Abstract
Objective
Recent studies demonstrated cutaneous phosphorylated α synuclein (p‐syn) deposition in idiopathic and some monogenetic Parkinson disease (PD) patients, suggesting synucleinopathy identical to that in the brain. Although the LRRK2 Gly2385Arg (G2385R) variant is a common PD risk factor in the Chinese population, the pathogenesis of PD with G2385R variant has not been reported. We investigated whether synucleinopathy and small fiber neuropathy (SFN) are associated with the G2385R variant.
Methods
We performed genotyping in 59 PD patients and 30 healthy controls from the skin biopsy database. The scale of SFN was assessed, as well as bright‐field immunohistochemistry against antiprotein gene product 9.5 (PGP9.5) and double‐labeling immunofluorescence with anti‐PGP9.5 and anti‐p‐syn.
Results
(1) p‐syn deposited in the skin nerve fibers of G2385R carrier PD patients, which was a different pattern from noncarriers, without no difference observed between proximal and distal regions; (2) decreased distal intraepidermal nerve fiber density was found in both the G2385R carrier and the noncarrier PD group, and was negatively correlated with composite autonomic symptom score‐31 item (COMPASS‐31) scores; (3) PD patients with the G2385R variant showed a more peculiar clinical profile than noncarriers with a higher nonmotor symptoms scale, COMPASS‐31 score, and levodopa equivalent dose, in addition to an increased prevalence of certain autonomic symptoms or rapid eye movement sleep behavior disorders.
Interpretation
Synucleinopathy is related to the LRRK2 G2385R genotype and implies a different pathogenesis in G2385R variant carriers and noncarriers. This study also extended the clinical profiles of PD patients with the G2385R variant.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease characterized by abnormal aggregation of α‐synuclein (α‐syn) forming Lewy bodies and neuronal loss in the substantia nigra pars compacta (SNpc). The gold standard criteria for the diagnosis of PD are based on clinical and pathological findings. 1 , 2 While most cases of PD with the confirmed genetic risks may not include α‐synuclein pathology (synucleinopathy), 3 this challenges the current notion that α‐syn aggregation is required for a pathological diagnosis of PD.
Mutations in the leucine‐rich repeat kinase 2 (LRRK2) gene are the most frequent cause of familial autosomal‐dominant PD, mostly producing a α‐synuclein‐type neuropathology. 4 , 5 The LRRK2 variations are also common risk factors of sporadic PD, among them the Gly2385Arg (G2385R, p.2385G>R) variant is the most common PD risk factor in the Asian population, which is known to increase the risk of PD by nearly 2‐fold in the Chinese population. 6 LRRK2 G2385R variant carriers are more likely to have a higher levodopa equivalent dose (LED) and specific motor and nonmotor phenotypes compared to idiopathic PD (iPD), including postural instability and gait dysfunction, motor fluctuations, rapid eye movement sleep behavior disorders (RBD), and fatigue 7 , 8 ; this indicates that LRRK2 G2385R variants have unique PD phenotypes. Nevertheless, neither an autopsy nor a biopsy with the LRRK2 G2385R variant has been reported; consequently, the pathogenesis of PD with the LRRK2 G2385R variant has not yet been reported.
In recent decades, cutaneous phosphorylated α synuclein (p‐syn) deposition and small fiber neuropathy (SFN) have been demonstrated in iPD patients by different research groups, suggesting potential in vivo biomarkers of PD. 9 , 10 , 11 , 12 , 13 A similar peripheral synucleinopathy was also reported in a couple of monogenetic PD patients, such as SNCA (α‐synuclein), PARK2 (parkin), and PARK7 (DJ‐1), corresponding to the Lewy body pathology in the brain. 14 , 15 , 16 These findings imply that a skin biopsy may be a useful tool in studying the peripheral pathogenesis of idiopathic and genetic PD.
In this study, we investigated whether synucleinopathy and small fiber degeneration was associated with the LRRK2 G2385R variant, and examined the relationship between them.
Materials and Methods
Subjects and clinical assessment
Fifty‐nine patients diagnosed with clinically established PD according to the Movement Disorder Society clinical diagnostic criteria for Parkinson’s disease (MDS PD Criteria), 17 who agreed to undergo a skin biopsy, were enrolled from the Department of Neurology in the First Affiliated Hospital of Zhengzhou University between January 2019 and December 2019. Thirty healthy subjects were recruited from the relatives and colleagues of the organizers, or volunteers. All subjects underwent a neurological examination, serological testing, and neuroimaging evaluation. Subjects were excluded if they had clinical symptoms or signs of large fiber neuropathy, or conditions potentially causing peripheral neuropathy such as diabetes, glucose intolerance, thyroid dysfunction, vitamin B12 or folic acid deficiency, hepatic or renal failure, HIV, connective tissue disorders, monoclonal gammopathy, or a history of specific medications.
Demographic data including age, gender, body mass index (BMI), age of onset, disease duration, symptoms of onset, and medication were recorded. The Movement Disorder Society Unified Parkinson’s Disease Ranking Scale part III (MDS‐UPDRS‐III) 18 was used to assess motor function. The severity of the disease was graded using the Hoehn and Yahr scale. 19 The SFN symptoms inventory questionnaire (SFN‐SIQ), 20 composite autonomic symptom score‐31 items (COMPASS‐31), 21 nonmotor symptoms scale (NMSS), 22 and mini‐mental state examination scale (MMSE) were used to evaluate SFN and other nonmotor symptoms. Information on response to levodopa (L‐Dopa), LED, and RBD was collected from a self‐report and patients’ family members. The criteria of responding well to L‐dopa were marked improvement with dose increases or marked worsening with dose decreases according to 2015 MDS PD Criteria. 17 Briefly, it can be either objectively (defined as >30% in UPDRS III with change in treatment), or subjectively with a clear history of marked changes provided by a reliable patient or caregiver.
The procedures in this study were approved by the human ethics committee at the First Affiliated Hospital of Zhengzhou University and followed the Helsinki Declaration regarding international clinical research involving human beings. All the subjects provided their written informed consent.
Genetic analysis
Genomic DNA was extracted from peripheral blood using the QIAamp DNA Blood Maxi Kit (QIAGEN, Valencia, CA). The LRRK2 G2385R variant was amplified by PCR with 5’‐TGCAGCTTTCAGTGATTCCA‐3’ (forward primer) and 5’‐TCAAAGCTAGCAGAGACTCCA‐3’ (reverse primer). All reactions were conducted in 25 μL reaction mixtures and by the following PCR protocol: 95°C for 5 min; 94°C for 30 sec, 66°C for 30 sec, 72°C for 30 sec, 15 cycles; 94°C for 30 sec, 51°C for 30 sec, 72°C for 30 sec, 25 cycles; 72°C for 7 min; 4°C forever. The PCR products were sequenced using an ABI 3730XL automated DNA sequencer (Applied Biosystems, Thermo Fisher Scientific Corp., Waltham, MA, USA) and analyzed with SeqMan software (ver.7.1). The G2385R mutation was known according to the LRRK2 sequence (rs.34778348 G>A).
Skin biopsy
Skin biopsies were taken with a 3‐mm skin punch at the distal leg (DL) (10 cm above the external ankle) and cervical C7 paravertebral area according to the previously described procedure. 9 Skin samples were taken from the more affected side, and were fixed in 4°C cold Zamboni (G2190, Solarbio, Beijing, China) for 24–48 h. Fifty‐micrometer‐thick serial sections were cut using a freezing microtome (Leica CM1950, Wetzlar, Germany). Free‐floating bright‐ and dark‐field immunohistochemistry was conducted separately, as described below.
Skin innervation
Free‐floating bright‐field immunohistochemistry was performed for skin innervation, as previously described. 23 For each patient, four serial 50 μm thickness sections, 200 μm apart, were incubated in pan‐axonal marker mouse antiprotein gene product 9.5 (PGP9.5) (1:1000, BIO‐RAD, Raleigh, NC, USA) overnight. The sections were washed and incubated in horse anti‐mouse IgG (1:50, Vectastain Elite ABC kits, Burlingame, CA, USA) followed by Avidin/Biotinylated HRP (1:50, Vectastain Elite ABC kits, USA) incubation, and were colored with sweat glands (SG) peroxidase substrate reaction (Vector SG kits, USA). The intraepidermal nerve fiber density (IENFD) was counted as the number of fibers crossing the dermal‐epidermal junction per mm with high magnification light microscopy (400×; Nikon ECLIPSE Ni‐U, Tokyo, Japan), according to a previous protocol. 23
Cutaneous phosphorylated α‐syn detection
Four additional 50 μm thickness sections at 200 μm interval from each skin samples were double‐immunostained with the following primary antibodies for detecting p‐syn deposition, including mouse anti‐PGP9.5 (1:2000) and rabbit anti‐alpha‐synuclein phosphorylated at Ser 129 (1:500, ab51253, Abcam, Cambridge, UK). The immunostaining should be repeated when negative p‐syn signal was found for the first time. All double‐immunostained sections were viewed with fluorescence microscopy (Nikon ECLIPSE Ni‐U, Tokyo, Japan) at high magnification (400×). Positive p‐syn nerve fibers were defined only when anti‐p‐syn immunostaining alpha‐synuclein depositions were co‐localized with anti‐PGP9.5 immunostaining nerve fibers.
Statistical analyses
Statistical analyses were carried out using SPSS Statistics 25 software for windows (IBM, Armonk, NY, USA). Data are expressed as mean ± standard deviation or percentages. Independent sample T test or the Mann–Whitney U test were used to analyze continuous variables. We used Pearson χ 2 (n ≥ 40 and T ≥ 5), continuous correction (n < 40 or T < 1), or Fisher’s exact probabilities (n ≥ 40 and 1 ≤ T ≤ 5) to test the categorical variables. Correlation was accessed by Pearson or Spearman linear correlation analysis. A significance level of 0.05 (two‐tailed) was set.
Results
Demographics and clinical manifestation
The DNA samples were analyzed for the frequency of the LRRK2 G2385R (c.7153G>A) mutation in 59 patients with PD. Among them, 12 subjects (20.3%) were identified as carrying the LRRK2 G2385R variant. Demographics including age, gender, and BMI were equivalent in G2385R carriers, G2385R noncarriers, and healthy controls (HC). Age of onset, disease duration, Hoehn and Yahr stage, MDS‐UPDRS‐Ⅲ score, annual UPDRS‐III progression, MMSE score, percentage of patients with onset symptoms, percentage of patients with nonmotor symptoms, and percentage of patients responding well to L‐dopa were also comparable between G2385R carrier and noncarrier groups. Nevertheless, the NMSS score (56.92 ± 21.37 vs. 29.87 ± 22.56, P < 0.001), percentage of patients with RBD (8/12 [66.7%] vs. 11/47 [23.4%], P = 0.012), and LED (512.5 ± 190.54 vs. 346.01 ± 138.27, P = 0.001) in the G2385R carrier group were higher than those in the noncarrier group (Table 1).
Table 1.
Demographic and clinical characteristics of PD patients and healthy controls.
| G2385R carriers | G2385R non‐carriers | Healthy controls | P value | |
|---|---|---|---|---|
| n = 12 | n = 47 | n = 30 | ||
| Age (years) | 63.00 ± 7.92 | 59.91 ± 8.84 | 58.73 ± 8.33 | 0.365 1 , 0.137 2 , 0.274 3 |
| Gender, male, n (%) | 4 (33.3%) | 23 (48.9%) | 16 (53.3%) | 0.333 1 , 0.241 2 , 0.707 3 |
| BMI | 25.25 ± 4.39 | 25.09 ± 3.16 | 25.05 ± 2.98 | 0.883 1 , 0.887 2 , 0.961 3 |
| Age of onset (years) | 57.83 ± 9.27 | 55.89 ± 9.70 | / | 0.665 |
| Symptoms of onset | ||||
| Resting tremor, n (%) | 5 (41.7%) | 21 (44.7%) | / | 0.851 |
| Bradykinesia‐rigidity, n (%) | 4 (33.3%) | 19 (40.4%) | / | 0.906 4 |
| Mixed symptoms, n (%) | 2 (16.7%) | 5 (10.64%) | / | 0.939 4 |
| Others, n (%) | 1 (8.33%) | 2 (4.26%) | / | 1.000 4 |
| Disease duration (years) | 5.17 ± 4.24 | 4.12 ± 3.10 | / | 0.461 |
| Hoehn & Yahr stage | 2.58 ± 0.73 | 2.35 ± 0.62 | / | 0.132 5 |
| MDS‐UPDRSⅢ score | 52.17 ± 26.29 | 36.55 ± 17.82 | / | 0.072 |
| Annual UPDRS‐III progression | 13.33 ± 6.50 | 15.57 ± 18.57 | / | 0.684 |
| Non‐motor symptoms, n (%) | 12 (100%) | 46 (97.87%) | / | 1.000 6 |
| NMSS score | 56.92 ± 21.37 | 29.87 ± 22.56 | / | <0.001 * |
| RBD, n (%) | 8 (66.7%) | 11 (23.4%) | / | 0.0124 |
| MMSE score | 27.42 ± 1.62 | 26.40 ± 2.10 | / | 0.121 |
| Response well to L‐Dopa, n (%) | 9 (75.0%) | 26 (55.3%) | / | 1.000 4 |
| LED (mg/day) | 512.5 ± 190.54 | 346.01 ± 138.27 | / | 0.001 * |
Others: weakness, pain, or constipation; PD, Parkinson disease; BMI, body mass index; MDS‐UPDRSIII, Movement Disorder Society Unified Parkinson’s Disease Ranking Scale part III; NMSS, Nonmotor Symptoms Scale; RBD, rapid eye movement sleep behavior disorder; LED, levodopa equivalent dose; /, none.
indicates the significant difference.
G2385R carriers versus G2385R noncarriers.
G2385R carriers versus Healthy Control.
G2385R noncarriers versus Healthy Control.
Continuous correction chi‐square test.
Mann–Whitney U test.
Fisher’s exact test.
SFN in G2385R carrier and noncarrier PD patients
SFN‐SIQ and COMPASS‐31 were used to assess the symptoms of SFN, focusing on total SFN and autonomic disorders, respectively. An SFN‐SIQ score >2 was considered positive SFN symptoms. The total percentage of patients with SFN symptoms was 12/12 (100%) in G2385R carriers, which was comparable with that in noncarriers 37/47 (78.7%) (Table 2). Both groups had a higher prevalence of SFN than HC (1/30, 3.33%; both P < 0001). The percentage of patients with independent paresthesia or autonomic symptoms was not different between the G2385R carrier and noncarrier group (Table 1).
Table 2.
Symptoms of small fiber neuropathy in LRRK2 G2385R variants carrier and noncarrier PD patients
| Whole group | G2385R carriers | G2385R non‐carriers | P value | |
|---|---|---|---|---|
| N = 59 | n = 12 | n = 47 | ||
| Paresthesia, n (%) | 27 (45.8%) | 7 (58.3%) | 20 (42.6%) | 0.327 |
| Sensitive skin at distal leg, n (%) | 3 (5.1%) | 1 (8.3%) | 2 (4.3%) | 0.372 1 |
| Burning feet, n (%) | 8 (13.6%) | 3 (25.0%) | 5 (10.6%) | 0.410 1 |
| Sheet intolerance, n (%) | 13 (22.0%) | 4 (33.3%) | 9 (19.1%) | 0.504 1 |
| Restless legs, n (%) | 18 (30.5%) | 5 (41.7%) | 13 (27.7%) | 0.347 |
| Autonomic disturbance, n (%) | 50 (84.7%) | 12 (100%) | 38 (80.9%) | 0.181 2 |
| Orthostatic intolerance, n (%) | 9 (15.3%) | 3 (25.0%) | 6 (12.8%) | 0.0471 |
| Vasomotor symptoms, n (%) | 2 (3.4%) | 1 (8.3%) | 1 (2.1%) | 0.105 1 |
| Secretomotor symptoms, n (%) | 36 (61.0%) | 11 (91.7%) | 25 (53.2%) | <0.0011 |
| Gastrointestinal symptoms, n (%) | 34 (57.6%) | 9 (75.0%) | 25 (53.2%) | 0.0021 |
| Bladder symptoms, n (%) | 17 (28.8%) | 7 (58.3%) | 10 (21.3%) | <0.001 * |
| Pupillomotor symptoms, n (%) | 17 (28.8%) | 4 (33.3%) | 13 (27.7%) | 0.539 1 |
| Small fiber neuropathy, n (%) | 49 (83.1%) | 12 (100%) | 37 (78.7%) | 0.105 |
| SFN‐SIQ score | 6.16 ± 2.80 | 6.42 ± 3.87 | 4.45 ± 3.60 | 0.130 |
| Compass‐31 score | 10.77 ± 11.23 | 16.57 ± 11.11 | 9.28 ± 10.88 | 0.044 * |
| Orthostatic intolerance score | 2.03 ± 6.31 | 4.67 ± 8.50 | 2.47 ± 7.00 | 0.357 |
| Secretomotor score | 3.92 ± 3.76 | 6.25 ± 2.50 | 3.33 ± 3.81 | 0.004 * |
| Gastrointestinal score | 2.77 ± 2.73 | 3.42 ± 2.46 | 2.60 ± 2.78 | 0.358 |
| Bladder score | 0.66 ± 1.24 | 1.48 ± 1.66 | 0.45 ± 1.03 | 0.061 |
LRRK2, leucine‐rich repeat kinase 2; PD, Parkinson disease; SFN‐SIQ, Small Fiber Neuropathy Symptoms Inventory Questionnaire; Compass‐31, the Composite Autonomic Symptom Score.
indicates the significant difference.
Continuous correction chi‐square test.
Fisher’s exact test.
The COMPASS‐31 scores of G2385R carriers were significantly higher than those of noncarriers, although no difference in the SFN‐SIQ scores was found (Table 1). The SFN‐SIQ or COMPASS‐31 scores in both the carrier and noncarrier group were significantly higher than those in HC (SFN‐SIQ: carrier vs. HC, 6.42 ± 3.87 vs. 0.27 ± 0.64, P < 0.001; noncarrier vs. HC, 4.45 ± 3.60 vs. 0.27 ± 0.64, P < 0.001; COMPASS‐31: carrier vs. HC, 16.57 ± 11.11 vs. 0.44 ± 1.10, P < 0.001; noncarrier vs. HC, 9.28 ± 10.88 vs. 0.44 ± 1.10) (Fig. 2A). Pearson linear correlation analysis demonstrated that the SFN‐SIQ score was positively correlated with the COMPASS‐31 score (ρ = 0.706, P < 0.001, Spearman correlation analysis) (Fig. 2B).
Figure 2.
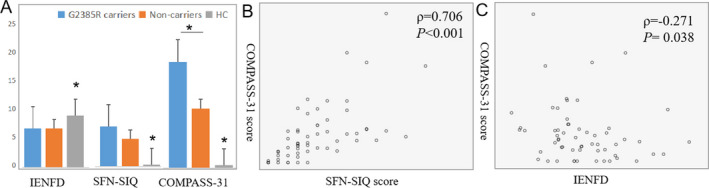
The correlation between IENFD, SFN‐SIQ and COMPASS‐31 in PD patients and healthy controls (HC). (A) The IENFD and SFN‐SIQ score in G2385R carriers was comparable with that in noncarrier group, P values were 0.966 and 0.130, respectively; whereas the COMPASS‐31 score was significantly higher than noncarrier group, P = 0.044; the IENFD at distal legs was decreased in carriers and noncarrier PD compared to HC, P = 0.01; SFN‐SIQ and COMPASS‐31 scores in both groups were significantly higher than HC, both P < 0.001. Spearman correlation analysis demonstrated a moderately positive correlation between SFN‐SIQ and COMPASS31 score (B); and a mildly negative correlation IENFD and COMPASS31 score (C). Carriers n = 12, noncarriers n = 47, HC n = 30, Error bars: +SE. IENFD, intraepidermal nerve fiber density; SFN‐SIQ, small fiber neuropathy symptoms inventory questionnaire; COMPASS‐31, composite autonomic symptom score‐31 items; PD, Parkinson disease. * indicates the significant difference between two or three groups.
The items/domains in the SFN‐SIQ and COMPASS‐31 were further analyzed. Each item of paresthesia was not different between the G2385R carrier and noncarrier group, including sensitive skin at the DL, burning feet, sheet intolerance, and restless legs (Table 2). One of the relatively more prevalent somatic symptoms was restless legs; the percentage in carriers was 5/12 (41.7%) and in noncarriers was 13/47 (27.7%). In addition, the prevalence of certain autonomic items in the carrier group was significantly higher than that in the noncarrier group, such as orthostatic intolerance, secretomotor symptoms, gastrointestinal symptoms, and bladder symptoms. Among these, the scores of secretomotor symptoms in the carrier group were also higher than that in the noncarrier group (6.25 ± 2.50 vs. 3.33 ± 3.81, P = 0.004) (Table 1).
Cutaneous innervation and IENFD
Under bright‐field immunohistochemisty, PGP9.5‐positive nerve fibers branching from subepidermal nerve plexus (SP), crossing the basal membrane formed intraepidermal nerve fibers (IENF) (Fig. 1Aa–Ca). Dermal nerve bundles and skin annexes including SG, blood vessels (BV), arrector pili muscles (APM), and the occasional hair follicle were also innervated by PGP9.5‐positive skin fibers. PGP9.5 immunostained SG demonstrated unique tubular or acinar structure (Fig. 1Ac–Cc), APM showed fine wavy PGP9.5 signals (Fig. 1D), BV demonstrated curlings or reticular formations surrounding the lumen (Fig. 1E), frequently accompanying nerve bundles, which had a long thin morphology with very compact PGP9.5 signals (Fig. 1F). The IENF, SP, dermal nerve bundles, and skin fibers innervating the skin annex were abundant in the HC group, while were relatively sparse in PD patients with or without the LRRK2 2385R variant, as shown for IENF and SG in Figure 1.
Figure 1.
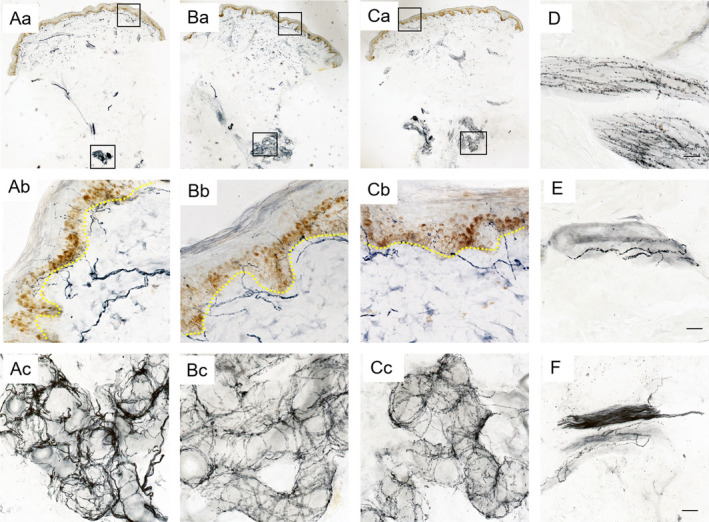
Cutaneous innervation in PD patients with and without LRRK2 G2385R variants and healthy controls. The epidermal and dermal innervation was abundant in the healthy control group (Aa), while seemed relatively sparse in PD patients with (Ba) or without LRRK2 2385R variant (Ca). Ab, Bb, and Cb showed the intraepidermal nerve fibers (IENF) penetrating the basal membrane of Aa, Ba, and Ca at high magnification, demonstrating decreased IENF in PD patients with or without LRRK2 2385R variant. Ac, Bc, and Cc showed the nerve fibers innervating the sweat glands in Aa, Ba, and Ca at high magnification, demonstrating sparse innervation of sweat glands in PD patients with or without LRRK2 2385R variant. (D–F) demonstrated PGP9.5 positive arrector pili muscles (APM), blood vessels (BV), and nerve bundles in the deep dermis of PD patients at high magnification, respectively. Scale bars: 200 um at low magnification, 20 μm at high magnification. PD, Parkinson disease; LRRK2, leucine‐rich repeat kinase 2.
The linear density of IENF under bright‐field immunohistochemistry was calculated as IENFD. The IENFD at the DL in G2385R non‐carrier PD patients was significantly lower than that of HC (fibers/mm, 6.17 ± 2.67 vs. 8.21 ± 4.08, P = 0.01); the difference was attenuated between the carrier group and HC (fibers/mm, 6.13 ± 3.40 vs. 8.21 ± 4.08, P = 0.069). Whereas the IENFD was not different between carrier and noncarrier PD patients (fibers/mm, 6.13 ± 3.40 vs. 6.17 ± 2.67, P = 0.971) (Fig. 2A). Two patients (2/17, 16.7%) showed distal IENFD below normal values in carriers compared to that in seven noncarriers (7/47, 14.9%); the IENFD values of both patients were approximately twice that of HC (2/30, 6.67%), despite no statistically significant difference.
The IENFD at the DL was negatively correlated with COMPASS‐31 scores in the whole PD group (ρ = −0.271, P = 0.038, Spearman correlation analysis) (Fig. 2C). We did not observe a correlation between IENFD at the DL and SFN‐SIQ (P = 0.147, Spearman correlation), disease duration (P = 0.36, Spearman correlation), Hoehn and Yahr stage (P = 0.138, Spearman correlation), UPDRS‐III (P = 0.664, Pearson correlation), or LED (P = 0.262, Pearson correlation).
Intraneuronal phosphorylated α‐synuclein deposition
By double‐labeling immunofluorescence, linear or dotted phosphorylated α‐synuclein (p‐syn) immunosignals were found to be co‐localized with PGP9.5 stained skin nerve fibers in both G2385R carrier and noncarrier PD patients (Fig. 3), while they were absent in HC. Single p‐syn signals or those not within nerve fibers were ignored. The following substructures of skin innervation demonstrated p‐syn deposition in both G2385R carriers and noncarriers: dermal bundles in the deep dermis, nerve fibers in SGs, APMs (Fig. 3), BV, and hair follicles, and the SP located just under the basal membrane (Fig. 3) and nerve bundles in the superficial dermis, which were supposed to be somatosensory. The IENF, fibroblasts, and adipocytes did not have p‐syn deposition.
Figure 3.
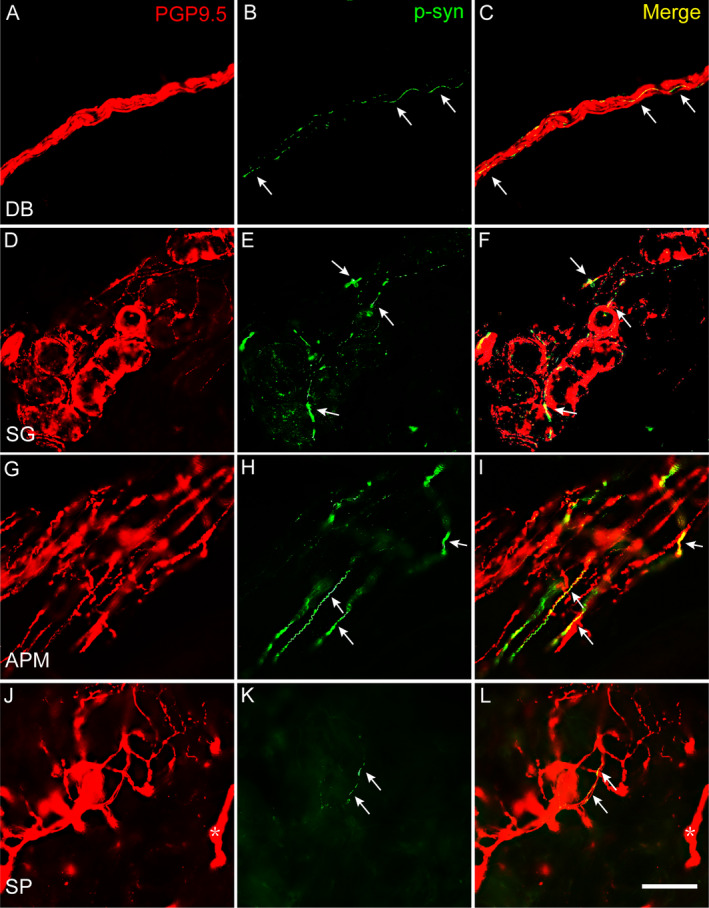
P‐syn deposited in skin nerve fibers of PD patients with and without G2385R variant. Double immunofluorescent staining demonstrated linear or dotted p‐syn immunosignals (green) colocalized with PGP9.5 signals in DB in deep dermis (A–C), nerve fibers innervating SG (D–F), APM (G–I), and SP and DB (asterisk) in superficial dermis (J–L). P‐syn, phosphoralated α synuclein; Arrows indicated p‐syn immunosignals; DB, dermal nerve bundles, SG, nerve fibers innervating sweat glands, APM, arrector pili muscles; SP, subepidermal plexus; PD, Parkinson disease. Scale bar: 50 μm.
Two biopsy sites were taken for each patient; the proximal cervical 7 region (C7) and DL. The positivity of p‐syn deposition in the whole group was 47/59 (79.7%) in total, 41/59 (69.5%) at C7, and 24/59 (40.7%) at DL. The positivity at C7 was significantly higher than that at DL in G2385R noncarriers (33/47 [70.2%] vs. 17/47 [36.2%], P = 0.001) and the whole PD group (41/59 [69.5%] vs. 24/59 [40.7%], P = 0.002), whereas it was similar with that at DL in carriers (8/12 [66.7%] vs. 7/12 [58.3%], P = 1.000) (Fig. 4A). The positivity of p‐syn was not different between G2385R carrier and noncarrier PD patients at each biopsy site.
Figure 4.
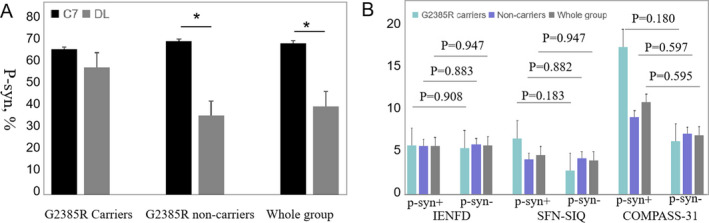
The p‐syn deposition in relation to biopsy sites and the small fiber neuropathy. (A) The positivity of p‐syn deposition at C7 was significantly higher than that at DL in G2385R non‐carriers (70.2% vs. 36.2%, *P = 0.001) and whole PD group (69.5% vs. 40.7%, *P = 0.002), whereas was similar with that at DL in carriers (66.7% vs. 58.3%, P = 1.000) (chi‐square and Fisher test). (B) The IENFD, SFN‐SIQ scores or COMPASS‐31 scores of patients with or without p‐syn deposition were not significantly different (all P > 0.05) (independent‐samples T test). C7, cervical 7 region; DL, distal leg; p‐syn+, with p‐syn deposition; p‐syn‐, without p‐syn deposition; PD, Parkinson disease; IENFD, intraepidermal nerve fiber density; SFN‐SIQ, small fiber neuropathy symptoms inventory questionnaire; COMPASS‐31, composite autonomic symptom score‐31 items. Error bars: +SE.
We also compared the IENFD between patients with and without p‐syn within the G2385R carrier group, noncarriers, and the whole PD group, to understand the relationship between p‐syn deposition and IENFD and obtained the following results: 6.19 ± 3.75 fibers/mm versus 6.16 ± 2.80 fibers/mm, P = 0.908; 6.14 ± 2.63 fibers/mm versus 6.28 ± 2.96 fibers/mm, P = 0.883; 6.15 ± 2.86 fibers/mm versus 6.21 ± 2.69 fibers/mm, P = 0.947, respectively (Fig. 4B). No difference was found within each group. Similarly, the SFN‐SIQ scores and COMPASS‐31 scores between patients with and without p‐syn in the three PD groups were not significantly different (Fig. 4B). The MDS‐UPDRS‐III scores (40.85 ± 22.58 vs. 35.33 ± 8.57, P = 0.187), Hoehn and Yahr stages (P = 0.132), disease duration (4.58 ± 3.58 vs. 3.35 ± 2.07, P = 0.261), or LED (392 ± 164.12 mg vs. 329.17 ± 155.52 mg, P = 0.231) were not different between PD patients with and without p‐syn deposition.
Discussion
The main findings of this study include the following: (1) LRRK2 G2385R carrier PD patients demonstrated a different p‐syn distribution pattern from noncarriers, showing abnormal deposits without a difference between proximal and distal skin sites; (2) decreased distal IENFD was found in G2385R carrier and noncarrier PD patients, which was negatively correlated with autonomic scale COMPASS‐31 scores; (3) LRRK2 G2385R carrier PD patients showed a more peculiar clinical profile than noncarriers with a higher NMSS, COMPASS‐31 score, LED, and increased prevalence of certain autonomic symptoms or RBD.
A recent study demonstrated that the G2385R variant altered the strength and quality of LRRK2 interactions and increased the rate of synaptic vesicle fusion, suggesting that the G2385R variant may behave like a loss‐of‐function mutation. 24 Nevertheless, patients with LRRK2 mutations cannot be distinguished on a clinical basis from iPD. Although the LRRK2 G2019S mutation was reported to be more likely to have LB, 25 to our knowledge, no neurophathological study with the LRRK2 G2385R variant has been reported. As in iPD, p‐syn was found in the skin nerve fibers of PD patients with the LRRK2 G2385R variant. This is the first neuropathological study concerning the LRRK2 G2385R variant, which may underlie synucleinopathy in LRRK2 G2385R variant PD. This is important in that interventions targeting α‐synuclein could be considered in LRRK2 G2385R variant PD, although this should be confirmed by further autopsies and a further large‐scale study.
The distribution pattern of p‐syn in PD in concern with biopsy sites had been published with controversy in recent years, indicating the heterozygous factors may influence the deposition. To our knowledge, two patterns had been reported, one was proximal‐distal gradient which was the main pattern reported by two groups, 9 , 10 , 26 , 27 , 28 , 29 the other was homogenous pattern reported by Doppler in 2018 and another group in Switzerland. 30 , 31 Although a more distal distribution tendency was found in Doppler’s article, no significant difference was found between the distal and proximal sites (12/25 vs. 8/25). The analysis of p‐syn positivity in PD, RBD, and MSA patients was performed between Vincenzo Donadio and Kathrin Doppler groups in 2019, and reported excellent reproducibility intra‐ and inter laboratory. 32 Because the different fixatives, different sources of primary antibody and different diameters of biopsy tissue were used in the two groups, these aspects that may influence the distribution pattern were excluded. PD patients with orthostatic hypotension (PD + OH) and without OH (PD‐OH) demonstrated both the above two distribution patterns recently, 28 suggesting sources of PD patients in concern with clinical profiles such as autonomic symptoms may also influence the p‐syn distribution pattern. The phenomenon was further supported by Donadio’s article in 2020, where all PD + OH patients displayed p‐syn deposits without a proximal–distal gradient. 29 Although the Doppler’s group and the Switzerland group reported a homogeneous p‐syn pattern, 30 , 31 none of the autonomic function especially OH was not clarified in the two articles. In this manuscript, we demonstrated a homogeneous distribution pattern of p‐syn in the G2385R carrier group, the percent of patients with complaint of orthostatic intolerance was higher (3/12) than that (6/47) in noncarrier PD patients. The percent of patients with additional autonomic symptoms including secretomotor symptoms, the gastrointestinal symptoms, bladder symptoms, and the score of autonomic scale COMPASS‐31 in this group was also higher than those in non‐carriers. All the findings supported the LRRK2 G2385R carrier group a unique clinical phenotype, and a relatively reported cutaneous p‐syn homogeneous distribution pattern.
SFN mainly based on a skin biopsy has been recently reported in iPD patients with immunofluorescence microscopy techniques. 9 , 10 , 31 , 33 , 34 In this study, the effect of the G2385R variant on SFN was firstly studied with bright‐field immunohistochemistry. Considering all the subjects were devoid of clinical symptoms or signs of large fiber neuropathy, or potential peripheral neuropathy causing conditions, the decreased distal IENFD and higher percentage of IENFD below normal values in PD with and without G2385R variants compared with HC highlighted small fiber involvement in both PD groups. Levodopa was reported to have a neurotoxic effect on peripheral large fibers by several research groups 35 , 36 , 37 , 38 ; a recent study demonstrated both large and small fiber pathology in PD patients free of L‐dopa treatment, but that SFN did not worsen in the treatment group, suggesting a selective neurotoxic effect of L‐dopa on peripheral neuropathy. 34 In our cohort, the decrease in IENFD did not worsen in the G2385R carrier group with higher LED, implying that the dosage of L‐dopa may not promote the progression of SFN in PD. This supports the theory that small fiber pathology is an intrinsic feature of PD, although solid conclusions cannot be made until the definite pathogenic mechanism is clarified.
Somatic SFN symptoms in relation to the LRRK2 G2385R variant have not been previously reported. The similar prevalence and frequency of somatosensory symptoms in G2385R carriers and noncarriers were coincident with the IENFD finding in this study, suggesting similar somatic small fiber involvement in regard to G2385R variants with iPD. On the contrary, higher prevalence and severity of autonomic symptoms in G2385R carriers by use of COMPASS‐31 were inconsistent with the results of a previous study demonstrating similar autonomic scores by means of the scale for outcomes in PD for autonomic symptoms (SCOPA‐AUT), in which only the frequency of autonomic symptoms was assessed. 21 Furthermore, COMPASS‐31 scores showed an excellent positive correlation with the SFN‐SIQ score, which assesses all SFN symptoms, and a mildly negative correlation with IENFD. When combined with the evaluation of severity, COMPASS‐31 may enhance the sensitivity of detection. However, conclusions on this cannot be made until a comparison study between COMPASS and SCOPA‐AUT has been conducted.
The question as to whether cutaneous p‐syn deposition correlates with SFN has not yet been answered. In this study, no difference in IENFD, SFN‐SIQ, or COMPASS‐31 was found between the p‐syn deposition subgroup and nondeposition subgroup in each PD group. This result appears to underlie the ambiguous relationship between SFN and p‐syn deposition in the skin. This was consistent with the former findings in iPD by Doppler and colleagues, 10 who demonstrated a reduction of distal IENFD in 7/16 patients with p‐syn deposition compared to 4/15 patients without deposits. By contrast, Donadio et al. reported an indirect correlation between p‐syn and IENFD, 9 in which the percentage of a‐synuclein deposits was inversely correlated with IENFD at the DL. Because of the diverse comparison methods, further large‐scale, well‐designed studies are needed to disclose the relationship between p‐syn deposition and SFN, which may accordingly unravel the mechanism of SFN in PD patients.
The equivalent demographics including age, gender, and BMI made the comparison of the SFN‐SIQ score, COMPASS‐31 score, and IENFD among G2385R carrier PD patients, noncarriers, and HC feasible. The similar age of onset, onset symptoms, disease, Hoehn and Yahr stage, MDS‐UPDRS‐Ⅲ scores, higher levodopa dosage, and increased percentage of patients with RBD were consistent with those in previous studies on G2385R mutation PD compared with iPD. 6 , 7 , 8 , 39 Higher nonmotor scores based on NMSS were first reported in this study, and was different from those in a previous report based on NMSQuest, which assessed the frequency of nonmotor symptoms without assessment of severity; a tendency of mildly higher NMSQuest scores was found in G2385R carriers without statistical significance. 7
It is important to clarify that we identified 12/59 (20.33%) of PD patients with a G2385R mutation, which is higher than the frequency varying from 5.96% (14/235) to 11.9% (117/1031) in previous large sample studies in the Chinese population. 6 , 7 , 40 The MDS PD criteria for clinically probable PD we used in this study could improve the diagnostic accuracy compared to the older criteria, which is attributed to the increased sensitivity (94.5% vs. 89.4%) and specificity (88.5% vs. 79.2%). 41 , 42
There were several limitations in this study. The relatively small sample of PD patients recruited from the skin biopsy database, and consequently the small sample of PD patients with G2385R variants should also be considered as a potential study limitation. An additional limitation is that the skin biopsy was only performed on one side (the more affected side). As it was reported that p‐syn could be unrelated to the more affected motor site in unilateral PD patients. 43 Biopsy on the more affected side may affect the number of patients showing p‐syn deposits and the pattern of p‐syn distribution.
In summary, we found a pronounced difference of the p‐syn distribution pattern with different clinical profiles in LRRK2 G2385R carriers compared with noncarriers, including a higher prevalence of autonomic symptoms, and similar intraepidermal SFN in the two groups. These findings propose that synucleinopathy is related to the LRRK2 G2385R genotype and implies a different pathogenesis in LRRK2 G2385R variant carriers and noncarriers. Accordingly, PD with the LRRK2 G2385R variant should not be included in the spectrum of iPD, which is possibly represented by an independent clinical variant of synucleinopathy.
Conflict of Interest
There are no conflicts of interest in this study.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China to Yuming Xu (81530037), the National Natural Science Foundation of China to Jing Yang (81600946), the Provincial and Ministry of Health Construction Committee of Henan Province to Jing Yang (SB201902012), and Key projects of higher education institutions in Henan Province to Jing Yang (16A320061).
Funding Information
This work was supported by grants from the National Natural Science Foundation of China to Yuming Xu (81530037), the National Natural Science Foundation of China to Jing Yang (81600946), the Provincial and Ministry of Health Construction Committee of Henan Province to Jing Yang (SB201902012), and Key projects of higher education institutions in Henan Province to Jing Yang (16A320061).
Funding Statement
This work was funded by Ministry of Health grants 16A320061 and SB201902012; National Natural Science Foundation of China grants 81530037 and 81600946.
Contributor Information
Jing Yang, Email: yangjing9527@126.com.
Yuming Xu, Email: xuyuming@zzu.edu.cn.
References
- 1. Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol 2009;8:1150–1157. [DOI] [PubMed] [Google Scholar]
- 2. Volpicelli‐Daley LA, Luk KC, Patel TP, et al. Exogenous alpha‐synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011;72:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schneider SA, Alcalay RN. Neuropathology of genetic synucleinopathies with parkinsonism: review of the literature. Mov Disord 2017;32:1504–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Fonzo A, Rohe CF, Ferreira J, et al. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson's disease. Lancet 2005;365:412–415. [DOI] [PubMed] [Google Scholar]
- 5. Whaley NR, Uitti RJ, Dickson DW, et al. Clinical and pathologic features of families with LRRK2‐associated Parkinson's disease. J Neural Transm Suppl 2006;70:221–229. [DOI] [PubMed] [Google Scholar]
- 6. An XK, Peng R, Li T, et al. LRRK2 Gly2385Arg variant is a risk factor of Parkinson's disease among Han‐Chinese from mainland China. Eur J Neurol 2008;15:301–305. [DOI] [PubMed] [Google Scholar]
- 7. Sun Q, Wang T, Jiang TF, et al. Effect of a leucine‐rich repeat kinase 2 variant on motor and non‐motor symptoms in Chinese Parkinson's disease patients. Aging Dis 2016;7:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu R, Cui SS, Du JJ, et al. Fatigue correlates with LRRK2 G2385R variant in Chinese Parkinson's disease patients. Parkinsonism Relat Disord 2017;44:101–105. [DOI] [PubMed] [Google Scholar]
- 9. Donadio V, Incensi A, Leta V, et al. Skin nerve alpha‐synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology 2014;82:1362–1369. [DOI] [PubMed] [Google Scholar]
- 10. Doppler K, Ebert S, Uceyler N, et al. Cutaneous neuropathy in Parkinson's disease: a window into brain pathology. Acta Neuropathol 2014;128:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zange L, Noack C, Hahn K, et al. Phosphorylated alpha‐synuclein in skin nerve fibres differentiates Parkinson's disease from multiple system atrophy. Brain 2015;138(Pt 8):2310–2321. [DOI] [PubMed] [Google Scholar]
- 12. Wang N, Gibbons CH, Lafo J, Freeman R. alpha‐Synuclein in cutaneous autonomic nerves. Neurology 2013;81:1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin CH, Chao CC, Wu SW, et al. Pathophysiology of small‐fiber sensory system in Parkinson's disease: skin innervation and contact heat evoked potential. Medicine 2016;95:e3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fadda L, Lombardi R, Soliveri P, et al. Skin nerve alpha‐synuclein deposits in a parkinsonian patient with heterozygous parkin mutation. Parkinsonism Relat Disord 2019;60:182–183. [DOI] [PubMed] [Google Scholar]
- 15. Carmona‐Abellan M, Gabilondo I, Murueta‐Goyena A, et al. Small fiber neuropathy and phosphorylated alpha‐synuclein in the skin of E46K‐SNCA mutation carriers. Parkinsonism Relat Disord 2019;65:139–145. [DOI] [PubMed] [Google Scholar]
- 16. Narendra DP, Isonaka R, Nguyen D, et al. Peripheral synucleinopathy in a DJ1 patient with Parkinson disease, cataracts, and hearing loss. Neurology 2019;92:1113–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 18. Goetz CG, Stebbins GT, Tilley BC. Calibration of unified Parkinson's disease rating scale scores to Movement Disorder Society‐unified Parkinson's disease rating scale scores. Mov Disord 2012;27:1239–1242. [DOI] [PubMed] [Google Scholar]
- 19. Gupta DK, Fahn S, Tatsuoka C, Kang UJ. Hoehn and Yahr stage 3 and postural stability item in the movement disorder society‐unified Parkinson's disease rating scale. Mov Disord 2018;33:1188–1189. [DOI] [PubMed] [Google Scholar]
- 20. Sun B, Li Y, Liu L, et al. SFN‐SIQ, SFNSL and skin biopsy of 55 cases with small fibre involvement. Int J Neurosci 2018;128:442–448. [DOI] [PubMed] [Google Scholar]
- 21. Pierangeli G, Turrini A, Giannini G, et al. Translation and linguistic validation of the composite autonomic symptom score COMPASS 31. Neurol Sci 2015;36:1897–1902. [DOI] [PubMed] [Google Scholar]
- 22. Chaudhuri KR, Martinez‐Martin P, Brown RG, et al. The metric properties of a novel non‐motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord 2007;22:1901–1911. [DOI] [PubMed] [Google Scholar]
- 23. Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:903–912, e44‐9. [DOI] [PubMed] [Google Scholar]
- 24. Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2‐associated Parkinson's disease: a case‐control study. Lancet Neurol 2008;7:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalia LV, Lang AE, Hazrati LN, et al. Clinical correlations with Lewy body pathology in LRRK2‐related Parkinson disease. JAMA Neurol 2015;72:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donadio V, Incensi A, Piccinini C, et al. Skin nerve misfolded alpha‐synuclein in pure autonomic failure and Parkinson disease. Ann Neurol 2016;79:306–316. [DOI] [PubMed] [Google Scholar]
- 27. Donadio V, Incensi A, El‐Agnaf O, et al. Skin alpha‐synuclein deposits differ in clinical variants of synucleinopathy: an in vivo study. Sci Rep 2018;8:14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donadio V, Incensi A, Del Sorbo F, et al. Skin nerve phosphorylated alpha‐synuclein deposits in Parkinson disease with orthostatic hypotension. J Neuropathol Exp Neurol 2018;77:942–949. [DOI] [PubMed] [Google Scholar]
- 29. Donadio V, Incensi A, Rizzo G, et al. Skin biopsy may help to distinguish multiple system atrophy‐parkinsonism from Parkinson's disease with orthostatic hypotension. Mov Disord 2020;35:1649–1657. [DOI] [PubMed] [Google Scholar]
- 30. Doppler K, Jentschke HM, Schulmeyer L, et al. Dermal phospho‐alpha‐synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson's disease. Acta Neuropathol 2017;133:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Melli G, Vacchi E, Biemmi V, et al. Cervical skin denervation associates with alpha‐synuclein aggregates in Parkinson disease. Ann Clin Transl Neurol 2018;5:1394–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donadio V, Doppler K, Incensi A, et al. Abnormal alpha‐synuclein deposits in skin nerves: intra‐ and inter‐laboratory reproducibility. Eur J Neurol 2019;26:1245–1251. [DOI] [PubMed] [Google Scholar]
- 33. Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain 2008;131(Pt 7):1903–1911. [DOI] [PubMed] [Google Scholar]
- 34. Nolano M, Provitera V, Manganelli F, et al. Loss of cutaneous large and small fibers in naive and l‐dopa‐treated PD patients. Neurology 2017;89:776–784. [DOI] [PubMed] [Google Scholar]
- 35. Toth C, Brown MS, Furtado S, et al. Neuropathy as a potential complication of levodopa use in Parkinson's disease. Mov Disord 2008;23:1850–1859. [DOI] [PubMed] [Google Scholar]
- 36. Toth C, Breithaupt K, Ge S, et al. Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol 2010;68:28–36. [DOI] [PubMed] [Google Scholar]
- 37. Rajabally YA, Martey J. Neuropathy in Parkinson disease: prevalence and determinants. Neurology 2011;77:1947–1950. [DOI] [PubMed] [Google Scholar]
- 38. Ceravolo R, Cossu G, Bandettini di Poggio M, et al. Neuropathy and levodopa in Parkinson's disease: evidence from a multicenter study. Mov Disord 2013;28:1391–1397. [DOI] [PubMed] [Google Scholar]
- 39. Marras C, Alcalay RN, Caspell‐Garcia C, et al. Motor and nonmotor heterogeneity of LRRK2‐related and idiopathic Parkinson's disease. Mov Disord 2016;31:1192–1202. [DOI] [PubMed] [Google Scholar]
- 40. Li C, Ting Z, Qin X, et al. The prevalence of LRRK2 Gly2385Arg variant in Chinese Han population with Parkinson's disease. Mov Disord 2007;22:2439–2443. [DOI] [PubMed] [Google Scholar]
- 41. Postuma RB, Poewe W, Litvan I, et al. Validation of the MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2018;33:1601–1608. [DOI] [PubMed] [Google Scholar]
- 42. Schrag A. Testing the MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2018;33:1518–1520. [DOI] [PubMed] [Google Scholar]
- 43. Donadio V, Incensi A, Rizzo G, et al. Spine topographical distribution of skin alpha‐synuclein deposits in idiopathic Parkinson disease. J Neuropathol Exp Neurol 2017;76:384–389. [DOI] [PubMed] [Google Scholar]


