Abstract
Massive skin defects caused by severe burn and trauma are a clinical challenge to surgeons. Timely and effective wound closure is often hindered by the lack of skin donor site. Bone marrow‐derived cells (BMDCs) have been shown to ‘differentiate’ into multiple tissue cells. In this study we focused on the direct manipulation of endogenous BMDCs, avoiding the immunocompatibility issues and complicated cell isolation, purification, identification and amplification procedures in vitro on wound repair. We found that mobilisation of the BMDCs into the circulation significantly increased the amount of BMDCs at the injury site which in turn accelerated healing of large open wound. We used a chimeric green fluorescent protein (GFP) mouse model to track BMDCs and to investigate their role in full‐thickness skin excisional wounds. We have shown that bone marrow mobilisation by granulocyte colony stimulating factor (G‐CSF) exerted multiple beneficial effects on skin repair, both by increasing the engraftment of BMDCs into the skin to differentiate into multiple skin cell types and by upregulating essential cytokine mRNAs critical to wound repair. The potential trophic effects of G‐CSF on bone marrow stem cells to accelerate wound healing could have a significant clinical impact.
Keywords: BMDCs, G‐CSF, Wound healing
Introduction
Massive skin defects caused by severe burn and trauma remain a challenge in clinical work. Early closure of open wounds is apparently the primary goal of treatment. Currently, surgeons usually perform the intermingled transplantation using autograft, allograft and micrografting techniques. However, this type of therapy often requires multiple operations and is subject to the limitation of available donor skin. Non‐invasive wound management is therefore a top concern for clinicians.
Wound repair is a highly complex biological process involving many kinds of cells, extracellular matrix and regulatory factors (1). The process of wound healing is highly dependent on some systemic signals, such as growth factors, cytokines, chemokines and proteolytic enzymes. Stem cells play a unique role in the above biological changes in response to skin injury, and thereby represent promising non‐invasive wound management. These stem cells could originally be located in the injured tissues or migrated to injured tissues from bone marrow, such as mesenchymal stem cells (MSCs) and haematopoietic stem cells. Bone marrow‐derived cells (BMDCs) have been shown to ‘differentiate’ into multiple tissue cells. The traditional concept of stem cell therapy includes the isolation of stem cells from patients, amplification and differentiation in vitro and subsequent re‐injection of autologous cells into the patient. The quantity of stem cells is very low in fresh bone marrow fluid aspirated from the ilium. Consequently, BMDCs should be cultured and amplified in vitro before clinical application. Such in vitro preparation could increase contamination risk, decrease pluripotency of MSCs and miss the optimal transplantation time, which limits its clinical usefulness. An alternate cell source could be the endogenous stem cells. Thus, scientists currently focus on particular on the convenient and safety usage of BMDCs on in vivo wound repair. However, the study by Fan et al.(2), using two different murine models, suggest that BMDCs do not significantly contribute to steady‐state epidermal homeostasis and are not required or responsible for providing keratinocyte stem cells and keratinocyte repopulation following skin injury. These data indicate that BMDCs have a limited role in wound repair whether skin is injured or not. In this study we hypothesise that mobilisation by granulocyte colony stimulating factor (G‐CSF) of BMDCs into the circulation can significantly increase the amount of BMDCs at injury site, which in turn accelerates wound healing after skin injury. To allow tracking of the BMDC, we used a chimeric mouse model in which bone marrow cells marked with enhanced green fluorescent protein (EGFP) were isolated from EGFP‐transgenic C57BL/6 donor mice and were transplanted into lethally irradiated wild‐type C57BL/6 mice to investigate the role of BMDCs in a full‐thickness skin excisional wound. Using this mouse model, we investigated the total contribution of the BMDCs after mobilisation to cutaneous wound healing.
We found that wound healing was accelerated after the G‐CSF induced bone marrow mobilisation with increased recruitment of BMDCs to wound site and expression of growth factors critical to wound healing.
Materials and methods
Chimeric mice preparation
All experiments were performed in accordance with the protocols approved by the Animal Care Committee of Second Military Medical University. Two‐month‐old 300 male C57BL/6 mice and 60 EGFP‐transgenic C57BL/6 donor mice were used. All mice were housed under identical conditions and given food and water ad libitum. The experimental design is summarised in Figure 1.
Figure 1.
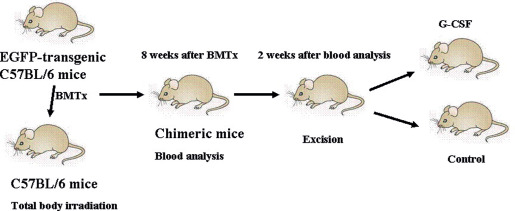
Experimental design. C57BL/6 mice after total body irradiation were transplanted with bone marrow cells acquired from enhanced green fluorescent protein (EGFP)‐transgenic C57BL/6 mice immediately. A small number of surviving mice had undergone blood analysis 8 weeks after bone marrow transplantation (BMTx) to evaluate the degree of chimerism. The rest of survivors received an excisional wound on the back 2 weeks after blood analysis. After excision, these mice were divided into two groups: granulocyte colony stimulating factor (G‐CSF) group (mobilisation group) and control group.
In order to prepare chimeric mice, bone marrow was collected from the tibia and femur of EGFP‐transgenic C57BL/6 donor mice (kindly provided by Professor Huang, Department of Cardiac Surgery, Changhai Hospital). A single‐cell suspension was prepared, and the cells were ready for immediate transplantation. Recipient adult C57BL/6 mice were treated with a lethal irradiation dosage of 8·5 Gy at 0·8737 Gy/min using a cobalt‐60 irradiator. Approximately 5 × 106 EGFP+ cells were prepared for transplantation by resuspension in phosphate buffered saline (PBS) and injected into recipient mice via the tail vein. Degree of chimerism was assessed by flow cytometry of circulating nucleated cells at 8 weeks after bone marrow transplantation.
Skin wound model and BMDCs mobilisation
Under sodium pentobarbital anesthesia, the dorsal hair was removed, and the skin was prepared for generation of standardised full‐thickness wounds including the panniculus carnosus muscle on the mid‐back. All chimeric mice were divided into two groups and a wound injury of 1·3 × 1·3 cm2 was created in them. Recombinant human G‐CSF was injected s.c., 50 µg/kg/day (Kirin Biopharmaceutical, Tokyo, Japan), for 5 days before and 3 days after wound creation in G‐CSF group. Saline instead of G‐CSF was injected in other wounded mice as control group.
Wound‐healing examination
In order to record wound healing, images of the residual wound area were obtained using a digital camera under the same photographic conditions. Average wound‐healing time was then determined. The digital analysis was performed by the investigators who were blinded to group assignments. Complete wound‐healing times were indicated by the shedding of scars and complete re‐epithelialisation of the wound bed. The re‐epithelialised but unhaired skin area was recorded as zero. The percentage of residual wound area relative to the original area was calculated using an image analysis software.
On days 0, 3, 6, 9, 12, 15 and 18 after wound injury, mice were anaesthetised and the entire wound with its surrounding skin within 2 mm was excised. The excised tissue was bisected along its craniocaudal axis into two parts. One of them was fixed in 2% paraformaldehyde and then embedded in OCT (Tissue‐Tek, Sakura, Torrance, CA) for histological analysis; the other one was snap‐frozen in liquid nitrogen for real‐time polymerase chain reaction (RT‐PCR) examination.
GFP+ cells detection in recipient wound in vivo by spectral fluorescence imaging
On days 0, 3, 6, 9, 12, 15, 18 and 21 after wound injury, mice were anaesthetised and the entire wounds were exposed. Fluorescence imaging was conducted using a whole‐animal imaging system (Maestro, CRi, Woburn, MA). Multiple images were acquired with fixed bandpass excitation and emission filters in place. Each image was acquired with identical exposure time. Each series of image was subsequently analysed using Maestro version 2.4 software. Images corresponding to GFP signals were used to quantify fluorescence signals.
Immunofluorescence staining
Immunofluorescence staining was performed on OCT frozen sections using standard staining protocols. Skin sections were stained with anti‐GFP Ab (Molecular Probes, Eugene, OR). In addition, skin sections were treated with primary Abs against CK10 (Santa Cruz Biotechnology), α‐smooth muscle actin (α‐SMA; Sigma Biotechnology, St. Louis, MO), and factor VIII(Santa Cruz Biotechnology, CA). Secondary Abs conjugated to tetramethyl rhodamine isothiocyanate (TRITC; Santa Cruz Biotechnology) or fluorescein isothiocyanate (FITC; Santa Cruz Biotechnology) were used for fluorescence staining detection. The samples were photographed using an Olympus microscope and an Optronics digital camera (Olympus, Melville, NY). Selected slides were also analysed by confocal microscopy (Spectra Confocal Microscope TCSSP2‐AOBS, equipped with leica confocal software; Leica Microsystems Inc., Bannockburn, IL).
Detection of growth factors mRNA expressions by quantitative real‐time PCR
Total cellular RNA was extracted from wound tissue with TRI‐zol reagent (Invitrogen, Carlsbad, CA) and then purified using a Qiagen RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturers' protocol, and nucleic acid concentrations were measured with a spectrophotometer (NanoDrop ND‐1000; Saveen & Werner, Kristiansand, Norway). Total RNA was reversely transcribed using SuperScript First‐Strand Synthesis kit (RT‐PCR; Invitrogen). The primers used for real‐time PCR are shown in Table 1. Reactions were performed using SYBR‐Green PCR master mix (Applied Biosystems, Foster City, CA) in a BioRad iCycler iQ Detection System. Quantification was performed using the ABI Prism 7900 Fast Sequence Detection System (Applied Biosystems) according to the comparative CT method.
Table 1.
Primers used for real‐time PCR
| Gene | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) |
|---|---|---|
| β‐actin | CCTCTATGCCAACACAGTGC | GTACTCCTGCTTGCTGATCC |
| bFGF | CGGCATCACCTCGCTTCC | CCGTGTGGGTCGCTCTTCTC |
| EGF | GGGGGAATATCGGTGCTGACTCTG | TGTGCCGTGCTTGATGCCTGATAA |
| VEGF | ATCCGCAGACGTGTAAATGTTCCT | TCACCGCCTTGGCTTGTCAC |
PCR, polymerase chain reaction.
Statistical analysis
All values are presented as mean ± SD. Statistical analysis was performed using the two‐tailed Student's t‐test or by analysis of variance (ANOVA). Significance was taken as P < 0·05. All statistical analyses were performed using SPSS 10.0 software (SPSS Inc., Chicago, IL).
Results
Generation of EGFP chimeric mouse
Only 11·5% of the EGFP chimeric mice died within the first 2 weeks of radiation. All mice surviving over 2 weeks had normal leukocyte counts 6 weeks after radiation (data not shown), with 87·2–99·1% of the circulating GFP+ cells by flow cytometry (mean: 94·1% ±2·8; Figure 2). None of the animals showed signs of graft‐versus‐host disease, immunologic signs of rejection or wound infection.
Figure 2.
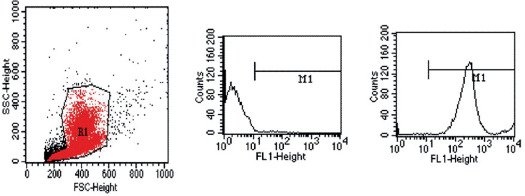
Flow cytometry histogram of circulating cells from a control mouse (middle) and an enhanced green fluorescent protein (EGFP) bone marrow‐transplanted mouse (right). Note the shift in emission spectra in the fluorescein isothiocyanate (FITC) channel, with 94·1% ±2·8 of the circulating cells fluorescing green.
Acceleration of wound healing by mobilised BMDCs
The mean time of wound healing in the G‐CSF‐treated mice was 12·5 ± 0·9 days while in control mice was 18·3 ± 0·8 days (P < 0·01, n = 6 wounds per group). As shown in Figure 3, wound closure was significantly faster in the G‐CSF‐treated mice than in control mice, supporting that mobilised BMDCs accelerated wound closure.
Figure 3.
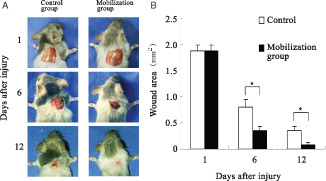
Effects of granulocyte colony stimulating factor (G‐CSF) mobilisation on wound closure. Wound measurement of control group (n = 6) and bone marrow mobilised group (n = 6) in mice. Wounds were excised on the dorsum of mice and treated with G‐CSF as described. Wounds were traced once a day and the area was determined after computer digitisation of the wounds. Each point represents the mean of six wounds. *P < 0·05.
In vivo cellular tracking by spectral fluorescence imaging
Images corresponding to GFP signals were used to quantify fluorescence signals. Measurements were taken from the computed images using a circular region of interested (ROI) placed over each GFP area. GFP signal intensity was quantified to indicate approximately the GFP+ cells per wound. To understand the cell migration to the wound after mobilisation, BMDCs expressing GFP were imaged repetitively over a 3‐week period in the same animals (n = 6). Control mice show lower GFP signal intensity (n = 6) compared with mobilised group during the whole wound‐healing process (Figure 4).
Figure 4.
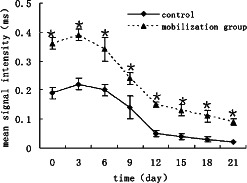
In vivo cellular tracking by spectral fluorescence imaging. Control mice show lower green fluorescent protein (GFP) signal intensity (n = 6) compared with mobilised group during the whole wound‐healing process. *P < 0·05.
Mobilised BMDCs contribution to wound healing
GFP+ cells were colocalised with cytokeratin 10 (CK 10), factor VIII (F‐VIII) and α‐SMA (Figure 5). Immunohistochemical analysis for GFP+ cells within the wound could also coexpress α‐SMA (on the 5th day after wounding), which indicates that BMDCs could differentiate into fibroblasts (Figure 5, left column). We also found incorporation of GFP+ cells in the walls of blood vessels (on the 5th day after wounding). These cells were typically found lining the luminal side of the vessels, and stained positive for factor VIII (Figure 5, middle column), thereby indicating that they undergo endothelial differentiation. Staining of the skin samples for GFP+ cells coexpressed CK 10 (1 week after wounding) as well (Figure 5, right column). Most GFP+ cells seen in the epidermis were closely associated with hair follicles, primarily in the region of the inner and outer root sheath and the dermal papillae and occasionally near the bulge region of the hair follicle. This suggests that the BMDCs could transdifferentiate into keratinocytes.
Figure 5.
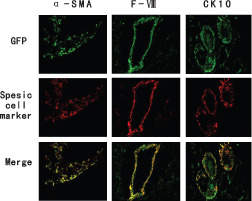
Immunohistochemical examination of bone marrow‐derived cells (BMDCs) integration and differentiation in the wound site after granulocyte colony stimulating factor (G‐CSF) treatment. Green fluorescent protein (GFP) positive cells (green) were colocalised with α‐smooth muscle actin (α‐SMA) (on the 5th day after wounding), factor VIII (on the 5th day after wounding) and CK 10 (1 week after wounding) (red).
Contribution of mobilised BMDCs to growth factor mRNA expression in wounds
We compared the mRNA expression of various growth factors in wound tissue of G‐CSF mobilisation group and control group by means of quantitative real‐time PCR. Figure 6 compares the mRNA expression levels of these growth factors in wound tissue during the wound‐healing period. In the control group, mRNA expression of epidermal growth factor (EGF)/basic fibroblast growth factor (bFGF) increased, reached the peak on the 3rd day after wound injury, and then declined to the normal level on the 6th day. In contrast, in the G‐CSF mobilisation group EGF/bFGF mRNA expression was enhanced throughout the first 6 days before dropping to the normal level. There was no statistical difference of the peak values between two groups. However, the time for high expression of EGF and bFGF mRNA was extended in mobilisation group compared with control group (Figure 6A,B).
Figure 6.
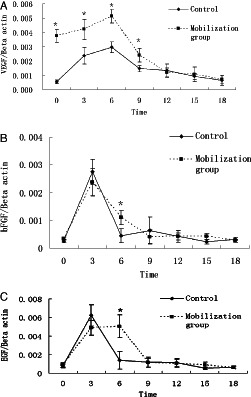
Effect of granulocyte colony stimulating factor (G‐CSF) therapy on growth factor expression in wounds. Experimental animals were divided and managed as described. On days 0, 3, 6, 9, 12, 15 and 18 after injury, tissue of wounds were excised, and changes of VEGF, bFGF and EGF were detected by real‐time quantitive polymerase chain reaction (RT‐PCR).*P < 0·05.
In the control group, mRNA expression of vascular endothelial growth factor (VEGF) increased and reached the peak on the 6th day after skin injury, and then decreased to the normal range. On 0, 3, 6, 9 days after G‐CSF mobilisation the mRNA expression of VEGF was significantly higher than the corresponding values in the control group (Figure 6C). These results indicate that G‐CSF mobilisation could up‐regulate VEGF mRNA expression and extend high expression time of EGF and bFGF mRNA in skin wounds.
Discussion
Wound healing is a complex process, initiated by interactions between many cell types, soluble factors and matrix components 3, 4, which involves multiple local and systemic signalling medicators, such as growth factors, cytokines, chemokines and proteolytic enzymes (5). The sum of these interrelated events determines the process of wound healing. Stem cells in bone marrow include haemopoietic stem cells, MSCs and multipotent adult progenitor cells (6). MSC therapy has emerged as a highly promising approach of regenerative cell therapy 7, 8, 9, 10, 11, 12. The stem cells can enter a transdifferentiation pathway to be programmed into specific cells required for regenerating damaged tissues. However, some results suggest that BMDCs do not significantly contribute to steady‐state epidermal homeostasis and are not required or responsible for providing keratinocyte stem cells and keratinocyte repopulation following skin injury (2). Fan and coworkers (2) found that in murine models keratinocytes derived from donor BMDCs re‐presented only one to three cells per 106 total epidermal cells obtained from healed wounded skin, a frequency of 0·0001% to 0·0003%. These data indicate that BMDCs have a minimal contribution to the repair process after skin injury. A reasonable explanation of this result may be that BMDCs in peripheral circulation is extremely limited, resulting in rare incorporation of BMDCs to the wounds. We therefore seek to find a new approach to increase BMDCs ‘releasing’ from bone marrow to the peripheral circulation by which to amplify the effect of BMDCs in wound healing.
G‐CSF is a cytokine synthesised by a variety of cell types, and is known to be involved in the proliferation and differentiation of granulocytes and their precursors, as well as in mediating haematopoietic stem cell and MSC mobilisation to peripheral blood 13, 14. Recent studies have indicated that G‐CSF may be effective in mobilising cells that contribute to organ repair 15, 16, 17. However, little is known with respect to skin regeneration after G‐CSF mobilisation. On basis of the observation that extra‐organ stem cells play a minor role in wound repair in the absence of cell‐mobilising factors such as G‐CSF, we hypothesise that BMDC mobilisation could be effective in accelerating wound healing after skin injury.
Green fluorescent protein (GFP) has been used for cell tracking and imaging gene expression in superficial or surgically exposed structures. In order to further trace the BMDCs, we used a GFP chimeric mouse model. For imaging the immunofluorescence, we used a stringent system of optical filters to exclude the possibility of autofluorescence and other conditions of false positive green fluorescence. GFP signal intensity was quantified to indicate the approximate number of GFP+ cells in the wound. Our results indicate that, after G‐CSF mobilisation there was substantial GFP+ cell infiltration in wounds during the acute early phase, which was detected by spectral fluorescence imaging. As the inflammation subsides, there was a discrete population of cells expressing GFP that persists up to 7 days after the wound was healed. However, mice in control group showed lower GFP signal intensity during the acute early phase and the GFP signal could hardly be detected after wound healing. From these data, it is clear that after bone marrow mobilisation there were a larger proportion of GFP+ cells originating from the bone marrow in the healing wound in comparison with the control mice. After G‐CSF mobilisation, the capability of BMDCs was enhanced to participate phenotypically in the restoration of BM‐derived myofibroblasts, hair follicles, dermal blood vessels and epidermal phenotype post‐injury, which were shown by morphology and immunoreactivity for CK 10, factor VIII and α‐SMA. Most GFP+ cells seen in the epidermis were closely associated with hair follicles, primarily in the region of the inner and outer root sheath and the dermal papillae and occasionally near the bulge region of the hair follicle. This finding confirms that after bone marrow mobilisation BMDCs are recruited non‐specifically via the inflammatory response and neovascularisation 18, 19, 20. In the wound environment, recruited BMDCs can differentiate into epidermal stem cells or transit‐amplifying cells near the region of hair follicles.
Growth factors play a crucial role in wound healing 21, 22, 23. Wound healing could be enhanced by a number of tissue repair growth factors secreted by both tissue‐borne cells and those recruited to the wound immediately after tissue injury. Therapeutic efficacy of systemical G‐CSF in wound healing might be related to increased growth factor production on wound site. Indeed, our RT‐PCR analysis of growth factor mRNA expression in G‐CSF‐treated group showed constitutive expression of VEGF, bFGF and EGF. VEGF is released by a variety of cells and stimulates multiple components of the angiogenic cascade. Experimental data supports the hypothesis that VEGF stimulates epithelialisation and collagen deposition in a wound (21). Fu et al.(24) investigated the effect of bFGF on burn wound in a randomised placebo‐controlled trial and showed that bFGF effectively decreased healing time and improved healing quality. Uchi et al.(25) evaluated the clinical efficacy of bFGF for diabetic ulcer and showed accelerated wound healing effects of bFGF.
The role of EGF has been extensively investigated in normal and pathological wound healing. It is implicated in keratinocyte migration, fibroblast function and the formation of granulation tissue (26). This investigation suggests that upregulation of growth factor expression in wound tissue following G‐CSF therapy plays a key role in promoting cellular processes such as chemotaxis, cell proliferation, cell signalling and angiogenesis that are critical to proper wound healing. The data obtained in this study indicate that the healing effect on full‐thickness wound resulting from G‐CSF treatment is associated with increased expression at the wound site of VEGF, bFGF and EGF by various cell populations, including macrophages, haemopoietic stem cells and MSCs.
A regenerative medicine approach for tissue repair focused on the direct manipulation of endogenous adult stem cells, avoiding the immunocompatibility issues and complicated in vitro procedures, is very appealing. The approach appears reliable and safe and is very promising in terms of effectively stimulating the repair process in injured tissue. We have shown that G‐CSF exerts multiple beneficial effects on skin repair, both by increasing the engraftment of BMDCs into the skin to differentiate into multiple skin cell types, and upregulating essential cytokines critical to wound repair. The potential trophic effects of G‐CSF on bone marrow stem cells to accelerate wound healing could have a dramatic clinical impact. Also this study does not discriminate the ultimate origin of the BMDCs which are participating in skin regeneration after mobilisation. We believe that the G‐CSF treatment is an appealing and functional potential therapy for improving wound healing because it shortens the course of wound healing and offers both ease of treatment and versatility. Further work on understanding the mechanism of G‐CSF mobilisation in accelerating wound healing and the long‐term outcomes of healed wounds will be critical for introduction of these findings into clinical practice.
Conclusion
Bone marrow mobilisation by G‐CSF exerted multiple beneficial effects on skin repair, both by increasing the engraftment of BMDCs into the skin to differentiate into multiple skin cell types and by upregulating essential cytokine mRNAs critical to wound repair. The potential trophic effects of G‐CSF on bone marrow stem cells to accelerate wound healing could have a significant clinical impact.
Acknowledgements
This work was supported by the National Nature Science Foundation of China Emphasis Grant No. 30730091; the National Scientific and Technological Support Projects Grant No. 2009BAI87B03 and the Science and Technology Commission of Shanghai Municipality Emphasis Grant No. 0841 1952800. The authors have no conflicting financial interests.
Wang and Sun contributed equally to this work.
References
- 1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 2. Fan Q, Yee CL, Ohyama M, Tock C, Zhang G, Darling TN, Vogel JC. Bone marrow‐derived keratinocytes are not detected in normal skin and only rarely detected in wounded skin in two different murine models. Exp Hematol 2006;34:672–79. [DOI] [PubMed] [Google Scholar]
- 3. Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol 2009;18:921–33. [DOI] [PubMed] [Google Scholar]
- 4. Gibran NS, Boyce S, Greenhalgh DG. Cutaneous wound healing. J Burn Care Res 2007;28:577–79. [DOI] [PubMed] [Google Scholar]
- 5. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 6. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz‐Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–9. [DOI] [PubMed] [Google Scholar]
- 7. Wollert KC. Cell therapy for acute myocardial infarction. Curr Opin Pharmacol 2008;8:202–10. [DOI] [PubMed] [Google Scholar]
- 8. Cuenca‐Lopez MD, Zamora‐Navas P, Garcia‐Herrera JM, Godino M, Lopez‐Puertas JM, Guerado E, Becerra J, Andrades JA. Adult stem cells applied to tissue engineering and regenerative medicine. Cell Mol Biol (Noisy-le-grand) 2008;54:40–51. [PubMed] [Google Scholar]
- 9. Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 2008;26:2201–10. [DOI] [PubMed] [Google Scholar]
- 10. Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology 2008;135: 438–50. [DOI] [PubMed] [Google Scholar]
- 11. van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell‐derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008;47:1634–1643. [DOI] [PubMed] [Google Scholar]
- 12. Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, Mareschi K, Testa L, Stecco A, Tarletti R, Miglioretti M, Fava E, Nasuelli N, Cisari C, Massara M, Vercelli R, Oggioni GD, Carriero A, Cantello R, Monaco F, Fagioli F. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol 2009. [DOI] [PubMed] [Google Scholar]
- 13. Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone‐marrow‐derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430–36. [DOI] [PubMed] [Google Scholar]
- 14. Szumilas P, Barcew K, Baskiewicz‐Masiuk M, Wiszniewska B, Ratajczak MZ, Machalinski B. Effect of stem cell mobilization with cyclophosphamide plus granulocyte colony‐stimulating factor on morphology of haematopoietic organs in mice. Cell Prolif 2005;38: 47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piscaglia AC, Shupe T, Gasbarrini A, Petersen BE. Microarray RNA/ DNA in different stem cell lines. Curr Pharm Biotechnol 2007;8: 167–75. [DOI] [PubMed] [Google Scholar]
- 16. Orlic D. BM stem cells and cardiac repair: where do we stand in 2004? Cytotherapy 2005;7:3–15. [DOI] [PubMed] [Google Scholar]
- 17. Schabitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, Sommer C, Schwab S. Neuroprotective effect of granulocyte colony‐stimulating factor after focal cerebral ischemia. Stroke 2003;34:745–51. [DOI] [PubMed] [Google Scholar]
- 18. Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, Ohsaka A. Granulocyte colony‐stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J 2005; 19:2005–7. [DOI] [PubMed] [Google Scholar]
- 19. Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg 2005;55:414–9. [DOI] [PubMed] [Google Scholar]
- 20. Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, Hoefer IE, Mueller‐Hoecker J, Franz WM. G‐CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J 2006;20:956–8. [DOI] [PubMed] [Google Scholar]
- 21. Bao P, Kodra A, Tomic‐Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dogan S, Demirer S, Kepenekci I, Erkek B, Kiziltay A, Hasirci N, Muftuoglu S, Nazikoglu A, Renda N, Dincer UD, Elhan A, Kuterdem E. Epidermal growth factor‐containing wound closure enhances wound healing in non‐diabetic and diabetic rats. Int Wound J 2009;6:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu C, Ding Y, Chen J, Liu D, Zhang Y, Ding M, Wang G. Basic fibroblast growth factor stimulates epithelial cell growth and epithelial wound healing in canine corneas. Vet Ophthalmol 2009;12:170–5. [DOI] [PubMed] [Google Scholar]
- 24. Fu X, Shen Z, Chen Y, Xie J, Guo Z, Zhang M, Sheng Z. Randomised placebo‐controlled trial of use of topical recombinant bovine basic fibroblast growth factor for second‐degree burns. Lancet 1998;352:1661–4. [DOI] [PubMed] [Google Scholar]
- 25. Uchi H, Igarashi A, Urabe K, Koga T, Nakayama J, Kawamori R, Tamaki K, Hirakata H, Ohura T, Furue M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol 2009;19:461–8. [DOI] [PubMed] [Google Scholar]
- 26. Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing–past, present and future perspectives. Surgeon 2008;6:172–7. [DOI] [PubMed] [Google Scholar]


