to patient. Also, the model has not been validated for replicating clinical levels of wound exudate, particularly for levels of exudate that decline over time as a result of wound healing. Therefore, the model cannot be used to predict exact dressing wear time or fluid‐handling capacity, but rather relative performance of the dressings given similar clinical conditions. This also highlights the main benefit of the in vivo model in that side‐by‐side comparisons of dressing performance are possible within a relatively short time frame and on a much smaller population of subjects than can be obtained in a clinical environment.
Research implications
As discussed previously, it is important to be mindful that the scope of this research is limited to the use of in vitro MVTR and in vivo wear time or fluid‐handling measurements for predicting relative performance of dressing wear time and fluid‐handling capacity. However, a discussion of the limitations and clinical implications of this research would not be complete without addressing wound healing.
Numerous studies have demonstrated the importance of creating a moist wound environment for proper wound healing 22, 23. One of the greatest fears of using moist wound healing is that it will increase bioburden within the wound which may possibly lead to infection. However, in a review of published studies reporting infection rates across a variety of dressing and wound types, the mean infection rate among 1085 wounds treated with non‐moist wound healing dressings was 7·1% compared with 2·6% for 3047 wounds treated with moist wound healing dressings (P < 0·001) 24. Furthermore, in a systematic literature review for the use of moist wound healing dressings compared with traditional non‐moist dressings for the treatment of skin graft donor wounds, it was concluded that wounds heal faster and with less pain and infection when treated with moist wound healing dressings 25. Clearly, there is a preponderance of literature supporting the use of moist wound healing dressings, but how much moisture is needed and how is this related to dressing in vitro MVTR or in vivo wear time or fluid‐handling capacity?
Work by Bolton et al. using an acute wound animal model provides a valuable reference point 23, 26. Using an in vivo real‐time MVTR methodology, this study clearly shows a statistically significant correlation between lower in vivo dressing MVTR measurements 24 hours after application to freshly excised wounds and day 7 wound‐healing outcomes for both partial‐thickness acute wounds (R 2 = 0·5729, P < 0·001) and full‐thickness acute wounds (R 2 = 0·6578, P < 0·001) 23, 26. However, it is difficult to compare these real‐time in vivo measurements with the in vitro measurements in this study because of potential differences in environmental test conditions and methodologies. Bolton et al. provided no environmental laboratory conditions in their study, and it is well understood that MVTR measurements are highly sensitive to environmental temperature and RH 19, 27, 28. Furthermore, none of the dressings in the study by Bolton et al. was moisture reactive, so it is unclear how this type of dressings might have performed in such a study. It is clear that with non moisture‐reactive dressings in this acute wound animal model the wounds covered with lower MVTR dressings exhibited faster rates of healing than dressings with higher MVTR.
It is tempting to extrapolate these findings to clinical applications and say that all wound care dressings should be as occlusive as possible in order to maximise healing rates, but this ignores the fact that we simply do not know what optimum moisture levels should be during the various phases of healing. Furthermore, extrapolating these data to chronic human wounds ignores other important aspects of chronic wound care including underlying aetiologies, wound bed preparation 6, control of dysregulated wound proteases 29, 30, 31 and biofilms 32, prevention of periwound maceration by adequately handling wound drainage and the economics of wound care through improved fluid‐handling capacity and dressing wear time. The intent of this work is simply to address the latter issue through side‐by‐side comparisons of in vitro MVTR with in vivo wear time or fluid‐handling capacity. Future work is clearly needed to better understand wound moisture and dressing MVTR requirements as it applies to dressing selection for chronic wounds.
Conclusion
Tegaderm HP Foam exhibited significantly higher inverted MVTR values relative to four of six comparator adhesive foam dressings and was adaptive to high or low moisture conditions. Furthermore, Tegaderm HP Foam exhibited significantly longer in vivo wear time and handled significantly more AWF than all six comparator dressings, suggesting that marked differences potentially exist between the dressings in terms of clinical wear time and fluid‐handling capacity. These results also suggest that high in vitro inverted MVTR measurements do not necessarily predict longer in vivo wear time or fluid‐handling capacity.
Acknowledgements
The authors wish to thank Mr James B Lutz, MS, Lutz Consulting LLC, Medical Writing Services who provided medical writing/editing services on behalf of 3M Company. CZ, DH, SS and S‐AW are employees of 3M Health. This study was funded by 3M Health Care.
References
- 1. Folestad A, Gilchrist B, Harding K, de Laat E, Lyder C, Meaume S, Phillips T, Price P, Romanelli M, Sibbald G, Vanscheidt W, Verdú J, Vowden K, Vowsen P. Wound exudate and the role of dressings. A consensus document. Int Wound J 2008;5(Suppl 1):iii–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phillips TJ, al‐Amoudi HO, Leverkus M, Park HY. Effect of chronic wound fluid on fibroblasts. J Wound Care 1998;7:527–32. [DOI] [PubMed] [Google Scholar]
- 3. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg 2006;117(7 Suppl):1e‐S–32e‐S. [DOI] [PubMed] [Google Scholar]
- 4. Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol 2007;25:19–25. [DOI] [PubMed] [Google Scholar]
- 5. Katz MH, Alvarez AF, Kirsner RS, Eaglstein WH, Falanga V. Human wound fluid from acute wounds stimulates fibroblast and endothelial cell growth. J Am Acad Dermatol 1991;25(6 pt 1):1054–8. [DOI] [PubMed] [Google Scholar]
- 6. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(Suppl 1):S1–S28. [DOI] [PubMed] [Google Scholar]
- 7. White R, Hartwell S, Brown S. Interim report on a study to assess the effectiveness and improved fluid uptake of new Allevyn. Wounds UK 2007;3:121–7. [Google Scholar]
- 8. Thomas S, Young S. Exudate‐handling mechanisms of two foam‐film dressings. J Wound Care 2008;17:309–15. [DOI] [PubMed] [Google Scholar]
- 9. Thomas S. Laboratory findings on the exudate‐handling capabilities of cavity foam and foam‐film dressings. J Wound Care 2010;19:192 194–9. [DOI] [PubMed] [Google Scholar]
- 10. Jones KR, Fennie K, Lenihan A. Chronic wounds: factors influencing healing within 3 months and nonhealing after 5–6 months of care. Wounds 2007;19:51–63. [PubMed] [Google Scholar]
- 11. 3M™ Tegaderm™ High Performance Foam Adhesive Dressing Product Brochure . URL http://solutions3mcom/wps/portal/3M/en_US/WW2/Country/ >Products & Services > Health Care > Medical: Skin and Wound Care > Products > Dressings/Foam > 3M™ Tegaderm™ High Performance Foam Adhesive Dressing > Resources [accessed on 14 August 2011]
- 12. BSI . British Standard BS EN 13726‐2. Test methods for primary wound dressings. Moisture vapour transmission rate of permeable film dressings. BSI, 2002.
- 13. Lutz JB, Zehrer CL, Solfest SE, Walters SA. A new in vivo test method to compare wound dressing fluid handling characteristics and wear time. Ostomy Wound Manage 2011;57:28–36. [PubMed] [Google Scholar]
- 14. Mulder GD. Quantifying wound fluids for the clinician and researcher. Ostomy Wound Manage 1994;40:66–9. [PubMed] [Google Scholar]
- 15. Thomas S, Fear M, Humphreys J, Disley L, Waring M. The effect of dressings on the production of exudate from venous leg ulcers. Wounds 1996;8:45–9. [Google Scholar]
- 16. World Medical Association Declaration of Helsinki . Ethical principles for medical research involving human subjects. URL http://wwwwmanet [accessed on 27 July 2011]
- 17. U.S. Food and Drug Administration . Good Clinical Practice. 21 CFR Parts 11, 40, 54, 56, 312, 314. URL http://www.fda.gov/oc/gcp/regulations.html [accessed on 27 July 2011]
- 18. U.S. Department of Health and Human Services . Standards for Privacy of Individually Identifiable Health Information; Final Rule, 45 C.F.R. Parts 160, and 164. Code of Federal Regulations. URL http://wedi.org/snip/public/articles/45CFR160&164.pdf [accessed on 27 July 2011]
- 19. Thomas S, Barry L, Fram P, Phillips PJ. The effect of temperature and humidity on the permeability of film dressings. J Wound Care 2011;20:484–9. [DOI] [PubMed] [Google Scholar]
- 20. Atwood NP, Solfest SE, Lutz JB. Wear time comparison of three absorbent dressings with human artificial wound models. Poster Presentation, Scientific and Clinical Abstracts from the 36th Annual Wound, Ostomy and Continence Conference, Tampa, June 5–9, 2004.
- 21. Brown‐Etris M, Milne C, Orsted H, Gates JL, Netsch D, Punchello M, Couture N, Albert M, Attrell E, Freyberg J. A prospective, randomized, multisite clinical evaluation of a transparent absorbent acrylic dressing and a hydrocolloid dressing in the management of Stage II and shallow Stage III pressure ulcers. Adv Skin Wound Care 2008;21:169–74. [DOI] [PubMed] [Google Scholar]
- 22. Bolton LL. Moist wound healing from past to present. In: Rovee DT, Maibach HI, editors. The epidermis in wound healing. Boca Raton: CRC Press, 2004:89–102. [Google Scholar]
- 23. Bolton LL, Monte K, Pirone LA. Moisture and healing: beyond the jargon. Ostomy Wound Manage 2000;46(1A Suppl):51S–62S quiz 3S‐4S. [PubMed] [Google Scholar]
- 24. Hutchinson JJ, McGuckin M. Occlusive dressings: a microbiologic and clinical review. Am J Infect Control 1990;18:257–68. [DOI] [PubMed] [Google Scholar]
- 25. Wiechula R. The use of moist wound‐healing dressings in the management of split‐thickness skin graft donor sites: a systematic review. Int J Nurs Pract 2003;9:S9–17. [DOI] [PubMed] [Google Scholar]
- 26. Pirone LA, Monte KA, Shannon RJ, Bolton LL. Wound healing under occlusion and non‐occlusion in partial‐thickness and full‐thickness wounds in swine. Wounds 1990;2:74–81. [Google Scholar]
- 27. Pinnagoda J. Standardization of measurements. In: Elsner P, Berardesca E, Maibach HI, editors. Bioengineering of the skin: water and the stratum corneum. Boca Raton, London: CRC, 1994:59–65. [Google Scholar]
- 28. Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 1990;22:164–78. [DOI] [PubMed] [Google Scholar]
- 29. Lobmann R, Zemlin C, Motzkau M, Reschke K, Lehnert H. Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing. J Diabetes Complications 2006;20:329–35. [DOI] [PubMed] [Google Scholar]
- 30. Mwaura B, Mahendran B, Hynes N, Defreitas D, Avalos G, Adegbola T, Adham M, Connolly CE, Sultan S. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase‐2, tissue inhibitor of matrix metalloproteinase‐2 and PDGF‐AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg 2006;31:306–10. [DOI] [PubMed] [Google Scholar]
- 31. Schultz GS, Mast BA. Molecular analysis of the environment of healing and chronic wounds: cytokines, proteases, and growth factors. Wounds 1998;10(Suppl F):1F–9F. [Google Scholar]
- 32. Bjarnsholt T, Kirketerp‐Moller K, Jensen PO, Madsen KG, Phipps R, Krogfelt K, Hoiby N, Givskov M. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008;16:2–10. [DOI] [PubMed] [Google Scholar]


