Abstract
Appropriate food intake requires exquisite coordination between the gut and the brain. Indeed, it has long been known that gastrointestinal signals communicate with the brain to promote or inhibit feeding behavior. Recent advances in the ability to monitor and manipulate neural activity in awake, behaving rodents has facilitated important discoveries about how gut signaling influences neural activity and feeding behavior. This review emphasizes recent studies that have advanced our knowledge of gut–brain signaling and food intake control, with a focus on how gut signaling influences in vivo neural activity in animal models. Moving forward, dissecting the complex pathways and circuits that transmit nutritive signals from the gut to the brain will reveal fundamental principles of energy balance, ultimately enabling new treatment strategies for diseases rooted in body weight control.
Keywords: gut–brain, vagus nerve, hypothalamus, hindbrain, dopamine, calcium imaging
The gut is commonly described as a second brain. The connection between the gut and the brain is invoked to describe deep, often unconscious feelings, as in a “gut instinct” or “going with your gut.” This metaphorical link is rooted in physiology—hundreds of millions of neurons connect the gut and the brain to regulate a plethora of bodily functions, including food intake and energy homeostasis.
Research on gut–brain communication emerged in the 1700s, when physicians began to document the importance of connectivity between organs, and in particular the gut and the brain (1). Since then, a tremendous amount has been discovered regarding the anatomy, physiology, and behavior of gut–brain signaling. Only recently, however, have advances in techniques to monitor in vivo neural activity dynamics provided a window into the living brain. Widespread adoption of new tools for in vivo electrophysiology, calcium imaging, and neurotransmitter sensing by the neuroscience community has revolutionized our ability to ask questions about how gut signaling affects the brain in real time.
The complex gut–brain mechanisms that underlie food intake—including satiation pathways, vagal and hormonal gut–brain signaling, and the development of food preferences—in both animal models and humans have been elegantly reviewed elsewhere (2-8). Here, we focus on the recent use of in vivo neural activity monitoring techniques in animal models to enhance our understanding of gut signaling effects on the brain. First, we provide an overview of techniques used to monitor neural activity. In particular, calcium monitoring and neurotransmitter (eg, dopamine) sensing have been most widely used to advance our knowledge of in vivo gut–brain signaling. Next, we will discuss studies that examine how gut signals (neural and endocrine) rapidly modulate neural activity in feeding-relevant neuron populations. Effects of gastrointestinal nutrient and hormonal signaling on calcium or neurotransmitter activity are described. Finally, we review recent literature on the effects of weight gain on in vivo neural activity, which demonstrate how these techniques can be leveraged to understand the etiology of obesity.
Techniques for Monitoring Neural Activity in Awake, Behaving Animals
This section provides an overview of the techniques that are commonly used to record neural activity in living rodents, as well as a brief summary of some advantages and disadvantages of each technique. For more thorough discussions on these experimental approaches, see refs: (9-13). Although not reviewed here, it is also worth noting that the effects of gut–brain signaling on neural activity in humans have been examined with functional magnetic resonance imaging or positron emission tomography (5, 14-19)
Monitoring Neural Activity of Cell Types
The most common techniques to monitor neural activity of genetically defined cells along the gut–brain axis involve measuring dynamics of genetically encoded calcium indicators. Calcium indicators change conformation and emit brighter fluorescence when bound to calcium that floods a cell when action potentials are generated, and therefore serve as a proxy for neural activity. While calcium imaging has been used for over 30 years to study neural activity (20, 21), more recent developments in transgenic experimental models and viral mediated gene transfer, as well as improvements in calcium indicators themselves, have facilitated the use of these techniques in awake, behaving mice. The most commonly used calcium indicator for in vivo monitoring, GCaMP, is available in many variants that differ in sensitivity and temporal resolution. The most recent variants, GCaMP6 (22) and GCaMP7 (23), are well suited for in vivo calcium monitoring of many different cell types. These calcium indicators can be expressed in neurons of interest through commercially available viral vectors (www.addgene.org), or genetically with transgenic mouse lines (24-28). Importantly, incorporation of fiber optic implants, gradient index (GRIN) lenses, or microprisms has enabled imaging of deep and/or difficult to access brain regions, like many of those involved in feeding behavior (29-32).
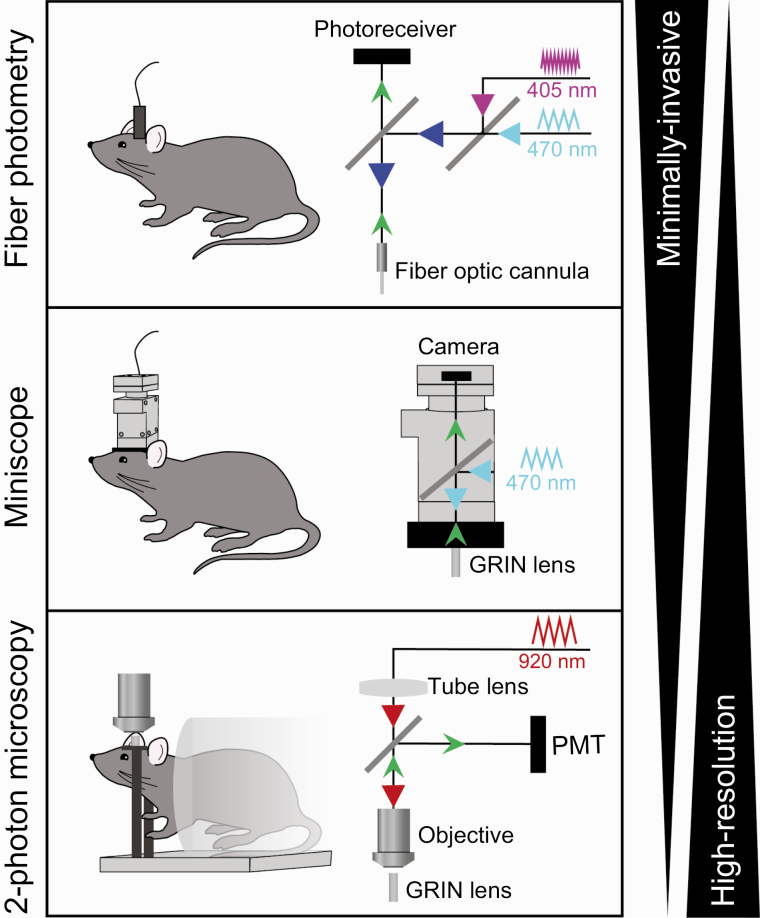
What is the optimal calcium imaging technique for monitoring GCaMP dynamics? The answer to this question depends on the cell population of interest as well as experimental goals, and the most common techniques are summarized in Fig. 1. Fiber photometry is commonly used to measure overall calcium activity in populations of neurons. This technique is relatively noninvasive, as it involves the implant of a small optic fiber and neural activity can be recorded while animals are freely moving. Because fiber photometry integrates activity of many neurons, it is best used to examine activity in populations that are relatively homogeneous in their responses to stimuli of interest. In contrast, calcium imaging using miniscopes (small, head-mounted microscopes/cameras) or 1- or 2-photon microscopy is advisable for neuron populations that are heterogeneous, or in experiments where measuring activity dynamics of individual neurons is necessary. Miniscope technology is ideal for calcium imaging during behavior, as relatively lightweight, head-mounted microscopes and cameras allow animals to freely move and behave. For greater spatial resolution, contrast, and the ability to image difficult to access areas such as lateral cortical regions (30) or peripheral vagal afferent neurons (33), 1- and 2-photon microscopy are optimal techniques. Additionally, microscopy offers the ability to image in multiple colors (34, 35), providing the opportunity to measure calcium dynamics in 2 cell populations. This technique could be applied to monitor multiple intermingled neuron populations in the same brain region (eg, hypothalamus) in the same subject. 2-photon imaging generally requires anesthesia or head fixation, and therefore is more difficult to adapt to experimental designs involving complex behavioral tasks. That being said, miniature head-mounted 2-photon microscopy enables high-resolution imaging in freely moving mice (36).
Figure 1.
Optical techniques for in vivo neural activity monitoring. Schematic diagrams of technical setups for fiber photometry (top), miniscope imaging (middle), and 2-photon microscopy (bottom). These techniques each have advantages and disadvantages with regard to invasiveness and resolution.
Overall, calcium monitoring has been widely adopted by the neuroscience community including several groups examining gut–brain signaling. Significant advantages include the ability to easily express calcium indicators in genetically defined cell types (using Cre-recombinase–expressing experimental animals) and to record from the same neurons across time, often for weeks or months. The latter is especially important to examine, for example, the neural association of sensory and nutritive information over time (37), or the long-term effects of diet on in vivo neural activity (38-40). Limitations of calcium imaging include the relatively slow temporal dynamics of GCaMP relative to other techniques (see below), as well as the inability to directly correlate in vivo increases in calcium with action potentials. To address this, studies have demonstrated that increases in GCaMP fluorescence correlate with action potential firing in individual genetically defined hypothalamic neurons (41, 42). The relationship between GCaMP signaling and in vivo action potential firing in other feeding-related neuron populations remains unknown.
In vivo electrophysiology, on the other hand, involves direct electrode recordings of action potentials at microsecond temporal resolution. This technique has been used for decades to measure spiking of neurons across the brain. More recently, the addition of “optical tagging” enables genetic identification of recorded neurons (43). Optical tagging uses an optrode (a combined optic fiber with electrodes) to record activity in cells that are identified by their responsivity to light that is driven by expression of a light-sensitive excitatory protein, such as channelrhodopsin-2 (ChR2). This approach has been used to understand the spiking properties of hypothalamic feeding populations in response to nutrients (42, 44). A limitation of this method is that ChR2-expressing cells can rapidly activate neighboring cells when stimulated, and so stringent criteria regarding spike latency must be applied to make interpretations about cell type–specific neural activity (45). Further, it is difficult to track individual neurons over time, as even small movements of the electrodes compromise the ability to track the same neurons. Unlike calcium imaging, it is impossible to visually confirm the identity of individual neurons, although computational methods can be applied to determine whether the electrode is recording from the same or different cells (46, 47).
In principle, imaging of genetically encoded voltage indicators resolves many of the limitations of calcium imaging and in vivo electrophysiology. Voltage monitoring can directly measure action potentials at millisecond time resolution in populations of (48) or individual (49) genetically identified neurons. However, the signal to noise ratios of voltage indicators are generally inferior to calcium indicators (9), and therefore voltage imaging has not been widely adopted by the field. If voltage indicators follow the same path of optimization as calcium indicators, they will become an important tool for understanding how gut–brain signaling influences temporal spiking dynamics in feeding neurons.
Monitoring Neurotransmitter Dynamics
In addition to monitoring neural activity dynamics, neurotransmitter sensors are useful tools to investigate brain activity. In the field of gut–brain signaling, the most commonly studied neurotransmitter in vivo is dopamine. For decades, dopamine sampling (eg, microdialysis) and electrochemistry (eg, fast-scan cyclic voltammetry, FSCV) have been used to measure in vivo dopamine signaling as well as other neurotransmitter signals. Microdialysis involves sampling of molecules from interstitial space that allows quantification of dopamine as well as other transmitters, peptides, and hormones in awake, behaving animals (50). The nature of the microdialysis probe also allows for infusion of drugs through the probe for pharmacological experiments. However, the slow temporal dynamics (minutes, compared to seconds/milliseconds for other techniques) prevents analysis of phasic dopamine signaling, a critical feature of reward signaling. In contrast, FSCV is well suited for capturing these phasic changes in dopamine signaling with millisecond resolution (51). FSCV involves applying voltage to an electrode to induce the oxidation and reduction of a chemical (eg, dopamine) which is detected as a current. Application of multiple voltages allows for multiple chemicals to be detected simultaneously (52). Disadvantages of both microdialysis and FSCV include the inability to monitor long-term signaling, which is important for understanding how neurotransmitter-mediated changes in behavior develop over time.
Neurotransmitter sensors combined with optical techniques provide an alternative strategy to monitor in vivo neurochemistry. There are 2 major types of neurotransmitter sensors: those based on G-protein–coupled receptors (GPCRs) and those based on periplasmic-binding protein (PBP). Modified fluorescent GPCRs have been successfully developed to sense neuromodulators such as dopamine, norepinephrine, and serotonin (12). For dopamine sensing, GRABDA (53) and dLight1 (54) are both based on human dopamine receptors; GRABDA is a modified dopamine D2 receptor, and dLight1 sensors are based on D1, D3, or D4 receptors. Because these sensors monitor brain neurochemistry with sub-second temporal dynamics, they are effective at monitoring phasic dopamine signaling. Neurotransmitter sensors can be packaged in viral vectors and monitored with fiber photometry, miniscopes, or 2-photon microscopy, and therefore offer several benefits. First, viral packaging enables expression of dopamine sensors in either a constitutive or cell type–specific manner. Second, laboratories that are set up to monitor calcium dynamics require no additional equipment for dopamine monitoring. Third, dopamine signaling can be monitored for weeks or months without losing fluorescence signal. Further, these sensors continue to be optimized for increased brightness and sensitivity, and fused to different fluorophores to be able to combine dopamine sensing with, for example, GCaMP imaging (55).
PBP-based sensors have a fluorescent protein fused near a PBP that binds a ligand (neurotransmitter or small molecule). Examples of these sensors include those for the fast neurotransmitters glutamate (iGluSnFR) (56) and gamma-aminobutyric acid (GABA, iGABASnFR) (57) that have been used for both in vivo and in vitro imaging. Similar to dopamine sensors, PBP-based sensors have been optimized for speed (58), altered for color (59), and expanded to be able to monitor other small molecules including acetylcholine (60) and glucose (61, 62). Protein engineering efforts will enable the continual innovation of biosensors to monitor other neurotransmitters and neuropeptides—potentially revolutionizing the ability to monitor brain signaling across circuits and systems. Unlike dopamine sensors, few studies have utilized other neurotransmitter sensors to examine in vivo gut–brain signaling. Looking forward, these new sensors will provide an opportunity to understand how gut–brain signaling influences neurotransmitter signaling and behavior.
While the aforementioned optical approaches to neurotransmitter monitoring have enhanced the resolution of neural signaling in awake, behaving animals, there are technical caveats that must be considered. In contrast to microdialysis and FSCV, optical monitoring of neurotransmitters is a relative measure, and therefore cannot detect absolute levels of these chemicals in the brain. Further, all sensors (including calcium indicators) that introduce modified proteins into cells can potentially modify endogenous signaling or cause ectopic signaling events. For example, modified GPCRs could initiate signaling cascades within cells of interest, causing off-target effects. While great care is taken during tool development to minimize these effects (eg, as in (53, 54)), this should be taken into consideration when choosing a sensor/indicator and planning experiments.
Gut Signaling Effects on In Vivo Neural Activity
Having provided an overview of current techniques utilized to monitor in vivo neural activity and neurotransmitter signaling, we will now review how this technology has been used in key populations of feeding-relevant neurons to advance our understanding of gut–brain signaling (Fig. 2).
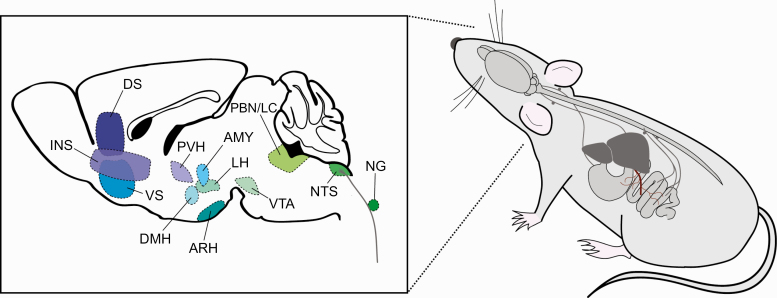
Figure 2.
Summary of brain regions and gut–brain pathways examined using in vivo activity monitoring techniques. Left, sagittal diagram of mouse brain depicting brain regions that have been monitored in response to feeding or gut signaling. Right, schematic depicting gut–brain pathways that influence in vivo neural activity, including vagal, spinal, and hepatic portal pathways. AMY, amygdala; ARH, arcuate hypothalamic nucleus; DS, dorsal striatum, DMH, dorsomedial hypothalamic nucleus; INS, insular cortex; LH, lateral hypothalamus; NG, nodose ganglion; NTS, nucleus tractus solitarius; PBN/LC, parabrachial nucleus/peri-locus coeruleus area; PVH, paraventricular hypothalamic nucleus; VS, ventral striatum; VTA, ventral tegmental area.
Vagal Signaling
The vagus nerve is a highway for sensory and motor signaling between visceral organs and the brain. In particular, the vagus nerve is well known for its role in gut–brain signaling as sensory afferents innervate the gastrointestinal tract. Cell bodies of afferent vagal neurons reside in the nodose ganglion, which is located outside of the skull in the jugular sheath. These neurons are bipolar, with the projection to the gut comprising of structurally distinct sensory terminals (63) and the projection to the hindbrain terminating in the nucleus of the solitary tract (NTS). Vagal afferent neurons respond to multiple qualities of food: these neurons are activated by gut stretch, nutrients, and gut-released satiation hormones (64).
Calcium imaging of molecularly distinct cell types has enabled the characterization and measurement of in vivo response properties of molecularly defined vagal cells. The location of the nodose ganglion in the periphery makes it particularly difficult to access to image in vivo. However, surgical exposure and immobilization of the ganglion has enabled imaging of vagal afferent neurons with gut-projections remaining intact (33, 65). All vagal sensory neurons express Vglut2 (66), and 1-photon imaging studies have characterized response properties of these neurons to postingestive gut stimulation (33, 65). Vglut2-expressing neurons of the nodose ganglion respond to gastric distension, intestinal distension, and intestinal nutrient application (33). Neurons detecting stretch vs chemosensory properties of nutrients are largely distinct. Interestingly, however, neurons responsive to nutrients are generally broadly tuned, with the majority of neurons responsive to glucose, amino acid, fatty acid, salt, and pH (33).
A separate study imaged Vglut2-expressing vagal afferent neurons in the context of sugar reinforcement (65). This study confirmed the rapid activation of nodose ganglion neurons in response to sugar but not artificial sweetener as in Williams et al. (33). The nonmetabolizable glucose analogue methyl-alpha-D-glucopyranoside (MDG) causes similar increases in vagal activity as glucose. Glucose and methyl-alpha-D-glucopyranoside are both substrates of the sodium-glucose linked transporter 1 (SGLT1), which is involved in glucose absorption from the intestinal lumen. Indeed, pharmacological blockade of SGLT1 inhibits vagal afferent activation by glucose as well as the development of glucose preference (65), similar to previous behavioral studies (4, 67, 68).
The in vivo activity responses of subtypes of vagal afferent neurons have also been studied. Neurons expressing the receptor for the satiation signal glucagon-like peptide-1 (GLP1R) primarily innervate the stomach and respond to stretch, capsaicin, and the satiation hormone cholecystokinin (CCK) (33). Surprisingly, these neurons have minimal response to nutrients or the GLP1R ligand, GLP1. In these experiments, GCaMP was genetically expressed by breeding transgenic mouse lines rather than by acute viral injection, therefore it is possible that the recorded neurons did not express GLP1R at the time of recording, potentially explaining this result. Alternately, it is possible that GLP1 affects GLP1R-expressing vagal afferent neural activity on a timescale beyond that which was measured, but given the short half-life of GLP1 (69), this is not likely the case. Reduced GLP1R expression in vagal afferent neurons increases meal size and decreases glucose tolerance (70), highlighting a behavioral role for this receptor to pair with the in vivo physiology. The orphan receptor GPR65 marks a neuron population that does not overlap with GLP1R and targets intestinal villi (33). GPR65 neurons respond to the satiation signal serotonin as well as duodenal nutrients. Interestingly, stimulation of GPR65 but not GLP1R vagal afferent neurons reduces gastric pressure, demonstrating a specific role for GPR65 neurons in an intestine–brain–stomach reflex controlling gastric emptying.
While in vivo calcium imaging studies confirm previous findings that vagal afferent neurons are activated by nutrients and satiation signals, other work indicates that there is also a direct synaptic connection between the gut and vagal afferent neurons (termed “neuropod” cells (71)). In vitro experiments using the glutamate sensor iGluSnFR show that glutamate is the neurotransmitter mediating fast transmission from enteroendocrine cells to neurons (71). These neuropod cells express CCK and they distinguish sugars from artificial sweeteners using SGLT1 (72). Furthermore, in vivo optogenetic inhibition of CCK-expressing enteroendocrine cells using a flexible, gut-implanted optic fiber, eliminates sugar preference (72).
Together, these studies highlight how in vivo optical techniques can transform our understanding of vagal gut–brain communication. However, they only begin to scratch the surface of how activity in the vagus nerve impacts our physiology and behavior. Moving forward, it will be important to further refine the cell types that transmit multiple modes of information from the gut to the brain via vagal afferent signaling, as well as their contributions to behavior. For example, GLP1R and GPR65 comprise only a small portion of all gut-innervating vagal afferent neurons. Multiple studies have elegantly characterized vagal afferent cell types using RNA sequencing (73-75), revealing clusters of cell types that aggregate based on the target organ of innervation (73). In fact, these studies have revealed populations of vagal afferent neurons that inhibit feeding as well as activity in hypothalamic hunger neurons (73). This work has only begun to determine how this signaling impacts behavior—the mechanisms and cell types that promote satiation are not fully understood. Additionally, vagal signaling is involved in food preference and reward in addition to satiation. However, while the vagus nerve mediates sugar (65, 76) and fat reinforcement (77) (see “Dopamine signaling” for more discussion)—it is unknown which cell types mediate these effects. Important future work must therefore examine the natural activity dynamics of other vagal cell types as well as their contributions to behavior.
Hindbrain Signaling
Hindbrain regions, such as the NTS and parabrachial nucleus (PBN) are integral to gut–brain signaling. Ascending gastrointestinal vagal afferents synapse in the caudal NTS, making the NTS the first central site of integration of direct gut signals. These signals are then transmitted to the PBN and other target sites in the brain including the hypothalamus. Although the hindbrain is notoriously difficult to access for in vivo calcium imaging, a few studies have successfully devised strategies to monitor activity in cell type–specific hindbrain neurons in awake (78, 79) or anesthetized (65) mice. While anesthesia virtually eliminates motion artifacts associated with movement, GRIN lens stabilization techniques (eg, adhering wires to the lens for stabilization) have enabled stable imaging in freely moving mice (78).
Glutamatergic calcium activity in NTS neurons was monitored with fiber photometry in anesthetized mice (65). Gut delivery of glucose but not artificial sweetener transiently increases calcium signaling in glutamatergic neurons, and this effect is dependent on vagal signaling (65). The NTS sends projections to the PBN, where multiple feeding-related cell types reside. In particular, calcitonin gene–related protein (CGRP)-expressing neurons receive projections from the NTS and are involved in anorexia/satiation (80-82) and food aversion (81). Miniscope calcium imaging of PBN CGRP neurons revealed the temporal activity dynamics of these neurons during a meal. PBN CGRP neuron activity is rapidly inhibited at the onset of a meal, and neural activity gradually increases as feeding progresses (79). PBN prodynorphin-expressing neurons also respond to food and are thought to be important regulators of postingestive feedback. Fiber photometry and 2-photon imaging of these prodynorphin neurons indicate that they are activated by gastric distention, an effect that is mediated by vagal signaling (83). Finally, a recent study imaged glutamatergic and GABAergic peri-locus coeruleus neurons, just medial to the PBN, and demonstrated that glutamatergic neurons selectively encode consummatory signals (78). While these studies begin to reveal hindbrain activity dynamics, overall, our understanding of how gut signals influence in vivo hindbrain neural activity is in its infancy.
Hypothalamic Signaling
The hypothalamus is the most-studied brain region for energy balance control, with the arcuate hypothalamic nucleus (ARH), dorsomedial hypothalamic nucleus (DMH), lateral hypothalamus (LH), and paraventricular hypothalamus (PVH) serving as critical nodes in the circuits that drive food intake. How does food intake influence in vivo neural activity in these hypothalamic feeding regions?
Within the ARH, 2 populations of neurons (agouti-related protein [AgRP]- and pro-opiomelanocortin [POMC]-expressing neurons) are essential for proper food intake and energy balance control (84-86). Activity in these 2 neuron populations results in opposing effects on feeding behavior: AgRP neuron activity drives feeding behavior, while POMC neuron activity inhibits feeding behavior (84, 85). As the ARH is adjacent to the median eminence, a circumventricular organ with privileged access to circulating factors, these neuron populations are thought to directly sense nutrient status. While energy status remains a critical factor for in vivo baseline activity in these populations (42, 87), 3 landmark studies in 2015 used complementary methods to show that neural activity in AgRP and POMC neurons is rapidly modulated by the sensory detection (eg, sight, smell, etc.) of food.
First, fiber photometry was used to monitor population calcium dynamics of AgRP and POMC neurons in hungry mice during food intake (88). Surprisingly, neural activity in both populations responded immediately to food presentation, even before the mouse tasted the food, demonstrating the ability of external sensory food cues to very rapidly influence brain activity in the ARH. Second, miniscope calcium imaging of individual AgRP neurons revealed the relative homogeneity of AgRP neuron responses to food (41). Indeed, AgRP neuron activity is high across single neurons during hunger, and activity rapidly decreases in the majority of AgRP neurons upon refeeding. Third, in vivo optrode recordings similarly demonstrated that AgRP and POMC neurons respond immediately to food cues and further revealed how neuron firing rates fluctuate across time/energy status (42).
Food is complex, with visual, olfactory, taste, texture, and nutrient components. What aspects of food intake are important for changes in hypothalamic neuron activity? Our work has demonstrated that nutrients detected in the gastrointestinal tract are the primary regulators of AgRP neuron activity. Although the original studies demonstrated that sensory food cues inhibit AgRP neuron activity, we showed that gut nutrients train sensory cues (ie, sight, smell, taste, etc.) to predict the caloric content of food in a single trial (37). Inputs to AgRP neurons from GABAergic DMH neurons likely mediate these effects, as these DMH neurons are rapidly activated upon food availability (89). Nutrients (fats, sugars, or amino acids) infused directly into the stomach are sufficient for sustained inhibition of AgRP neuron activity, and this is mediated in part by satiation signaling (37, 90). More recently, we have demonstrated that different macronutrients utilize distinct gut–brain pathways to communicate with AgRP neurons (91, 92). Both fat and sugar are detected in the small intestine and absorption from the intestinal lumen is required for effects on AgRP neurons, as lipase or SGLT1 inhibitors block the effects of intestinal fat or sugar, respectively (92). Interestingly, vagal signaling is required for fat but not glucose to inhibit AgRP neuron activity. Glucose, on the other hand, inhibits AgRP neurons through spinal afferent signaling that likely originates in the small intestine and the hepatic portal vein (92). Indeed, direct glucose infusion into the hepatic portal vein significantly inhibits AgRP neuron activity compared with saline or fat infusion. Taken together, these data demonstrate that fat and sugar utilize distinct pathways to communicate with AgRP neurons, and highlight an underappreciated role for spinal afferent signaling in mediating in vivo gut–AgRP glucose signaling.
Of course, there are many feeding-relevant hypothalamic populations outside of the arcuate nucleus that contribute to energy balance control. Within the PVH there are multiple subpopulations of neurons that influence feeding behavior. A recent study used in vivo fiber photometry to characterize responses to food in 4 of these neuron populations (neurons expressing oxytocin, GLP1, melanocortin-4 receptor, and corticotrophin-releasing factor) and demonstrated differential calorie- and state-dependent neural activity changes in these populations in response to food (93). Monitoring individual PVH neurons demonstrates the heterogeneity of single cells in response to food or gut-derived peptides (94). Neuron populations within the LH are also known to drive feeding behavior (95-97). GABAergic neurons within the LH can be divided into at least 2 subpopulations that encode appetitive or consummatory behavior, as defined using bidirectional neural activity manipulations and miniscope calcium imaging (32). Separately, optrode recordings indicate that LH neurons that project to the ventral tegmental area (VTA) are responsive to sugar consumption, and activation of the GABAergic projection to the VTA increases feeding (44) and dopamine signaling (98). Extrahypothalamic regions, such as the cortex, also receive projections via multisynaptic circuits from the hypothalamus. In vivo 2-photon imaging with a microprism revealed that neurons of the insular cortex respond to food based on physiological state (30). Indeed, activity in individual insular cortex neurons increases or decreases in response to food cues in hungry mice or in mice receiving AgRP neuron stimulation, but these responses are abolished in sated mice (30). Interestingly, similar activity changes in insular cortex neurons are observed in thirsty mice provided with water, suggesting that these neurons may be general interoceptors rather than specific to feeding (99). Overall, while these studies reveal several neural populations in or connected to the hypothalamus that are responsive to food, future studies are required to determine if and how neural activity is modulated by gut signaling.
Dopamine Signaling
Midbrain dopamine circuits are activated by natural (eg, food, water, sex) as well as drug rewards and have been extensively studied in the context of food reward. It is commonly thought that food tastes better when hungry. There are nods to this in art and literature, for example, the novel Don Quixote by Miguel de Cervantes quotes “Hunger is the best sauce in the world.” How does hunger affect dopamine signaling, and how have in vivo neural activity measurements informed our knowledge on the intersection of gut signaling and reward?
Food intake increases dopamine signaling in the ventral striatum (also known as nucleus accumbens) (100, 101). The first in vivo evidence that hunger influences reward came from experiments examining how energy status modulates the dopamine response to food. In these studies, microdialysis or FCSV was used to measure dopamine spikes to food in hungry or sated animals. Food-evoked dopamine release in the ventral striatum is increased in hunger (102, 103), and the same effect is observed following injection of ghrelin, a gut-derived hunger hormone (102). Conversely, activation of receptors for satiation signals such as GLP1 (104) and amylin (105) reduce dopamine signaling. We therefore sought to identify a neural pathway at the interface of hunger and reward. Because AgRP neurons are activated by ghrelin (88) and by physiological energy deficit (41, 42, 87, 88), we reasoned that AgRP neurons may mediate the interaction between hunger and dopamine signaling. To test this hypothesis, we chemogenetically activated AgRP neurons while monitoring ventral striatum dopamine using fiber photometry recordings of a genetically encoded dopamine sensor. AgRP neuron activation potentiated the dopamine response to food, demonstrating that these neurons are a conduit between energy status and reward signaling (91). AgRP neuron activation did not influence baseline dopamine levels (91), indicating this interaction is not mediated by a direct synaptic connection. Consistently, there are little or no direct projections from AgRP neurons to regions where dopamine neurons or projections exist, such as the VTA and striatum (39, 106, 107). Interestingly, AgRP neuron stimulation also potentiates the dopamine response to drugs of abuse (ie, alcohol and nicotine) (91), adding to the growing literature on the overlap in systems mediating food and drug reward (108).
These studies focused on the dopamine response to a food reward, but do isolated gut signals influence brain reward systems? To answer this question, studies have monitored calcium signaling in dopamine neurons while nutrients were infused directly into the gastrointestinal tract. We demonstrated that VTA dopamine neurons are activated by intragastric infusions of fat (91). Separate work showed that intragastric infusion of sugar (sucrose) but not artificial sweetener (sucralose) increases calcium dynamics in VTA dopamine neurons, and this activation is dependent on an intact vagus nerve (76).
In addition to VTA neuron activity, striatal dopamine signaling has also been monitored in response to nutrient signaling. While oral consumption of sugar and artificial sweetener increases striatal dopamine signaling measured with microdialysis, only sugar increases dopamine signaling in taste-blind mice (109), suggesting a postoral reward effect of calories. Indeed, intragastric glucose is sufficient to elevate dopamine signaling, and these increases are observed in both the dorsal and ventral striatum (110, 111). Striatal dopamine is also increased by a taste previously paired with intragastric sugar (112), demonstrating that dopamine neurons, similar to AgRP neurons (37), are entrained to use sensory cues to predict the caloric value of food. Bypassing the gut entirely, glucose infusions into the hepatic portal vein can elevate dorsal striatum dopamine efflux (113). These data complement our recent finding (mentioned above) demonstrating that portal glucose inhibits AgRP neuron activity (92) and highlight this pathway in mediating gut–brain nutrient signaling. Together, these studies demonstrate the ability for postingestive sugar sensing to increase VTA neuron activity and dopamine signaling in both the dorsal and ventral striatum.
Interestingly, intragastric fat increases dopamine signaling in the dorsal but not the ventral striatum (91, 114), and this effect is dependent on vagal signaling (77). In fact, optogenetic stimulation of the right but not left vagus nerve, even in the absence of fat in the gut, is sufficient to drive dorsal striatum dopamine release (77). It is thought that the dorsal and ventral striatum encode nutritive and hedonic values of food, respectively, potentially explaining these findings (115). In contrast to fat and sugar, the effects of intragastric protein/amino acids on dopamine signaling remain largely unknown.
Overall, simulation of dopamine release by postingestive signals highlights the power of the gut in modulating reward. However, the ability for gut nutrient signaling to stimulate dopamine release does not provide a causal link between dopamine and feeding behavior. Studies that have manipulated dopamine signaling during the formation of food preferences determined that dopamine signaling is required for this behavior (115-118). Ultimately, combining neural activity manipulations with dopamine monitoring and behavioral assays in the same subjects will directly address this gap.
In addition to projections to the dorsal and ventral striatum, dopamine neurons reach a range of target regions including the amygdala and prefrontal cortex (119, 120). Although extrastriatal dopamine signaling can also encode the reward value of food in a state-dependent manner (121), the ability for gut signaling to increase in vivo dopamine release in these regions remains unexplored.
Monitoring gut–brain neural activity dynamics in obesity
In addition to providing insight into how gut signaling affects the brain, in vivo techniques for monitoring neural activity can be used to help determine the etiology and pathology of disease. A few recent studies have demonstrated the power of these tools to reveal neural activity changes that result from obesity.
First, activity dynamics of glutamatergic LH neurons were tracked with 2-photon microscopy during the development of obesity (40). Before high-fat diet exposure, a subpopulation of LH glutamatergic neurons increases activity in response to sucrose, and these neural activity changes weaken over the development of obesity. This attenuated effect is observed after 2 weeks on high-fat diet (prior to significant elevations in body weight), and is more robust after 12 weeks (40). Second, 2 recent studies used fiber photometry to examine how obesity influences AgRP neuron activity (38, 39). Maintenance on high-fat diet causes an attenuation of AgRP neuron inhibition in response to food, gastrointestinal hormones, and intragastric fat (but not sugar). Strikingly, these effects occur after just 24 hours on high-fat diet (39). Attenuated responses to food are also observed in AgRP input neurons from the dorsomedial hypothalamus. Overall, these studies suggest a potential widespread dampening of hypothalamic neuron activity in response to high-fat diet and obesity.
Finally, monitoring dopamine signaling in obesity or with surgical obesity treatments reveals an interaction between dopamine and disease. High-fat diet exposure reduces food-evoked ventral striatum dopamine signaling (39). Additionally, it disrupts the ability for AgRP neuron activity to augment dopamine signaling to food (39). In the dorsal striatum, there are also links between dopamine signaling and obesity. High-fat diet maintenance prevents the ability for intragastric fat to elevate dopamine signaling (122). This is reversed by infusion of the lipid messenger oleoylethanolamine (122). Interestingly, gastric bypass surgery in obese mice stimulates oleoylethanolamine synthesis and potentiates dorsal striatum dopamine release in response to high-fat diet (123), perhaps partially explaining the success of bariatric surgery for weight loss. A review of how bariatric surgery alters central feeding circuits was recently published (124). Overall, these in vivo findings suggest that alteration of hypothalamic activity and striatal dopamine signaling may contribute to the pathology of obesity.
Conclusion
In summary, the adoption of complementary in vivo neural activity monitoring technologies has revolutionized our ability to understand how gut signaling influences neural activity with precise temporal resolution. Moving forward, combining activity monitoring of optimized biosensors with neural activity manipulations and behavior will establish important causal links between gut signaling, neural activity, and behavior.
Acknowledgments
The author thanks Michael Krashes, J. Nicholas Betley, Kuei-Pin Huang, and Nitsan Goldstein for comments on the manuscript.
Financial Support: Amber L. Alhadeff is funded by the NIH (R00DK119574), Klingenstein Fund and Simons Foundation, Monell Chemical Senses Center, and Penn Institute for Diabetes, Obesity and Metabolism.
Glossary
Abbreviations
- AgRP
agouti-related protein
- ARH
arcuate hypothalamic nucleus
- CCK
cholecystokinin
- CGRP
calcitonin gene–related
- ChR2
channelrhodopsin-2
- DMH
dorsomedial hypothalamic nucleus
- FSCV
fast-scan cyclic voltammetry
- GABA
gamma-aminobutyric acid
- GLP1R
glucagon-like peptide-1 receptor
- GRIN
gradient index
- G-
protein–coupled receptors
- LH
lateral hypothalamus
- NTS
nucleus of the solitary tract
- PBN
parabrachial nucleus
- PBP
periplasmic-binding protein
- POMC
pro-opiomelanocortin
- PVH
paraventricular hypothalamus
- SGLT1
sodium-glucose linked transporter 1
- VTA
ventral tegmental area
Additional Information
Disclosures: A.L.A. has no conflicts of interest to declare.
Data Availability
No datasets were generated during the preparation of this review.
References
- 1. Miller I. The gut-brain axis: historical reflections. Microb Ecol Health Dis. 2018;29(1):1542921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clemmensen C, Müller TD, Woods SC, Berthoud HR, Seeley RJ, Tschöp MH. Gut-brain cross-talk in metabolic control. Cell. 2017;168(5):758-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20(Suppl 1):64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sclafani A. Gut-brain nutrient signaling. Appetition vs. satiation. Appetite. 2013;71:454-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Araujo IE, Schatzker M, Small DM. Rethinking food reward. Annu Rev Psychol. 2020;71:139-164. [DOI] [PubMed] [Google Scholar]
- 6. Moran TH. Gut peptide signaling in the controls of food intake. Obesity (Silver Spring). 2006;14(Suppl 5):250S-253S. [DOI] [PubMed] [Google Scholar]
- 7. Shechter A, Schwartz GJ. Gut-brain nutrient sensing in food reward. Appetite. 2018;122:32-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Lartigue G. Putative roles of neuropeptides in vagal afferent signaling. Physiol Behav. 2014;136:155-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mollinedo-Gajate I, Song C, Knöpfel T. Genetically encoded fluorescent calcium and voltage indicators. Handb Exp Pharmacol. 2019;260:209-229. [DOI] [PubMed] [Google Scholar]
- 10. Yang HH, St-Pierre F. Genetically encoded voltage indicators: opportunities and challenges. J Neurosci. 2016;36(39):9977-9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin MZ, Schnitzer MJ. Genetically encoded indicators of neuronal activity. Nat Neurosci. 2016;19(9):1142-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pal A, Tian L. Imaging voltage and brain chemistry with genetically encoded sensors and modulators. Curr Opin Chem Biol. 2020;57:166-176. [DOI] [PubMed] [Google Scholar]
- 13. Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18(4):222-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D’Agostino AE, Small DM. Neuroimaging the interaction of mind and metabolism in humans. Mol Metab. 2012;1(1-2):10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dalenberg JR, Patel BP, Denis R, et al. Short-term consumption of sucralose with, but not without, carbohydrate impairs neural and metabolic sensitivity to sugar in humans. Cell Metab. 2020;31(3):493-502.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thanarajah SE, Backes H, DiFeliceantonio AG, et al. Food intake recruits orosensory and post-ingestive dopaminergic circuits to affect eating desire in humans. Cell Metab. 2019;29(3):695-706.e4. [DOI] [PubMed] [Google Scholar]
- 17. Rebollo I, Devauchelle AD, Beranger B, Tallon-Baudry C. Stomach-brain synchrony reveals a novel, delayed-connectivity resting-state network in humans. Elife. 2018;7:e3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azzalini D, Rebollo I, Tallon-Baudry C. Visceral signals shape brain dynamics and cognition. Trends Cogn Sci. 2019;23(6):488-509. [DOI] [PubMed] [Google Scholar]
- 19. Veldhuizen MG, Babbs RK, Patel B, et al. Integration of sweet taste and metabolism determines carbohydrate reward. Curr Biol. 2017;27(16):2476-2485.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Connor JA. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc Natl Acad Sci U S A. 1986;83(16):6179-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tank DW, Sugimori M, Connor JA, Llinás RR. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988;242(4879):773-777. [DOI] [PubMed] [Google Scholar]
- 22. Chen TW, Wardill TJ, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dana H, Sun Y, Mohar B, et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods. 2019;16:649–657. [DOI] [PubMed] [Google Scholar]
- 24. Sato M, Kawano M, Ohkura M, Gengyo-Ando K, Nakai J, Hayashi Y. Generation and imaging of transgenic mice that express G-CaMP7 under a tetracycline response element. PLoS One. 2015;10(5):e0125354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zariwala HA, Borghuis BG, Hoogland TM, et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci. 2012;32(9):3131-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madisen L, Garner AR, Shimaoka D, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85(5):942-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dana H, Chen TW, Hu A, et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS One. 2014;9(9):e108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Q, Cichon J, Wang W, et al. Imaging neural activity using Thy1-GCaMP transgenic mice. Neuron. 2012;76(2):297-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reed WA, Yan MF, Schnitzer MJ. Gradient-index fiber-optic microprobes for minimally invasive in vivo low-coherence interferometry. Opt Lett. 2002;27(20):1794-1796. [DOI] [PubMed] [Google Scholar]
- 30. Livneh Y, Ramesh RN, Burgess CR, et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 2017;546(7660):611-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gunaydin LA, Grosenick L, Finkelstein JC, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jennings JH, Ung RL, Resendez SL, et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160(3):516-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166(1):209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Inoue M, Takeuchi A, Manita S, et al. Rational engineering of XCaMPs, a multicolor GECI suite for in vivo imaging of complex brain circuit dynamics. Cell. 2019;177(5):1346-1360.e24. [DOI] [PubMed] [Google Scholar]
- 35. Akerboom J, Carreras Calderón N, Tian L, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope. high-resolution brain imaging in freely moving animals. Neuron. 2001;31(6):903-912. [DOI] [PubMed] [Google Scholar]
- 37. Su Z, Alhadeff AL, Betley JN. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 2017;21(10):2724-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beutler LR, Corpuz TV, Ahn JS, et al. Obesity causes selective and long-lasting desensitization of AgRP neurons to dietary fat. Elife. 2020;9:e55909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mazzone CM, Liang-Guallpa J, Li C, et al. High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nat Neurosci. 2020;23(10):1253-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossi MA, Basiri ML, McHenry JA, et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science. 2019;364(6447):1271-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Betley JN, Xu S, Cao ZFH, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521(7551):180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mandelblat-Cerf Y, Ramesh RN, Burgess CR, et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. 2015;4:e07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lima SQ, Hromádka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One. 2009;4(7):e6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nieh EH, Matthews GA, Allsop SA, et al. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160(3):528-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beyeler A, Namburi P, Glober GF, et al. Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron. 2016;90(2):348-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dickey AS, Suminski A, Amit Y, Hatsopoulos NG. Single-unit stability using chronically implanted multielectrode arrays. J Neurophysiol. 2009;102(2):1331-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tolias AS, Ecker AS, Siapas AG, Hoenselaar A, Keliris GA, Logothetis NK. Recording chronically from the same neurons in awake, behaving primates. J Neurophysiol. 2007;98(6):3780-3790. [DOI] [PubMed] [Google Scholar]
- 48. Marshall JD, Li JZ, Zhang Y, et al. Cell-type-specific optical recording of membrane voltage dynamics in freely moving mice. Cell. 2016;167(6):1650-1662.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adam Y, Kim JJ, Lou S, et al. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature. 2019;569(7756):413-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis. Curr Protoc Neurosci. 2009;47(1):7.1.1-7.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fortin SM, Cone JJ, Ng-Evans S, McCutcheon JE, Roitman MF. Sampling phasic dopamine signaling with fast-scan cyclic voltammetry in awake, behaving rats. Curr Protoc Neurosci. 2015;70:7.25.1-7.25.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76(19):5697-5704. [DOI] [PubMed] [Google Scholar]
- 53. Sun F, Zeng J, Jing M, et al. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell. 2018;174(2):481-496.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patriarchi T, Cho JR, Merten K, et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science. 2018;360(6396):eaat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun F, Zhou J, Dai B, et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat Methods. 2020;17(11):1156-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marvin JS, Borghuis BG, Tian L, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10(2):162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marvin JS, Shimoda Y, Magloire V, et al. A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nat Methods. 2019;16(8):763-770. [DOI] [PubMed] [Google Scholar]
- 58. Helassa N, Dürst CD, Coates C, et al. Ultrafast glutamate sensors resolve high-frequency release at Schaffer collateral synapses. Proc Natl Acad Sci U S A. 2018;115(21):5594-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu J, Abdelfattah AS, Zhou H, et al. Genetically encoded glutamate indicators with altered color and topology. ACS Chem Biol. 2018;13(7):1832-1837. [DOI] [PubMed] [Google Scholar]
- 60. Kazemipour A, Novak O, Flickinger D, et al. Kilohertz frame-rate two-photon tomography. Nat Methods. 2019;16(8):778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mita M, Ito M, Harada K, et al. Green fluorescent protein-based glucose indicators report glucose dynamics in living cells. Anal Chem. 2019;91(7):4821-4830. [DOI] [PubMed] [Google Scholar]
- 62. Keller JP, Marvin JS, Lacin H, et al. In vivo glucose imaging in multiple model organisms with an engineered single-wavelength sensor. bioRxiv. 2019:571422. [DOI] [PubMed] [Google Scholar]
- 63. Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol. 2013;10(5):286-296. [DOI] [PubMed] [Google Scholar]
- 64. Dockray GJ. The versatility of the vagus. Physiol Behav. 2009;97(5):531-536. [DOI] [PubMed] [Google Scholar]
- 65. Tan HE, Sisti AC, Jin H, et al. The gut-brain axis mediates sugar preference. Nature. 2020;580(7804):511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal sensory neuron subtypes that differentially control breathing. Cell. 2015;161(3):622-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and nonmetabolizable sugar analogs. Am J Physiol Regul Integr Comp Physiol. 2013;305(7):R840-R853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sclafani A, Koepsell H, Ackroff K. SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):R631-R639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409-1439. [DOI] [PubMed] [Google Scholar]
- 70. Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34-43. [DOI] [PubMed] [Google Scholar]
- 71. Kaelberer MM, Buchanan KL, Klein ME, et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361(6408):eaat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Buchanan KL, Rupprecht LE, Sahasrabudhe A, et al. A gut sensor for sugar preference. bioRxiv. 2020:2020.2003.2006.981365. [Google Scholar]
- 73. Bai L, Mesgarzadeh S, Ramesh KS, et al. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179(5):1129-1143.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kupari J, Häring M, Agirre E, Castelo-Branco G, Ernfors P. An Atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 2019;27(8):2508-2523.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Egerod KL, Petersen N, Timshel PN, et al. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol Metab. 2018;12:62-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fernandes AB, Alves da Silva J, Almeida J, et al. Postingestive modulation of food seeking depends on vagus-mediated dopamine neuron activity. Neuron. 2020;106(5):778-788.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Han W, Tellez LA, Perkins MH, et al. A neural circuit for gut-induced reward. Cell. 2018;175(3):887-888. [DOI] [PubMed] [Google Scholar]
- 78. Gong R, Xu S, Hermundstad A, Yu Y, Sternson SM. Hindbrain double-negative feedback mediates palatability-guided food and water consumption. Cell. 2020;182(6):1589-1605.e22. [DOI] [PubMed] [Google Scholar]
- 79. Campos CA, Bowen AJ, Roman CW, Palmiter RD. Encoding of danger by parabrachial CGRP neurons. Nature. 2018;555(7698):617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial CGRP neurons control meal termination. Cell Metab. 2016;23(5):811-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci. 2015;35(11):4582-4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun. 2016;7:11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim DY, Heo G, Kim M, et al. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature. 2020;580(7803):376-380. [DOI] [PubMed] [Google Scholar]
- 84. Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Krashes MJ, Koda S, Ye C, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683-685. [DOI] [PubMed] [Google Scholar]
- 87. Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146(3):1043-1047. [DOI] [PubMed] [Google Scholar]
- 88. Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160(5):829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Garfield AS, Shah BP, Burgess CR, et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci. 2016;19(12):1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Beutler LR, Chen Y, Ahn JS, Lin YC, Essner RA, Knight ZA. Dynamics of gut-brain communication underlying hunger. Neuron. 2017;96(2):461-475.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Alhadeff AL, Goldstein N, Park O, Klima ML, Vargas A, Betley JN. Natural and drug rewards engage distinct pathways that converge on coordinated hypothalamic and reward circuits. Neuron. 2019;103(5):891-908.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Goldstein N, McKnight AD, Carty JRE, Arnold M, Betley JN, Alhadeff AL. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 2021:S1550-4131(20)30716-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li C, Navarrete J, Liang-Guallpa J, et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 2019;29(3):681-694.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xu S, Yang H, Menon V, et al. Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles. Science. 2020;370(6514):eabb2494. [DOI] [PubMed] [Google Scholar]
- 95. Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77(2):323-324. [DOI] [PubMed] [Google Scholar]
- 96. Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19(2):198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Delgado JM, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol. 1953;172(1):162-168. [DOI] [PubMed] [Google Scholar]
- 98. Nieh EH, Vander Weele CM, Matthews GA, et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron. 2016;90(6):1286-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Livneh Y, Sugden AU, Madara JC, et al. Estimation of current and future physiological states in insular cortex. Neuron. 2020;105(6):1094-1111.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44(4-5):599-606. [DOI] [PubMed] [Google Scholar]
- 101. Taber MT, Fibiger HC. Feeding-evoked dopamine release in the nucleus, accumbens: regulation by glutamatergic mechanisms. Neuroscience. 1997;76(4):1105-1112. [DOI] [PubMed] [Google Scholar]
- 102. Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34(14):4905-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15(7 Pt 2):5169-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Konanur VR, Hsu TM, Kanoski SE, Hayes MR, Roitman MF. Phasic dopamine responses to a food-predictive cue are suppressed by the glucagon-like peptide-1 receptor agonist Exendin-4. Physiol Behav. 2020;215:112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mietlicki-Baase EG, Reiner DJ, Cone JJ, et al. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology. 2015;40(2):372-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang D, He X, Zhao Z, et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12(11):638-651. [DOI] [PubMed] [Google Scholar]
- 109. de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57(6):930-941. [DOI] [PubMed] [Google Scholar]
- 110. Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30(23):8012-8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Han W, Tellez LA, Niu J, et al. Striatal dopamine links gastrointestinal rerouting to altered sweet appetite. Cell Metab. 2016;23(1):103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mark GP, Smith SE, Rada PV, Hoebel BG. An appetitively conditioned taste elicits a preferential increase in mesolimbic dopamine release. Pharmacol Biochem Behav. 1994;48(3):651-660. [DOI] [PubMed] [Google Scholar]
- 113. Zhang L, Han W, Lin C, Li F, de Araujo IE. Sugar metabolism regulates flavor preferences and portal glucose sensing. Front Integr Neurosci. 2018;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ferreira JG, Tellez LA, Ren X, Yeckel CW, de Araujo IE. Regulation of fat intake in the absence of flavour signalling. J Physiol. 2012;590(4):953-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tellez LA, Han W, Zhang X, et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci. 2016;19(3):465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol Behav. 2011;104(1):64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D1 but not D2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2001;68(4):709-720. [DOI] [PubMed] [Google Scholar]
- 118. Touzani K, Bodnar R, Sclafani A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur J Neurosci. 2008;27(6):1525-1533. [DOI] [PubMed] [Google Scholar]
- 119. Beier KT, Steinberg EE, DeLoach KE, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162(3):622-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1-6):321-353. [DOI] [PubMed] [Google Scholar]
- 121. Lutas A, Kucukdereli H, Alturkistani O, et al. State-specific gating of salient cues by midbrain dopaminergic input to basal amygdala. Nat Neurosci. 2019;22(11):1820-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tellez LA, Medina S, Han W, et al. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341(6147):800-802. [DOI] [PubMed] [Google Scholar]
- 123. Hankir MK, Seyfried F, Hintschich CA, et al. Gastric bypass surgery recruits a gut PPAR-α-Striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017;25(2):335-344. [DOI] [PubMed] [Google Scholar]
- 124. Hankir MK, Seyfried F, Miras AD, Cowley MA. Brain feeding circuits after Roux-en-Y gastric bypass. Trends Endocrinol Metab. 2018;29(4):218-237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated during the preparation of this review.