Abstract
Objective
The aim of this paper was to report the 2‐year follow‐up in type I patients treated with Nusinersen and to assess whether possible changes in motor function are related to the subtype, age, or SMN2 copy number.
Methods
Sixty‐eight patients, with ages ranging from 0.20 to 15.92 years (mean: 3.96; standard deviation: +3.90) were enrolled in the study. All patients were assessed using the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) and the developmental section of the Hammersmith Infant Neurological Examination (HINE‐2) at the time they started treatment and 12 and 24 months after that.
Results
For both CHOP and HINE‐2 repeated measures analysis of variance showed a significant difference (P < 0.001) between baseline and 12 months, 12 months and 24 months, and baseline and 24‐month scores for the whole group. When age subgroups (<210 days, <2 years, 2–4 years, 5–11 years, 12–18 years) were considered, on the CHOP INTEND the difference was significant between baseline and 24 months in all age subgroups. On the HINE‐2, the difference between baseline and 24 months was significant in all the subgroups before the age of 4 years. Age was predictive of changes on both scales (P < 0.05), whereas SMN2 copy number and decimal classification were not.
Interpretation
Our results suggest that some improvement of motor function can be observed even after the first year of treatment. This is more obvious in the infants treated in the first 2 years but some improvement can also be found in older children.
Introduction
Several papers have reported real‐world data using Nusinersen in type I infants and children. 1 , 2 , 3 , 4 , 5 The findings, obtained in different countries are quite consistent, showing an improvement in functional scores, with larger improvements observed in younger infants. Most of these studies have reported data obtained in the first year after treatment initiation.
A recent study, reporting the long‐term follow‐up of infants enrolled in the pivotal trial ENDEAR and transitioned in the SHINE extension study has suggested that an increase in functional scores and the achievement of new milestones can also be achieved after the first year of treatment. 6 Less is known on the long‐term follow‐up of a wider group of patients with infantile‐onset treated with nusinersen, including those with the severe form with neonatal‐onset or patients treated after the age of 210 days, who had not been included in the ENDEAR study. 7 Establishing whether nusinersen‐treated patients have further improvements after the first year has become particularly important at the time new therapeutical approaches are becoming available for patients who are already treated with nusinersen, and there will soon be the need to understand possible differences with patients who may decide to switch to another therapeutic option or, when possible, to combine them.
The aim of this paper was to report the follow‐up of a cohort of type I patients treated with nusinersen for at least 2 years and to assess whether the possible changes are related to the subtype, age, and SMN2 copy number.
Methods
Patients included in the study were part of an EAP approach in Italy including five Italian centers previously involved in nusinersen trials. 8 The results of the first 6 and 12 months have already been published. 5 , 9 The study was approved by the institutional review board (ethics committee) in each center. Written informed consent was obtained from all participants (or guardians of participants) in the study.
All patients were assessed using both the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND), 10 , 11 which includes 16 items with a total score between 0 and 64, and the developmental section of the Hammersmith Infant Neurological Examination (HINE‐2), 12 which includes eight selected motor items scored accordingly to the gradient of normal development. Sitting position was defined as a score of 3 or 4 on the “Sitting” item of the HINE‐2 and maintained for at least 30 seconds.
Measures were performed by trained clinical evaluators. Details of the training and reliability sessions have already been reported. 13 Each center had a different schedule of assessments, according to their routine clinical practice but it was agreed that all patients should have at least one assessment after 12 months from the first dose of nusinersen, between the 6th and the 7th dose of nusinersen, and another assessment around 24 months, between the 10th and 11th dose.
Statistical analysis
The cohort was stratified according to the criteria used in the 6‐ and 12‐month follow‐up study. 5 , 9 This included subdividing patients according to the SMN2 copy number, to the age when they started treatment, and to the severity of the disease. The severity was calculated using the Dubowitz decimal classification, 14 classifying as 1.1 the infants at the more severe end of the spectrum, with severely reduced mobility at birth and early respiratory and bulbar difficulties; as 1.5 as those with the most common phenotype in type I SMA, with an inability to raise the legs against gravity or maintain the head posture but having, at diagnosis, no difficulty with feeding and swallowing, and no obvious respiratory distress; and as 1.9 the mildest phenotypes, often diagnosed after the first few months, with the ability to achieve some head control and having less respiratory compromise.
The cohort was also subdivided in age subgroups: <210 days, <2 years, 2–4 years, 5–11 years, 12–18 years, in agreement with the cut off reported in clinical trials (<210 days) 7 and to the previous data published after 1 year of treatment. 3
Variables were described by mean and standard deviation (SD). Repeated measures analysis of variance (ANOVA) was used to compare the mean score of both the CHOP INTEND and HINE‐2, between baseline 12‐ and 24‐month follow‐up.
Mixed effect linear regression model was used to evaluate possible predictive variables (age, SMN2 copy number, decimal classification) of changes.
SPSS software (SPSS, Inc.) V23 was used for all statistical analyses, setting the significance at P < 0.05.
Results
Of the initial 122 SMA patients with infantile onset reported in the paper on the Italian Nusinersen Expanded Access Program (EAP), 9 85 had been followed up in the first 12 months. Of those, after the first year, 11 stopped the treatment because the results did not meet their expectations, three because of the side effects of the procedures and four to be enrolled in a clinical trial.
Of the remaining 67, one died and two moved to other centers. Four additional patients who were too young at the time our 12‐month follow‐up paper was published but who have now completed the 24‐month follow‐up were added to the original cohort. This resulted in 68 patients with 24‐month follow‐up. Figure 1 provides the details of all the patients enrolled since baseline. 9
Figure 1.
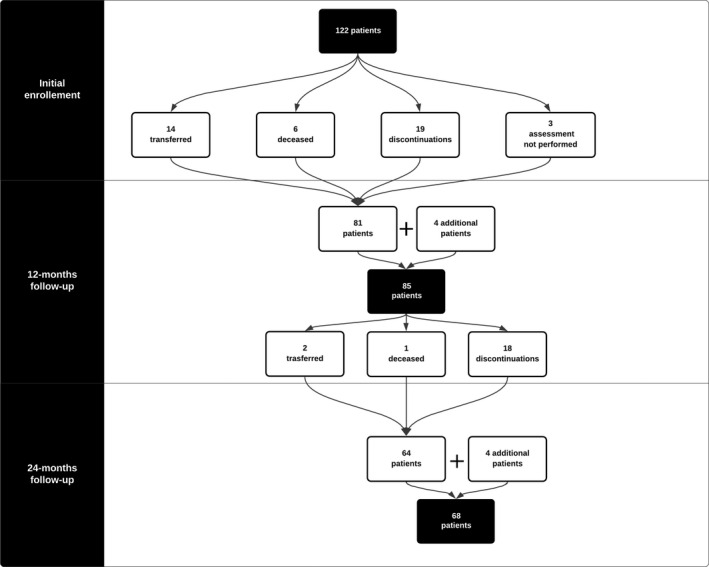
Enrollment flowchart from baseline paper to this study. 9
The 68 patients enrolled in the study had an age range at baseline between 0.20 and 15.92 years (mean: 3.96; SD: 3.90). Two patients had 1 SMN2 copy, 48 had 2, 17 had 3, and 1 had 4.
Based on the Dubowitz decimal classification, seven of the 68 were classified as 1.1, 36 as 1.5, and 25 as 1.9.
CHOP‐INTEND
The mean score in the whole cohort was 18.09 (±14.22) at baseline, 24.81 (±18.85) at 12 months, and 26.75 (±19.45) at 24 months.
The mean changes were 6.72 (±8.33) between baseline and 12 months, 1.94 (±3.69) between 12 and 24 month, and 8.66 (±9.35) between baseline and 24 months.
Table 1 and Figure 2 shows details of the scores in the age related and decimal classification subgroups.
Table 1.
Descriptive statistics on age and CHOP INTEND scores subdivided by age group and Dubowitz decimal classification.
| CHOP INTEND | Scores | Changes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | 24 months | 0–12 | P | 12–24 | P | 0–24 | P | |||
| ALL | ALL (n:68) | Mean ± SD | 18.09 ± 14.22 | 24.81 ± 18.85 | 26.75 ± 19.45 | 6.72 ± 8.33 | <0.001 | 1.94 ± 3.69 | <0.001 | 8.66 ± 9.35 | <0.001 |
| Min; Max | 0; 52 | 0; 64 | 0; 64 | −6; 32 | −8; 12 | −3; 37 | |||||
| 1.1 (n:7) | Mean ± SD | 6.86 ± 5.4 | 12.29 ± 15.18 | 14 ± 16.99 | 5.43 ± 11.56 | 1.71 ± 3.15 | 7.14 ± 13.32 | ||||
| Min; Max | 0; 14 | 0; 45 | 0; 51 | −1; 31 | −4; 6 | 0; 37 | |||||
| 1.5 (n:36) | Mean ± SD | 12.69 ± 11.86 | 18.97 ± 17.56 | 21.64 ± 18.5 | 6.28 ± 8.78 | 2.67 ± 3.77 | 8.94 ± 9.82 | ||||
| Min; Max | 0; 42 | 1; 56 | 1; 58 | −6; 32 | −4; 12 | −2; 35 | |||||
| 1.9 (n:25) | Mean ± SD | 29 ± 12.16 | 36.72 ± 15.47 | 37.68 ± 16.56 | 7.72 ± 6.79 | 0.96 ± 3.61 | 8.68 ± 7.63 | ||||
| Min; Max | 4; 52 | 5; 64 | 5; 64 | −3; 31 | −8; 11 | −3; 31 | |||||
| <210 days | ALL (n:7) | Mean ± SD | 23 ± 9.8 | 46.14 ± 7.56 | 51.29 ± 4.46 | 23.14 ± 7.82 | <0.001 | 5.14 ± 3.85 | <0.001 | 28.29 ± 7.18 | <0.001 |
| Min; Max | 14; 42 | 34; 56 | 44; 58 | 14; 32 | 0; 10 | 16; 37 | |||||
| 1.1 (n:1) | Mean ± SD | 14 ± N/A | 45 ± N/A | 51 ± N/A | 31 ± N/A | 6 ± N/A | 37 ± N/A | ||||
| Min; Max | 14; 14 | 45; 45 | 51; 51 | 31; 31 | 6; 6 | 37; 37 | |||||
| 1.5 (n:5) | Mean ± SD | 24.6 ± 10.97 | 44.6 ± 7.92 | 50.6 ± 5.08 | 20 ± 6.96 | 6 ± 3.81 | 26 ± 7.07 | ||||
| Min; Max | 14; 42 | 34; 56 | 44; 58 | 14; 32 | 2; 10 | 16; 35 | |||||
| 1.9 (n:1) | Mean ± SD | 24 ± N/A | 55 ± N/A | 55 ± N/A | 31 ± N/A | 0 ± N/A | 31 ± N/A | ||||
| Min; Max | 24; 24 | 55; 55 | 55; 55 | 31; 31 | 0; 0 | 31; 31 | |||||
| <2 years | ALL (n:23) | Mean ± SD | 25.69 ± 14.52 | 34.86 ± 18.15 | 36.82 ± 18.66 | 8.45 ± 6 | <0.001 | 2.25 ± 4.15 | 0.003 | 10.7 ± 7.2 | <0.001 |
| Min; Max | 0; 52 | 0; 64 | 0; 64 | −1; 19 | −4; 12 | −2; 25 | |||||
| 1.1 (n:3) | Mean ± SD | 6 ± 6.56 | 7.67 ± 6.66 | 6.67 ± 6.51 | 1.67 ± 3.79 | −1 ± 2.65 | 0.67 ± 1.15 | ||||
| Min; Max | 0; 13 | 0; 12 | 0; 13 | −1; 6 | −4; 1 | 0; 2 | |||||
| 1.5 (n:11) | Mean ± SD | 21.00 ± 11.92 | 30.18 ± 15.20 | 32.90 ± 14.64 | 9.18 ± 9.03 | 2.73 ± 4.67 | 11.91 ± 8.67 | ||||
| Min; Max | 4; 37 | 7; 51 | 11; 57 | −1; 28 | −4; 12 | −2; 27 | |||||
| 1.9 (n:9) | Mean ± SD | 38 ± 6.75 | 49.67 ± 7.25 | 51.56 ± 8.52 | 11.67 ± 2.74 | 1.89 ± 3.76 | 13.56 ± 4.22 | ||||
| Min; Max | 31; 52 | 41; 64 | 41; 64 | 8; 15 | −2; 11 | 9; 21 | |||||
| 2–4 years | ALL (n:14) | Mean ± SD | 18.78 ± 14.62 | 21.92 ± 15.86 | 22.71 ± 15.83 | 3.14 ± 2.44 | 0.027 | 0.79 ± 2.78 | 1.000 | 3.93 ± 2.73 | 0.012 |
| Min; Max | 1; 43 | 1; 47 | 3; 47 | −1; 7 | −5; 6 | −1; 10 | |||||
| 1.1 (n:2) | Mean ± SD | 4 ± 4.24 | 5.5 ± 6.36 | 8.5 ± 6.36 | 1.5 ± 2.12 | 3 ± 0 | 4.5 ± 2.12 | ||||
| Min; Max | 1; 7 | 1; 10 | 4; 13 | 0; 3 | 3; 3 | 3; 6 | |||||
| 1.5 (n:4) | Mean ± SD | 8.75 ± 7.36 | 9.75 ± 7.80 | 11 ± 8.91 | 1.00 ± 1.83 | 1.25 ± 2.63 | 2.25 ± 2.50 | ||||
| Min; Max | 1; 16 | 3; 17 | 3; 21 | −1; 3 | −1; 5 | −1; 5 | |||||
| 1.9 (n:8) | Mean ± SD | 25.75 ± 14.54 | 29.5 ± 15.71 | 29 ± 16.34 | 3.75 ± 3.24 | −0.5 ± 2.07 | 3.25 ± 2.55 | ||||
| Min; Max | 5; 43 | 5; 47 | 7; 47 | −3; 7 | −5; 2 | −1; 7 | |||||
| 5–11 years | ALL (n:22) | Mean ± SD | 10.27 ± 9.95 | 12.27 ± 10.91 | 14.36 ± 11.24 | 2 ± 3.31 | 0.097 | 2.09 ± 3.45 | 0.042 | 4.09 ± 3.82 | 0.001 |
| Min; Max | 0; 32 | 1; 32 | 1; 36 | −6; 8 | −8; 8 | −3; 13 | |||||
| 1.1 (n:1) | Mean ± SD | 8 ± N/A | 7 ± N/A | 10 ± N/A | −1 ± N/A | 3 ± N/A | 2 ± N/A | ||||
| Min; Max | 8; 8 | 7; 7 | 10; 10 | −1; −1 | 3; 3 | 2; 2 | |||||
| 1.5 (n:15) | Mean ± SD | 4.6 ± 3.62 | 5.93 ± 3.31 | 8 ± 3.85 | 1.33 ± 3.06 | 2.07 ± 2.87 | 3.4 ± 2.75 | ||||
| Min; Max | 0; 12 | 1; 14 | 1; 14 | −6; 7 | −2; 8 | −1; 8 | |||||
| 1.9 (n:6) | Mean ± SD | 24.83 ± 5.23 | 29 ± 2.68 | 31 ± 5.66 | 4.17 ± 3.31 | 2 ± 5.18 | 6.17 ± 5.71 | ||||
| Min; Max | 18; 32 | 26; 32 | 20; 36 | −1; 8 | −8; 6 | −3; 13 | |||||
| 12–19 years | ALL (n:2) | Mean ± SD | 3 ± 1.41 | 5.5 ± 0.71 | 7.5 ± 3.54 | 2.5 ± 0.71 | N/A | 2 ± 4.24 | N/A | 4.5 ± 4.95 | N/A |
| Min; Max | 2; 4 | 5; 6 | 5; 10 | 2; 3 | −1; 5 | 1; 8 | |||||
| 1.5 (n:1) | Mean ± SD | 2 ± N/A | 5 ± N/A | 10 ± N/A | 3 ± N/A | 5 ± N/A | 8 ± N/A | ||||
| Min; Max | 2; 2 | 5; 5 | 10; 10 | 3; 3 | 5; 5 | 8; 8 | |||||
| 1.9 (n:1) | Mean ± SD | 4 ± N/A | 6 ± N/A | 5 ± N/A | 2 ± N/A | −1 ± N/A | 1 ± N/A | ||||
| Min; Max | 4; 4 | 6; 6 | 5; 5 | 2; 2 | −1; −1 | 1; 1 | |||||
Figure 2.
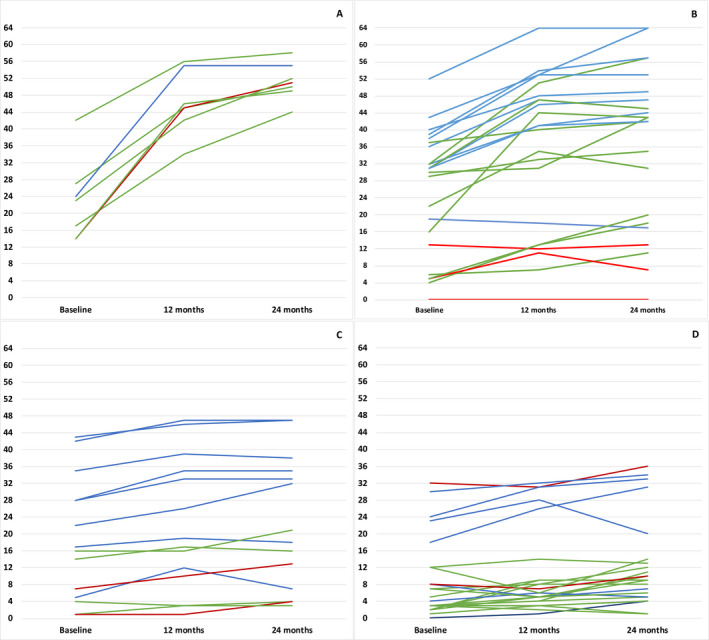
CHOP INTEND individual trajectories over 24 months. (A) <210 days (B) <2 years (C) 2–4 years (D) >4 years. Key to figure: Red line: 1.1; Green line: 1.5; Blue line: 1.9.
HINE – 2
The mean score was 0.88 (±1.33) at baseline, 2.75 (±3.87) at 12 months, and 3.50 (±4.96) at 24 months.
The mean changes were 1.87 (±3.18) between baseline and 12 months, 0.75 (±1.92) between 12 and 24 months, and 2.62 (±4.39) between baseline and 24 months (Table 2, Fig. 3). Table 2 and Figure 3 show details of the scores in the age related and decimal classification subgroups.
Table 2.
Descriptive statistics on age and HINE‐2 scores subdivided by age group and Dubowitz decimal classification.
| HINE ‐II | Scores | Changes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | 24 months | 0–12 | P‐value | 12–24 | P‐value | 0–24 | P‐value | |||
| ALL | ALL (n:68) | Mean ± SD | 0.88 ± 1.33 | 2.75 ± 3.87 | 3.5 ± 4.96 | 1.87 ± 3.18 | <0.001 | 0.75 ± 1.92 | <0.001 | 2.62 ± 4.39 | <0.001 |
| Min; Max | 0; 5 | 0; 16 | 0; 18 | −3; 12 | −3; 8 | −3; 17 | |||||
| 1.1 (n:7) | Mean ± SD | 0.14 ± 0.38 | 1.29 ± 3.4 | 1.29 ± 3.4 | 1.14 ± 3.02 | 0 ± 0 | 1.14 ± 3.02 | ||||
| Min; Max | 0; 1 | 0; 9 | 0; 9 | 0; 8 | 0; 0 | 0; 8 | |||||
| 1.5 (n:36) | Mean ± SD | 0.22 ± 0.64 | 1.5 ± 2.81 | 2.31 ± 4.48 | 1.28 ± 2.75 | 0.81 ± 1.92 | 2.08 ± 4.39 | ||||
| Min; Max | 0; 3 | 0; 10 | 0; 18 | −3; 9 | 0; 8 | −3; 17 | |||||
| 1.9 (n:25) | Mean ± SD | 2.04 ± 1.46 | 4.96 ± 4.4 | 5.84 ± 5.23 | 2.92 ± 3.62 | 0.88 ± 2.17 | 3.8 ± 4.56 | ||||
| Min; Max | 0; 5 | 0; 16 | 0; 16 | −1; 12 | −3; 7 | −2; 12 | |||||
| <210 days | ALL (n:7) | Mean ± SD | 0.86 ± 0.69 | 7.43 ± 3.36 | 10.86 ± 3.58 | 6.57 ± 3.21 | 0.002 | 3.43 ± 2.99 | 0.023 | 10.00 ± 3.46 | <0.001 |
| Min; Max | 0; 2 | 2; 12 | 8; 18 | 2; 11 | 0; 8 | 7; 17 | |||||
| 1.1 (n:1) | Mean ± SD | 1 ± N/A | 9 ± N/A | 9 ± N/A | 8 ± N/A | 0 ± N/A | 8 ± N/A | ||||
| Min; Max | 1; 1 | 9; 9 | 9; 9 | 8; 8 | 0; 0 | 8; 8 | |||||
| 1.5 (n:5) | Mean ± SD | 0.80 ± 0.84 | 6.20 ± 3.03 | 11 ± 4.24 | 5.40 ± 2.88 | 4.80 ± 2.28 | 10.20 ± 4.09 | ||||
| Min; Max | 0; 1 | 2; 10 | 8; 18 | 2; 9 | 2; 8 | 7; 17 | |||||
| 1.9 (n:1) | Mean ± SD | 1 ± N/A | 12 ± N/A | 12 ± N/A | 11 ± N/A | 0 ± N/A | 11 ± N/A | ||||
| Min; Max | 1; 1 | 12; 12 | 12; 12 | 11; 11 | 0; 0 | 11; 11 | |||||
| <2 years | ALL (n:23) | Mean ± SD | 1.39 ± 1.62 | 4.35 ± 4.81 | 5.73 ± 6.09 | 2.95 ± 3.44 | <0.001 | 1.39 ± 2.14 | 0.005 | 4.35 ± 4.82 | <0.001 |
| Min; Max | 0; 5 | 0; 16 | 0; 19 | 0; 12 | 0; 7 | 0; 15 | |||||
| 1.1 (n:3) | Mean ± SD | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||
| Min; Max | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | |||||
| 1.5 (n:11) | Mean ± SD | 0.45 ± 0.69 | 2.00 ± 2.89 | 2.45 ± 3.85 | 1.55 ± 2.54 | 0.45 ± 1.21 | 2.00 ± 3.49 | ||||
| Min; Max | 0; 2 | 0; 8 | 0; 12 | 0; 7 | 0; 4 | 0; 11 | |||||
| 1.9 (n:9) | Mean ± SD | 3 ± 1.32 | 8.67 ± 4.18 | 11.67 ± 4.09 | 5.70 ± 3.17 | 3.00 ± 2.45 | 8.70 ± 3.59 | ||||
| Min; Max | 1; 5 | 5; 16 | 7; 19 | 3; 12 | 0; 7 | 3; 12 | |||||
| 2–4 years | ALL (n:14) | Mean ± SD | 1.00 ± 1.25 | 1.71 ± 2.05 | 2.36 ± 2.89 | 0.72 ± 1.81 | 0.127 | 0.64 ± 1.27 | 0.083 | 1.36 ± 2.34 | 0.039 |
| Min; Max | 0; 3 | 0; 6 | 0; 8 | −3; 5 | 0; 4 | −3; 6 | |||||
| 1.1 (n:2) | Mean ± SD | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||
| Min; Max | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | |||||
| 1.5 (n:4) | Mean ± SD | 0.75 ± 1.50 | 0.25 ± 0.50 | 0.25 ± 0.50 | −0.50 ± 1.73 | 0 ± 0 | −0.50 ± 1.73 | ||||
| Min; Max | 0; 3 | 0; 1 | 0; 1 | −3; 1 | 0; 0 | −3; 1 | |||||
| 1.9 (n:8) | Mean ± SD | 1.38 ± 1.19 | 2.88 ± 2.03 | 4.00 ± 2.88 | 1.50 ± 1.77 | 1.13 ± 1.55 | 2.63 ± 2.13 | ||||
| Min; Max | 0; 3 | 0; 6 | 0; 8 | 0; 5 | 0; 4 | 0; 6 | |||||
| 5–11 years | ALL (n:22) | Mean ± SD | 0.55 ± 1.14 | 0.64 ± 1.33 | 0.45 ± 1.01 | 0.09 ± 0.43 | 0.329 | −0.18 ± 0.66 | 0.213 | −0.09 ± 0.53 | 0.427 |
| Min; Max | 0; 4 | 0; 4 | 0; 4 | −1; 1 | −3; 0 | −2; 1 | |||||
| 1.1 (n:1) | Mean ± SD | 0 ± N/A | 0 ± N/A | 0 ± N/A | 0 ± N/A | 0 ± N/A | 0 ± N/A | ||||
| Min; Max | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | |||||
| 1.5 (n:15) | Mean ± SD | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||
| Min; Max | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | |||||
| 1.9 (n:6) | Mean ± SD | 2.00 ± 1.41 | 2.33 ± 1.63 | 1.67 ± 1.37 | 0.33 ± 0.82 | −0.67 ± 1.21 | −0.33 ± 1.03 | ||||
| Min; Max | 0; 4 | 0; 4 | 0; 4 | −1; 1 | −3; 0 | −2; 1 | |||||
| 12–19 years | ALL (n:2) | Mean ± SD | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | — | ||
| Min; Max | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | |||||
| 1.5 (n:1) | Mean ± SD | 0 ± N/A | 0 ± N/A | 0 ± N/A | 0 ± N/A | 0 ± N/A | 0 ± N/A | ||||
| Min; Max | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | |||||
| 1.9 (n:1) | Mean ± SD | 0 ± N/A | 0 ± N/A | 0 ± N/A | 0 ± N/A | 0 ± N/A | 0 ± N/A | ||||
| Min; Max | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | 0; 0 | |||||
Figure 3.
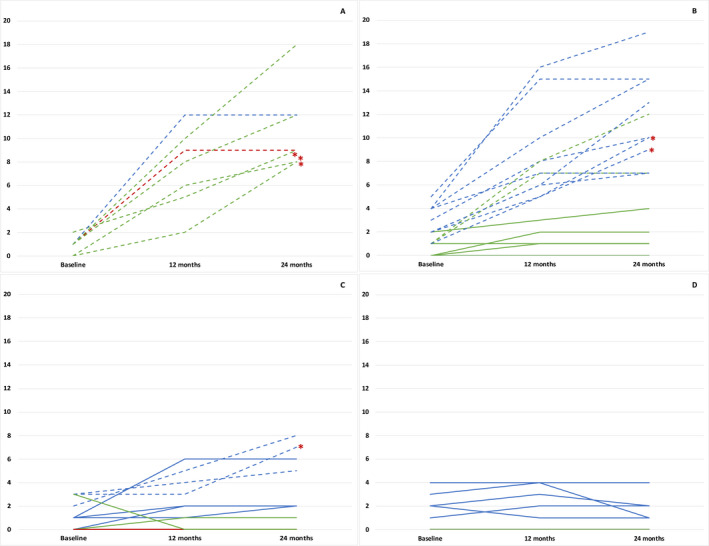
HINE‐2 individual trajectories over 24 months. (A) <210 days (B) <2 years (C) 2–4 years (D) >4 years. Key to figure: Red line: 1.1; Green line: 1.5; Blue line: 1.9. Dashed line: Sitters; Plain line: Nonsitter. Red asterisks: patients who achieved sitting position 24 months from treatment initiation.
Statistical analysis
For both CHOP and HINE2 repeated measures ANOVA showed a significant difference between baseline and 24‐month scores for the whole group (P < 0.001). Using Bonferroni post hoc test, the difference was also significant between baseline and 12‐month scores (P < 0.001) and between 12 and 24 months (P < 0.001).
When age subgroups were considered, on the CHOP INTEND the difference was significant between baseline and 24 months in all age subgroups. Table 1 shows details of the difference between baseline and 12 months and between 12 and 24 months in the different age subgroups.
On the HINE 2, the difference between baseline and 24 months, between baseline and 12 months was significant in patients < 210 days and between 210 days and 2 years but not in those older than 2 years. (Table 2).
Using mixed effect linear regression analysis, we found that age was predictive of changes on both scales (P < 0.05), whereas SMN2 copy number and decimal classification were not.
Sitting position
Of the 68 patients in whom 24‐month data were available, 21 reached the sitting position. In 16 of the 21 sitting was reached in the first year of treatment and in six in the second year. Sitting was achieved in all the seven patients treated before 210 days of age (100%), in 11 of the 23 treated between 210 days and 2 years (55%), and in 3 of the 14 treated between 2 and 4 years of age (17.64%) (Table 3, Fig. 4).
Table 3.
Baseline characteristics of nonsitters and sitters patients.
| Whole cohort | <210 days | <2 years | 2–4 years | |||||
|---|---|---|---|---|---|---|---|---|
| Nonsitter (N = 47) | Sitter (N = 21) | Nonsitter (N = 0) | Sitter (N = 7) | Nonsitter (N = 12) | Sitter (N = 11) | Nonsitter (N = 11) | Sitter (N = 3) | |
| Age at treatment (years) | ||||||||
| Mean (SD) | 5.32 (±3.94) | 0.90 (±0.70) | N/A | 0.26 (±0.14) | 1.13 (±0.50) | 0.95 (±0.37) | 3.12 (±0.96) | 2.23 (±0.13) |
| Min‐Max | 0.53–15.92 | 0.02–2.36 | N/A | 0.02–0.45 | 0.53–1.96 | 0.21–1.78 | 2.05–4.70 | 2.11–1.78 |
| CHOP‐INTEND (scores) | ||||||||
| Mean (SD) | 11.72 (±10.76) | 32.33 (±10.17) | N/A | 23.00 (±9.80) | 15.50 (±12.05) | 36.82 (±6.59) | 13.64 (±11.31) | 37.67 (±8.39) |
| Min‐Max | 0–37 | 14–52 | N/A | 14–27 | 0–37 | 31–52 | 1–35 | 28–43 |
| HINE – II (scores) | ||||||||
| Mean (SD) | 0.45 (±0.95) | 2.05 (±3.14) | N/A | 0.86 (±0.69) | 0.25 (±0.62) | 2.64 (±1.43) | 0.54 (±0.93) | 2.67 (±1.58) |
| Min‐Max | 0–4 | 0–5 | N/A | 0–1 | 0–2 | 1–5 | 0–3 | 1–3 |
| Decimal classification (N) | ||||||||
| 1.1 | 6/47 (12.77%) | 1/21 (4.76%) | N/A | 1/7 (14.28%) | 3/12 (25.00%) | 0/12 (0.00%) | 2/11 (18.18%) | 0/3 (0.00%) |
| 1.5 | 29/47 (61.70%) | 7/21 (33.33%) | N/A | 5/7 (71.43%) | 6/12 (50.00%) | 2/12 (16.66%) | 7/11 (63.63%) | 0/3 (0.00%) |
| 1.9 | 12/47 (25.53%) | 13/21 (61.91%) | N/A | 1/7 (14.28%) | 0/12 (0.00%) | 9/12 (75.00%) | 5/11 (35.72%) | 3/3 (100.00%) |
Figure 4.
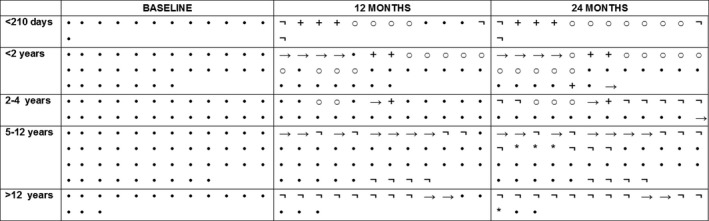
Individual details on sitting position from baseline to 24 months. ○: sitter •: nonsitter →: transferred; +:deceased; : nusinersen discontinuation; *: enrollment in a clinical trial.
This percentage is higher than that reported in the ENDEAR study, 7 but the data cannot be easily compared for several reasons. First the duration of the follow‐up in our study was longer than in ENDEAR and in four of the seven sitting was achieved in the second year. Furthermore, we only considered patients who completed the 24‐month follow‐up and we were unable to retrieve information on a number of patients lost at follow‐up because they entered clinical trials or, when Nusinersen became commercially available in Italy, decided to move to a local center closer to their residence than the EAP centers.
Other motor progresses
Of the 21 patients who reached the sitting position, 10 were also able to half roll to both sides independently, five to roll from prone to supine and/or viceversa, one was able to maintain “prone on elbows” position independently, one was able to maintain four‐point kneeling independently, one was able to stand with support, one was able to walk with support, and one was able to walk independently.
Survival and Respiratory support
Of the 125 patients enrolled in the Italian EAP project, six died within the first year and one in the second year (Fig. 5).
Figure 5.
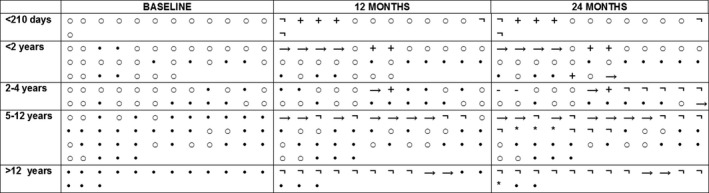
Individual details on ventilator status from baseline to 24 months. ○: Spontaneous breathing or NIV <16 h; •: NIV ≥16 h or tracheostomy; →: transferred; +:deceased; : nusinersen discontinuation; *: enrollment in a clinical trial.
At baseline, of the 68 patients reported in this study, 20 patients had a tracheostomy and seven used noninvasive ventilation for more than 16 h/day. At 24 months, 23 patients had a tracheostomy and eight used noninvasive ventilation for more than 16 h/day. There was no difference between baseline and 24 months (P = 0.862).
Nutritional support
At baseline, 32 patients were fed orally, and 36 had tube feeding.
At 24 months, 24 patients remained orally fed, and 44 had tube feeding. Seven of the eight who had a tube inserted after baseline were still able to swallow and eat solids or semi‐solids orally. There was no difference between baseline and 24 months (P = 0.222).
Discussion
The availability of different therapeutical options has highlighted the need to have reliable data on the short‐ and long‐term data in order to better understand the efficacy of each of the individual approaches and of the possible effect of combinational therapies or therapeutical changes. We report the results of motor functional changes over 2 years in a cohort of infantile‐onset SMA patients treated with Nusinersen, including patients with a wider spectrum of severity and age compared to the pivotal study in infantile‐onset SMA. 7 We recently reported preliminary data in this real‐world cohort showing a significant increase in functional scores after 12 months of treatment. 5 After an additional year of treatment, the difference between baseline and 2 years was still significant on both CHOP INTEND and HINE scores (P < 0.001). The improvement between the first and the second year after treatment initiation, even if smaller than that observed between baseline and 12 months, was also significant. This held true even when age subgroups were considered. In all the subgroups, there was an improvement in CHOP INTEND scores in the second year after treatment initiation. The difference was always significant with the exception of the subgroup treated between 2 and 4 years of age. Interestingly, in the subgroup treated between 5 and 11 years of age the difference did not reach significance in the first year after treatment initiation but reached significance in the second year, suggesting that in this age group more time may be needed to see a significant effect.
Even if the changes were more obvious in the type 1.9 patients, the severity of phenotype, expressed using the decimal scale, did not appear to predict the CHOP INTEND changes.
On the HINE2, a module designed to assess the development of motor milestones, there was also a difference between baseline and 24 months for the whole cohort, but it is not surprising that the changes were mostly observed in younger patients, up to the age of 4 years. In older patients, who are in a more chronic phase, are often full time ventilated and have very little residual functional abilities, often associated with contractures, even the partial achievement of a milestone can be more challenging than acquiring single aspects of functional abilities as detected by the CHOP INTEND. This is also reflected by the number of patients who achieved independent sitting. All the seven patients treated before the age of 210 days who had a 24‐month follow‐up achieved independent sitting and reported an increase in the HINE‐2 (mean: 10.00, SD: 3.50, range: 7–17).
On the other hand, we also included type 1.1 patients, that is, infants with neonatal onset who were excluded by the pivotal study.
The percentage of infants achieving independent sitting was still high (55%) in the group treated between 6 and 24 months of age who completed the 24‐month follow‐up. A recent study reported that several factors, including baseline values, may help to predict the achievement of sitting. 1 In their study, Aragon‐Gawinska and coworkers described that sitting was achieved in 31% of patients treated with nusinersen. The results are not easily comparable with our data as in their study the mean age treatment initiation was 21–23 months and no details of changes according to age were provided. In our cohort infants below 210 days achieved sitting, irrespective of their baseline values, whereas in those treated after 210 days months, sitting was more often achieved in the infants with higher CHOP INTEND scores. It is of note that sitting was only achieved in a few patients older than 2 years, all originally classified as 1.9, with relatively higher CHOP INTEND scores (28; 43; 42) at baseline.
Both our results and those from the previous study appear to indicate that while, as also observed in recent clinical trials, 7 , 15 age is an important predictor of response to treatment, functional level should also be considered. This is particularly true for infants with neonatal onset, who were not included in the clinical trials, who are already severely affected at a very early age and may have a lower chance of showing dramatic improvements compared to infants with the same age but higher functional scores.
Our prospective study was mainly focused on motor aspects but some information on respiratory and feeding function could be extracted by clinical notes. At variance with motor function, there was no obvious improvement, with no significant differences between baseline and 24 months after treatment. While this is partly expected in the older patients who were in a more chronic stable phase, it is of note that, even within young patients, the number of those who died or required tracheostomy or NIV >16 h after treatment started was relatively small compared to the natural history data. Similarly, no difference was found in feeding and while there was an increase in the number of patients who required tube feeding, in most of them this was mainly due to failure to thrive as the ability to eat by mouth was preserved. These aspects are being further investigated developing new tools providing more systematic assessments.
In conclusion, our results suggest that in infants with the infantile form of SMA treated with nusinersen, some improvement of motor function can be observed even after the first year of treatment. This is more obvious in the infants treated before the age of 24 months, but some improvement can also be observed in children treated at an older age. Unfortunately, at the time the study was performed, there were no specific tools for older weak type 1 patients and these were assessed with CHOP INTEND that had been designed for younger patients. New more appropriate tools are now being developed and they will hopefully provide a better understanding of possible changes.
Despite these limitations, our results can be of help at the time new therapeutic options are becoming available. As an increasing number of patients are considering switching to a new treatment or, when possible, to add one of the new treatments to nusinersen, the information on the long‐term effect of Nusinersen in different age groups reported in our paper will help to better understand the difference with the functional changes observed following the introduction of a new or concomitant drug. More generally, as the number of patients who undergo treatment with nusinersen or the other available drugs is considerably high, especially among new diagnosis (between 80% and 90% in the centers contributing to our registry 16 ), these data will contribute to describe the “new natural history” in treated patients and will help and modify the approach to care according to the new phenotypes.
Authors' Contributions
Marika Pane, Giorgia Coratti, Valeria A Sansone, Sonia Messina, Claudio Bruno, Enrico Bertini, and Eugenio Mercuri had a major role in the conception and design of the study, in the analysis and interpretation of data and in the drafting of the work for important intellectual content; Michela Catteruccia, Maria Sframeli, Emilio Albamonte, Marina Pedemonte, Adele D'Amico, Beatrice Berti, Concetta Palermo, Daniela Leone, Giorgia Brigati, Simona Lucibello, Maria Carmela Pera, and Gianluca Vita coordinated the work in within each center and had a major role in the interpretation of the data and in the drafting of the work for important intellectual content; Chiara Bravetti, Paola Tacchetti, Francesca Salmin, Roberto De Sanctis, Marco Piastra, Orazio Genovese, and Francesco Danilo Tiziano had a major role in the acquisition and the interpretation of data and in the review of the work for important intellectual content.
Conflict of Interest
GC, RDS, MP, SM, ADA, EB, VAS, CB, and EM has been a consultant for BIOGEN S.R.L. which owns patent rights to nusinersen that was used in this study.
Supporting information
Appendix S1. Italian EAP working group.
Acknowledgment
The authors gratefully acknowledge Famiglie SMA for supporting the Italian SMA Network (Telethon UILDM project GSP 13002).
A full list of the Italian EAP working group is provided in the supplemental file.
Funding Information
This work was supported by Fondazione Telethon, (Grant/Award Number: 'GSP 13002').
Funding Statement
This work was funded by Fondazione Telethon grant GSP 13002.
REFERENCES
- 1. Aragon‐Gawinska K, Daron A, Ulinici A, et al. Sitting in patients with spinal muscular atrophy type 1 treated with nusinersen. Dev Med Child Neurol 2020;62:310–314. [DOI] [PubMed] [Google Scholar]
- 2. Aragon‐Gawinska K, Seferian AM, Daron A, et al. Nusinersen in patients older than 7 months with spinal muscular atrophy type 1: a cohort study. Neurology 2018;91:e1312–e1318. [DOI] [PubMed] [Google Scholar]
- 3. Pechmann A, Langer T, Schorling D, et al. Evaluation of children with SMA type 1 under treatment with nusinersen within the expanded access program in Germany. J Neuromuscul Dis 2018;5:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pechmann A, Langer T, Wider S, Kirschner J. Single‐center experience with intrathecal administration of Nusinersen in children with spinal muscular atrophy type 1. Eur J Paediatr Neurol 2018;22:122–127. [DOI] [PubMed] [Google Scholar]
- 5. Pane M, Coratti G, Sansone VA, et al. Nusinersen in type 1 spinal muscular atrophy: twelve‐month real‐world data. Ann Neurol 2019;86:443–451. [DOI] [PubMed] [Google Scholar]
- 6. Finkel R, Kirschner J, Mercuri E, et al. Longer‐term effects of Nusinersen on notor funciton outcomes based on age at treatment initiation. Neuromuscul Disord 2020;30:s123. [Google Scholar]
- 7. Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile‐onset spinal muscular atrophy. N Engl J Med 2017;377:1723–1732. [DOI] [PubMed] [Google Scholar]
- 8. Messina S, Pane M, Sansone V, et al. Expanded access program with Nusinersen in SMA type I in Italy: strengths and pitfalls of a successful experience. Neuromuscul Disord 2017;27:1084–1086. [DOI] [PubMed] [Google Scholar]
- 9. Pane M, Palermo C, Messina S, et al. Nusinersen in type 1 SMA infants, children and young adults: preliminary results on motor function. Neuromuscul Disord 2018;28:582–585. [DOI] [PubMed] [Google Scholar]
- 10. Glanzman AM, Mazzone E, Main M, et al. The Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord 2010;20:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glanzman AM, McDermott MP, Montes J, et al. Validation of the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND). Pediatr Phys Ther 2011. Winter;23:322–326. [DOI] [PubMed] [Google Scholar]
- 12. Haataja L, Mercuri E, Regev R, et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr 1999;135(2 Pt 1):153–161. [DOI] [PubMed] [Google Scholar]
- 13. Glanzman AM, Mazzone ES, Young SD, et al. Evaluator training and reliability for SMA global Nusinersen trials1. J Neuromuscul Dis 2018;5:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubowitz V. Chaos in classification of the spinal muscular atrophies of childhood. Neuromuscul Disord 1991;1:77–80. [DOI] [PubMed] [Google Scholar]
- 15. Mendell JR, Al‐Zaidy S, Shell R, et al. Single‐dose gene‐replacement therapy for spinal muscular atrophy. N Engl J Med 2017;377:1713–1722. [DOI] [PubMed] [Google Scholar]
- 16. Mercuri E, Finkel R, Scoto M, et al. Development of an academic disease registry for spinal muscular atrophy. Neuromuscul Disord 2019;29:794–799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Italian EAP working group.


