Abstract
BACKGROUND
Uromodulin modulates the sodium-potassium-two-chloride transporter in the thick ascending limb of the loop of Henle, and its overexpression in murine models leads to salt-induced hypertension. We hypothesized that individuals with higher baseline levels of urine uromodulin would have a greater increase in systolic blood pressure (SBP) for the same increase in sodium compared with those with lower uromodulin levels.
METHODS
We used data from 157 subjects randomized to the control diet of the Dietary Approaches to Stop Hypertension (DASH)-Sodium trial who were assigned to 30 days of low (1,500 mg/d), medium (2,400 mg/d), and high salt (3,300 mg/d) diets in random order. Blood pressure was measured prerandomization and then weekly during each feeding period. We evaluated the association of prerandomization urine uromodulin with change in SBP between diets, as measured at the end of each feeding period, using multivariable linear regression.
RESULTS
Baseline urine uromodulin stratified by tertiles was ≤17.64, 17.65–31.97, and ≥31.98 µg/ml. Across the tertiles, there were no significant differences in SBP at baseline, nor was there a differential effect of sodium diet on SBP across tertiles (low to high, P = 0.81). After adjusting for age, sex, body mass index, and race, uromodulin levels were not significantly associated with SBP change from low to high sodium diet (P = 0.42).
CONCLUSIONS
In a randomized trial of different levels of salt intake, higher urine uromodulin levels were not associated with a greater increase in blood pressure in response to high salt intake.
Keywords: blood pressure, DASH, hypertension, salt sensitivity, sodium, uromodulin
Uromodulin, the most abundant protein in the urine of healthy adults, has been postulated to have a number of important functional roles, including renal modulation of sodium absorption and blood pressure regulation. Overexpression of uromodulin in transgenic mice leads to salt-sensitive hypertension and a robust decrease in blood pressure in response to furosemide therapy.1 In contrast, UMOD-knockout mice have lower blood pressure and are resistant to salt-induced hypertension.2 Uromodulin modulates phosphorylation of the sodium-potassium-two-chloride cotransporter through a tumor necrosis factor signaling pathway, thereby increasing its functional activity and consequently, enhancing salt reabsorption.3 In humans, genome-wide association studies have found susceptibility variants for hypertension in the UMOD gene.4
Prior literature has evaluated genetic alterations in UMOD and its downstream effects on sodium transporters, but whether or not urine uromodulin concentrations are themselves associated with salt-sensitive hypertension is unknown. Clinically, it is difficult to know the degree to which an individual patient is salt sensitive, and therefore, a urine biomarker that could provide this information would be of great utility. We hypothesized that individuals with higher urine concentrations of uromodulin would have greater increases in systolic blood pressure (SBP) in response to a high dietary sodium intake, compared with those with lower uromodulin concentrations. We tested this hypothesis using stored specimens collected as part of the Dietary Approaches to Stop Hypertension (DASH)-Sodium trial.
METHODS
The DASH-Sodium trial was a randomized trial designed to evaluate the effect of 2 dietary patterns and 3 dietary sodium levels on blood pressure among adults with higher than optimal baseline blood pressure.5 A total of 412 participants were randomly assigned to either the DASH diet or an isocaloric control diet. In random order, participants were fed diets with 3 sodium levels: high (3,300 mg/d = 150 mmol), intermediate (2,400 mg/d = 100 mmol), and low (1,500 mg/d = 50 mmol) for 30 consecutive days each (Supplementary Figure S1 online).6 There was an interval of about 5 days between each sodium diet intervention arm. Key exclusion criteria included use of antihypertensive medications and an estimated glomerular filtration rate <60 ml/min/1.73 m2. Uromodulin was measured, using the MesoScale Diagnostics R-plex assay (Item S1 online), in 24-hour urine samples collected at the final screening visit in participants in the control diet arm with available urine samples (n = 157). Blood pressure was measured in triplicate at each visit, and all 3 measurements were averaged for each day. Measurements were made at the baseline of each study arm, weekly during the first 3 weeks of each sodium intervention, and again at 5 clinic visits during the last 9 days of the intervention (Supplementary Figure S1 online).5 As per the study protocol, the average of the last 5 days was used to calculate mean end-of-intervention SBP. Our primary outcome was the difference in end-of-intervention SBP between the low and high sodium diets. As secondary outcomes, we evaluated SBP differences within each dietary sodium phase, from baseline to the end-of-intervention, as well as from week 1 to week 4 of each intervention.
RESULTS
The median 24-hour urine uromodulin concentration at baseline was 23.8 µg/ml (interquartile range 15.0–40.9 µg/ml). Across uromodulin tertiles, there were no significant differences in participant age, sex, race, body mass index, systolic or diastolic blood pressure, and prior use of blood pressure medication (Supplementary Table S1 online).
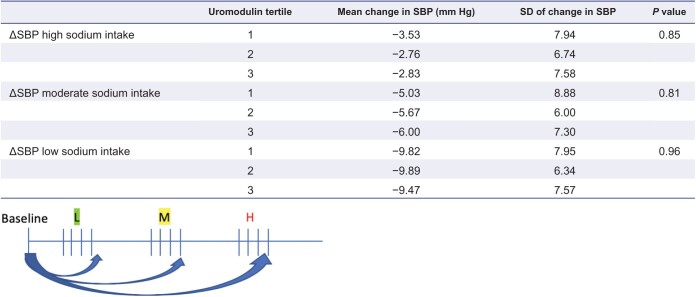
In an analysis of end-of-intervention SBP, no significant interaction was found between dietary sodium level and urine uromodulin concentration (P = 0.86). Additionally, there was no significant difference in the end-of-intervention SBP by baseline urine uromodulin concentrations (P = 0.81). Results were similar after adjusting for age, sex, body mass index, and race (P = 0.42, Supplementary Table S2 online). Change in SBP from week 1 to week 4 of each intervention was stratified by uromodulin tertile and no significant difference was found across the groups (Supplementary Figure S2 online). SBP decreased within each intervention arm (by mean of −3.04 mm Hg in the high sodium diet, −5.67 mm Hg in the intermediate sodium diet, and −9.73 mm Hg in the low sodium diet). Again, there was no significant difference in within intervention SBP changes based on baseline uromodulin concentrations (Table 1). Results were similar when urine uromodulin was indexed to urine creatinine and when total 24-hour urine uromodulin amount (µg) was used (data not shown).
Table 1.
Change in systolic blood pressure (SBP) within randomized groups from baseline to end-of-intervention, stratified by uromodulin tertiles
DISCUSSION
Our results demonstrate that within the DASH-Sodium trial, uromodulin is not associated with change in SBP in response to an increase in sodium intake. The lack of an association of uromodulin protein concentrations with blood pressure responses observed here has multiple possible explanations. First, recent data on post-translational modifications of uromodulin show that a serine protease, hepsin, affects the release of uromodulin and its polymerization within the urine.7,8 Thus, despite normal UMOD expression and normal concentrations of uromodulin in the urine, misprocessing of the protein may limit functional activity. Second, while our study benefitted from a randomized trial design testing different dietary sodium intake levels, the study sample was relatively modest in size, and blood pressure was measured over only 4 weeks. A larger study and/or longer-term follow-up may have led to different results.
Our study has a number of limitations. We do not have data on UMOD genotype which precludes analysis of genetic alterations and differential blood pressure responses to dietary sodium intake. Additionally, uromodulin was measured at baseline during the screening period, when dietary sodium intake was variable. Differences in sodium intake may influence urine concentrations of uromodulin.9 Also, because there was no designated washout period between sodium interventions, there may be carryover effects on blood pressure from one diet to the next, particularly during week 1. This may have biased the results of our secondary outcome. Finally, the sample size was relatively small, since we restricted the study to persons in the control arm to avoid the effect of other nonsodium aspects of the DASH diet on blood pressure. The strength of this study is the rigorous crossover design of the DASH-Sodium trial, where 24-hour urine collections were obtained at the end of each intervention to monitor dietary compliance.5
Within the DASH-Sodium trial, the effect of different dietary sodium intake levels on SBP was similar irrespective of baseline urine uromodulin concentrations. Although this analysis does not support the measurement of urine uromodulin as a biomarker of salt sensitivity in humans, studies specifically designed to address this question are needed to confirm this finding.
Supplementary Material
FUNDING
The authors would like to acknowledge the following funding sources: Veterans Medical Research Foundation—Research Development Pilot Award 2018 (P.G.), NIDDK 1K23DK114556-01A1 (P.G.), and NIDDK 5T32DK104717-04 (C.B.).
DISCLOSURE
Dr Garimella has received clinical trial support from Kadmon Inc. and speaker fees from Otsuka. Dr Ix is Principal Investigator of an Investigator Initiated Research Project from Baxter International.
REFERENCES
- 1. Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, Citterio L, Demaretz S, Trevisani F, Ristagno G, Glaudemans B, Laghmani K, Dell’Antonio G, Loffing J, Rastaldi MP, Manunta P, Devuyst O, Rampoldi L; SKIPOGH team . Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med 2013; 19:1655–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Graham LA, Padmanabhan S, Fraser NJ, Kumar S, Bates JM, Raffi HS, Welsh P, Beattie W, Hao S, Leh S, Hultstrom M, Ferreri NR, Dominiczak AF, Graham D, McBride MW. Validation of uromodulin as a candidate gene for human essential hypertension. Hypertension 2014; 63:551–558. [DOI] [PubMed] [Google Scholar]
- 3. Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, Raffi H, Rampoldi L, Uchida S, Hille C, Dosche C, Kumar S, Castañeda-Bueno M, Gamba G, Bachmann S. Activation of the bumetanide-sensitive Na+,K+,2Cl− cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem 2011; 286:30200–30210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Padmanabhan S, Melander O, Johnson T, Di Blasio AM, Lee WK, Gentilini D, Hastie CE, Menni C, Monti MC, Delles C, Laing S, Corso B, Navis G, Kwakernaak AJ, van der Harst P, Bochud M, Maillard M, Burnier M, Hedner T, Kjeldsen S, Wahlstrand B, Sjögren M, Fava C, Montagnana M, Danese E, Torffvit O, Hedblad B, Snieder H, Connell JM, Brown M, Samani NJ, Farrall M, Cesana G, Mancia G, Signorini S, Grassi G, Eyheramendy S, Wichmann HE, Laan M, Strachan DP, Sever P, Shields DC, Stanton A, Vollenweider P, Teumer A, Völzke H, Rettig R, Newton-Cheh C, Arora P, Zhang F, Soranzo N, Spector TD, Lucas G, Kathiresan S, Siscovick DS, Luan J, Loos RJ, Wareham NJ, Penninx BW, Nolte IM, McBride M, Miller WH, Nicklin SA, Baker AH, Graham D, McDonald RA, Pell JP, Sattar N, Welsh P, Munroe P, Caulfield MJ, Zanchetti A, Dominiczak AF; Global BPgen Consortium . Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet 2010; 6:e1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Svetkey LP, Sacks FM, Obarzanek E, Vollmer WM, Appel LJ, Lin P-H, Karanja NM, Harsha DW, Bray GA, Aickin M, Proschan MA, Windhauser MM, Swain JF, McCarron PB, Rhodes DG, Laws RL. The DASH diet, sodium intake and blood pressure trial (DASH-Sodium). J Am Diet Assoc 1999; 99:S96–S104. [DOI] [PubMed] [Google Scholar]
- 6. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, Karanja N, Lin P-H, Aickin M, Most-Windhauser MM, Moore TJ, Proschan MA, Cutler JA. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344:3–10. [DOI] [PubMed] [Google Scholar]
- 7. Olinger E, Lake J, Sheehan S, Schiano G, Takata T, Tokonami N, Debaix H, Consolato F, Rampoldi L, Korstanje R, Devuyst O. Hepsin-mediated processing of uromodulin is crucial for salt-sensitivity and thick ascending limb homeostasis. Sci Rep 2019; 9:12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brunati M, Perucca S, Han L, Cattaneo A, Consolato F, Andolfo A, Schaeffer C, Olinger E, Peng J, Santambrogio S, Perrier R, Li S, Bokhove M, Bachi A, Hummler E, Devuyst O, Wu Q, Jovine L, Rampoldi L. The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. Elife 2015; 4:e08887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torffvit O, Melander O, Hultén UL. Urinary excretion rate of Tamm-Horsfall protein is related to salt intake in humans. Nephron Physiol 2004; 97:31–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.