Abstract
Based on publicly available data, we reevaluated current algorithms for stratifying the risk of progressive multifocal leukoencephalopathy (PML) in natalizumab‐treated patients with multiple sclerosis, and found that there are a number of issues. First and foremost, our analysis highlights the necessity of separate PML incidence assessments for the U.S. versus Europe, and indicates that the risk in John Cunningham virus (JCV) antibody‐negative patients may be higher than previously communicated. Additionally, we advocate introducing a low‐risk JCV index threshold of 0.45 for individuals with prior exposure to an immunosuppressant, and setting the low‐risk threshold at 0.6 instead of 0.9 for those without such pretherapies. On the other hand, the risk of PML on natalizumab, in general, appears to not only plateau but to actually decrease after about 5 years of continuous dosing.
Introduction
In 2014, Plavina and colleagues reported that higher levels of John Cunningham virus (JCV) antibody in serum are associated with an increased risk of progressive multifocal leukoencephalopathy (PML) during therapy with natalizumab. 1 Subsequently, clinicians incorporated the JCV index into the PML risk‐stratification triad, namely, JCV serostatus, prior immunosuppressant (IS) exposure, and duration of treatment, that had been proposed 2 years earlier. 2 More recently, at ECTRIMS 2016, Biogen presented retrospective analyses of data from four clinical studies—STRATA, STRATIFY‐2, TOP, and TYGRIS—, 3 according to which the cumulative risk of PML with a positive JCV serostatus following 72 natalizumab infusions is roughly 27 per 1000 with prior IS exposure, and 17 per 1000 without. Those analyses were published in the fall of 2017, in a paper by Ho and colleagues. 4 The article in hand critically evaluates that updated risk algorithm, and discusses the findings in context with the current literature. Our results are relevant to shared decision making by patients and their treating neurologists.
Methods
The methods we employed to quantify the risk of PML are precisely the ones also used by Biogen (Kaplan–Meier, lifetable, Bayes), while the data, too, are mostly from the four trials pooled by Ho et al. for their analysis. 4 The only exceptions are Figures 2 and 4: the former shows the instantaneous—as opposed to cumulative—risk of PML, utilizing data from the TOUCH patient registry, 5 whereas the latter is based on work by John Foley. 6 Some of the calculations were performed with the assistance of the R statistical software package.
Figure 2.
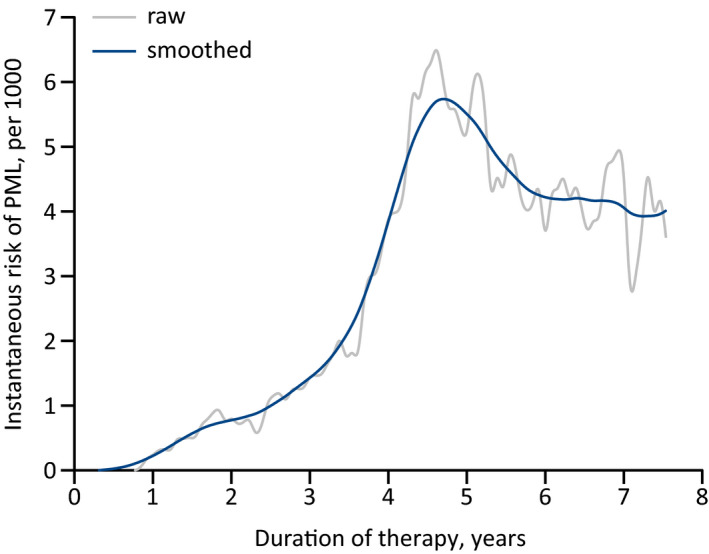
Hazard function of the annual risk of PML in the U.S. for JCV‐seropositive natalizumab‐treated patients—with or without prior IS exposure—, based on the analysis of the risk with standard‐interval dosing in the TOUCH registry utilizing the tertiary definition. 5 Although the ‘raw’ curve starts to oscillate from about the middle of the seventh treatment year due to the falling sample size, the incidence of PML did decrease in that cohort: in years 5, 6, 7, and 8 of treatment it was, respectively, 6.2 per 1000, 4.9 per 1000, 3.8 per 1000, and 3.6 per 1000. Also, the 95% confidence interval of the incidence during the fifth year is 4.1–8.4 per 1000, so that the point estimates of the incidence during the seventh and eighth years are actually both below the lower limit of that interval. IS, immunosuppressant; JCV, John Cunningham virus; PML, progressive multifocal leukoencephalopathy.
Figure 4.
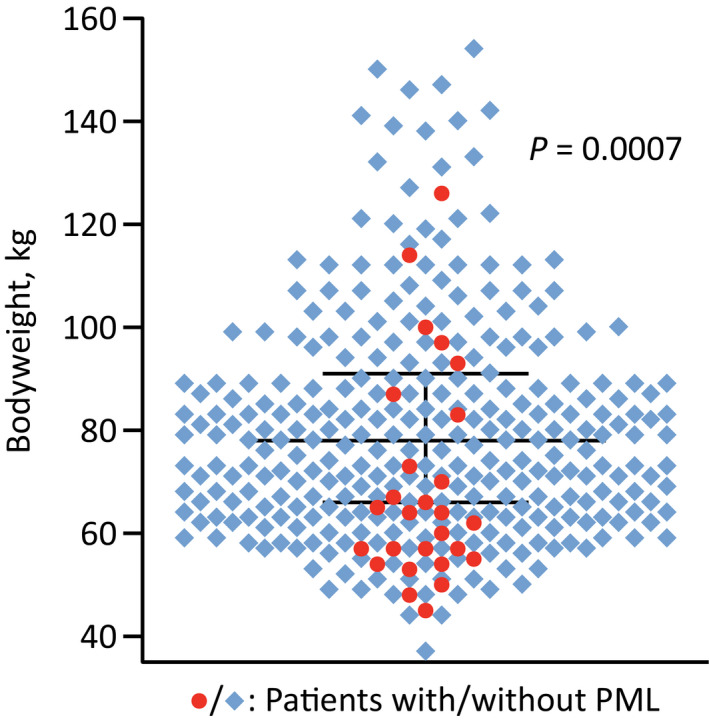
Association of PML and bodyweight. Case–control study of 328 natalizumab‐treated patients in the U.S., including 27 with PML. 6 PML, progressive multifocal leukoencephalopathy.
Geostratifying the risk of PML
A major weakness of the latest seminal risk assessment 4 is that, throughout, the estimates were computed jointly for all regions worldwide, disregarding in particular the large difference in the incidence of natalizumab‐associated PML between Europe and America. For example, during the TYGRIS trial, 7 just 3 of 2207 participants in the U.S. developed PML as against 41 of 4301 elsewhere (P < 0.0001). We therefore believe that it is essential to also consider patients’ geographic origin. Indeed, over the past several years, Biogen have always used only U.S. exposure data to calculate the U.S. risk of PML (as shown, e.g., in the labeling of Tysabri); consequently, only European data should be used to calculate the European risk.
However, Ho et al.’s publication is actually sufficiently detailed to derive regionally stratified Kaplan–Meier curves, for natalizumab‐treated patients with a positive JCV serostatus but no prior IS exposure having received up to 72 infusions (Fig. 1). What is crucial, for the U.S., the resulting estimate of the cumulative risk, of 12 per 1000, is very much consistent with the occurrence of PML in routine medical practice. 5 , 9 Furthermore, the concluding findings from TYGRIS are now published, 7 and though the PML incidences were obtained using an inadequate statistical method and on partially inaccurate assumptions—described later in this article—, when correcting for these, the cumulative risk in the same constellation as above comes out at around 9 per 1000 in the U.S. versus 25 per 1000 in Europe and Canada (Table 1).
Figure 1.
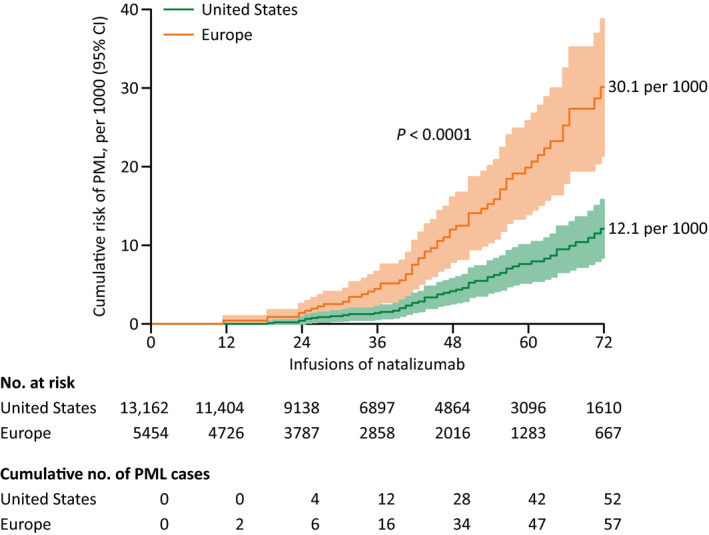
Kaplan–Meier curves of the risk of natalizumab‐associated PML in the U.S. versus Europe, for JCV‐seropositive patients without prior IS exposure. Derived from Figure 2A in Ho et al.’s publication, 4 by branching the blue curve into two limbs via the regional JCV seroprevalence and the proportion of prior IS exposure in their cohort; 8 the continental distribution of PML cases was then approximated using information provided in Table 1 of the same source (Data S1). IS, immunosuppressant; JCV, John Cunningham virus; PML, progressive multifocal leukoencephalopathy.
Table 1.
Incidence of PML in TYGRIS, stratified by region
| Incidence of PML per 1000 JCV+ natalizumab‐treated patients, by region | ||||
|---|---|---|---|---|
| with prior IS exposure | without prior IS exposure | |||
| Infusions | United States 1 | Europe and Canada | United States | Europe and Canada |
| 1–24 | 0.0 | 0.0 | 0.0 | 0.9 |
| 25–48 | 0.0 | 15.8 | 1.4 | 9.2 |
| 49–72 | 0.0 | 6.9 2 | 7.3 | 15.1 |
Incidence of natalizumab‐associated PML in TYGRIS among patients with a positive JCV antibody status, 7 estimated using the lifetable method. For completeness, that method has been superseded by the Kaplan–Meier estimator, which is more precise as it does not assume that the event of interest as well as withdrawal happen uniformly over the period considered. 10 So lifetables should really only be used anymore when the respective raw exposure data are actually unavailable (as is the case here). IS, immunosuppressant; JCV, John Cunningham virus; JCV+, JCV‐seropositive; PML, progressive multifocal leukoencephalopathy.
Though PML did not occur among U.S. study participants with prior IS exposure, there were only 309 such patients in total in the cohort. 7
The seemingly decreasing incidence after 48 infusions is again presumably due to the small sample size in that range; also, there were two further cases in patients receiving 73–77 infusions. 7
Carefully integrating the JCV index into risk estimation
In order to refine the stratification of the risk of PML in JCV‐seropositive patients without prior IS exposure, Ho et al. further integrated antibody titers into their algorithm. 4 While in principle helpful, there are issues with the way this was carried out. To begin with, the thresholds chosen are not best possible, especially the pooling of all natalizumab users with a JCV index ≤0.9. Numerically, the incidence of PML among the roughly 20% having an index ≤0.6 was in fact zero, 8 so that the risk of the 11% with an index in the range of 0.6–0.9 is not quite three times greater than that reported. 4 For example, in the latter category, the cumulative risk with 72 natalizumab infusions is not 1.6 per 1000, but rather 4.6 per 1000 (Table 2).
Table 2.
Bayesian analysis to stratify the cumulative risk of PML during therapy with natalizumab by JCV antibody titer
| Infusions | Cumulative risk of PML per 1000 patients, by JCV index | |||||
|---|---|---|---|---|---|---|
| with prior IS exposure | without prior IS exposure | |||||
| Index ≤0.45 | >0.45 | ≤0.6 | 0.6–1.0 | 1.0–1.7 | >1.7 | |
| 12 | 0.0 | 0.4 | 0.0 | 0.0 | 0.1 | 0.2 |
| 24 | 0.0 | 0.9 | 0.0 | 0.2 | 0.4 | 1.2 |
| 36 | 0.0 | 5.0 | 0.0 | 0.6 | 1.3 | 3.9 |
| 48 | 0.0 | 14.3 | 0.0 | 1.7 | 3.6 | 11.0 |
| 60 | 0.3 | 24.1 | 0.3 | 2.9 | 6.3 | 19.1 |
| 72 | 0.3 | 30.6 | 0.3 | 4.6 | 9.8 | 29.7 |
Estimated cumulative risk of PML on natalizumab by JCV antibody index only and using improved boundary thresholds, based on the data from Ho et al.’s pooled study cohort, 4 , 8 for both patients with and without prior IS exposure. Confidence intervals are omitted as calculating such intervals in this particular situation is somewhat tricky 1 , 4 and, at any rate, would necessitate the raw index data. We stress that the numbers in this table are overestimates by U.S. standards, and underestimates by European standards (Fig. 1). Additionally, although there were no cases of PML in Ho et al.’s cohort among patients without prior IS exposure and a JCV index ≤0.6 during the first 4 years of natalizumab therapy, 8 one such case has since been reported, 9 in a JCV‐seropositive patient with an antibody index of 0.34 having received 36 infusions (for the risk with a negative JCV serostatus, cf. Figure 3 also). IS, immunosuppressant; JCV, John Cunningham virus; PML, progressive multifocal leukoencephalopathy.
What is more, the non‐PML index distribution was extrapolated from a cohort consisting largely of subjects recruited from a low‐incidence population: of the 8170 patients in question, 11 approximately 95% were participants in the (U.S. only) STRATIFY trials. 4 That in itself might already have produced inaccurate ratios, for the median JCV index in high‐incidence cohorts of natalizumab recipients (two German and one Dutch) 12 , 13 , 14 was actually 2.2, 2.4, and 2.1, respectively, versus 1.7 in Biogen’s study. 4 An additional factor skewing the risk estimates was the collection of PML patients also from outside clinical trials, anyway with the ‘test’ dataset; 11 thus cases and controls were not directly comparable. Likewise, for each case of PML, all—four, on average—index samples (measured >6 months before diagnosis) were included, notwithstanding that benefit–risk reevaluations are generally based on a patient’s maximum index over time.
With respect to those with prior IS exposure, although it has been widely accepted that JCV titers were not useful in discriminating the risk in that patient population, 1 upon closer examination, this may not be entirely so. Specifically, in the relevant case–control study used by Ho et al., 11 no one developing PML whose prior IS status was either positive (26/101 subjects) or unknown (7/101) had ever had a JCV index ≤0.45 versus about 12% of JCV‐seropositive controls with prior IS exposure. 15 This suggests that even the cumulative risk of PML on natalizumab is low so long as a patient’s JCV antibody index remains consistently at or below 0.45—whether or not they were previously exposed to an IS (Table 2).
A gentle risk slope after 5 years of natalizumab therapy
In their study, Ho et al. do not furnish any figures of the risk of PML beyond 72 natalizumab infusions, 4 arguing that the incidence in their cohort (implausibly) decreased thenceforth, supposedly owing to the shrinking sample size. 8 At least in the U.S., however, that is apparently what happens; the hazard function peaks toward the end of the fifth treatment year, and starts to slowly decline thereafter (Fig. 2). Certainly, despite the fact that the number of patients was small and there could be a hidden bias, these data, too, indicate that the risk of natalizumab‐associated PML does not rise any further after 5–6 years.
About the risk with a negative JCV antibody status
As for PML among JCV‐seronegative natalizumab users, that risk was assessed on the basis of a single case, 8 clearly an insufficient number. Therefore, it should be informative to also calculate the risk in this setting via Bayes’s theorem. For example, in STRATA, the cumulative risk over 6 years of therapy was 23 per 1000 and the JCV seroprevalence in non‐PML subjects 67%; 16 assuming that 2% of cases are false‐negatives, the risk of a JCV‐seronegative patient is 1.4 per 1000 (Fig. 3).
Figure 3.
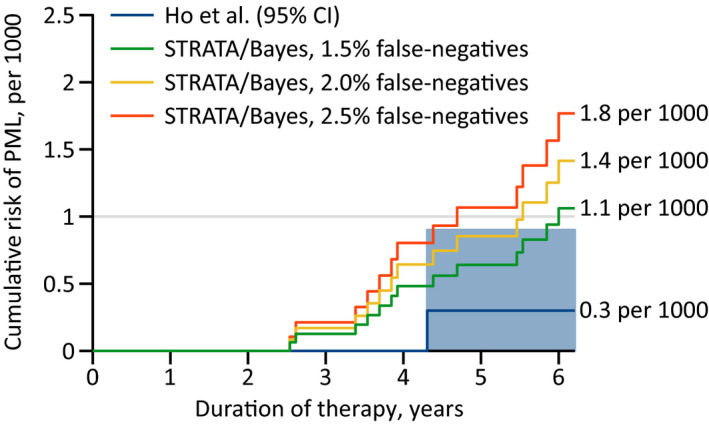
Risk of PML during natalizumab therapy with a negative JCV antibody status. Estimated on the basis of the Kaplan–Meier curve of the incidence in STRATA 16 and Bayes’s theorem, assuming a false‐negative rate of 1.5%, 2.0%, and 2.5%. NB: Biogen’s latest reports merely state that the proportion of such cases “has consistently been below the estimated false‐negative rate of 2.2%” of their antibody assay (https://medinfo.biogen.com), without giving any additional detail, while Ho et al.’s article does not discuss the question of the false‐negative rate at all. 4 By contrast, the original poster from ECTRIMS 2016 expressly says that the assay “was configured with detection and inhibition steps to confirm the presence of JCV‐specific antibodies with an analytical false negative rate of 3%”. 3 JCV, John Cunningham virus; PML, progressive multifocal leukoencephalopathy.
Another critical aspect is the long mean prediagnostic phase of PML, which in one (nationwide) study turned out to be almost half a year. 17 Recalling that it typically takes 16 weeks for the effects of natalizumab to vanish, 18 it would accordingly seem appropriate to count anyone developing PML not just within 6 months but within 12 months of a negative test result as false‐negative—after all, the idea is that a natalizumab‐treated patient who becomes JCV‐seropositive can still avoid PML by stopping the agent. Similarly, with the estimation of the risk by antibody index, samples collected less than a year before diagnosis should best be excluded.
Natalizumab induces JCV seroconversion?
Past analyses have repeatedly demonstrated that the JCV seroprevalence in the adult population grows by 5–10 percentage points per decade of life. 19 , 20 Hence, to the individual, the odds of converting from negative into positive are 1–2% annually. By contrast, several workgroups have observed much higher rates during natalizumab therapy, of up to 10%. 20 This has led to the proposition that the drug itself catalyzes JC‐viral replication, thereby aiding JCV seroconversion and raising antibody titers as a consequence of an anamnestic response. 12
However, if natalizumab increased the conversion rate three‐ or fourfold as postulated, 20 that would surely show up on a cross‐sectional analysis. For example, the JCV seroprevalence in the fourth treatment year should necessarily be greater than at baseline, which was not the case in STRATIFY‐1. 21 This apparent contradiction has a simple resolution, though, in that the abovementioned researches usually only assessed what occurred in those on natalizumab, but did not track multiple sclerosis patients receiving other therapies. Fortunately, STRATIFY‐2 partly unravels this mystery since JCV antibody testing was performed biannually, and thus revealed the probability of seroconverting over 24 months to be 12.2% with natalizumab use versus 13.4% without. 22 Additionally, in the ASCEND trial, there was no difference in the conversion rates between the placebo and treatments arms either, 23 although follow‐up was also a mere 2 years.
The notion of confirmed PML
The use of the term “confirmed” cases of natalizumab‐associated PML 3 , 4 raises concerns, as it implies that cases may have been overlooked and, a fortiori, not included in the risk analysis. Moreover, a ruleset for diagnosing PML deviating from the AAN consensus criteria 24 appears to be applied in practice. 25 Therefore, at a minimum, the drugmaker should not only report the number of confirmed cases but also those of “low‐suspect” and “high‐suspect” ones. 26 Furthermore, in North America anyway, the adjudication of cases is determined by a single Biogen‐employed physician, while most other pharmaceutical companies with agents on the market potentially causing PML rely on committees of independent experts. The latter approach is more likely to yield complete ascertainment, especially with suspected PML in JCV‐seronegative individuals.
Quantifying the incidence of PML in TYGRIS
As mentioned in the beginning, there are two problems with the computation of the incidence of PML in TYGRIS. 7 Firstly, although this has been rectified with Ho et al.’s analysis 4 compared to the earlier ones, 1 , 2 the method of survival analysis does not account for censoring, that is, incomplete follow‐up or patients stopping natalizumab during a given treatment period (“dropouts”). 27 Secondly, with each at‐risk population, the authors included only patients with known‐positive JCV serostatus, 7 neglecting that the serostatus was unavailable for a sizable proportion of study participants—almost 40% in the case of the Euro‐Canadian subcohort—, many of whom were doubtless positive. The former causes the PML incidence to be underestimated, whereas the latter actually leads to overestimates (Data S2). We therefore recalculated the incidence in TYGRIS for the same combinations of risk factors and treatment epochs as the investigators did in their publication, but using the lifetable method and with the missing values on the JCV serostatus imputed (Table 1).
Discussion
In conclusion, the updated algorithm, 4 albeit a step in the right direction, remains insufficient to comprehensively inform on the risk of PML from natalizumab. In particular, while Biogen’s JCV antibody assay has proven valuable, 28 , 29 the risk with a negative serostatus—or a low index—warrants further investigation (Fig. 3), not least because the patient population concerned is precisely that in whom natalizumab is nowadays prescribed primarily. For example, in the freshly published 10‐year data from TOP, 30 among just 36 patients with known JCV serostatus 6 months prior to developing PML, as in Ho et al.’s cohort, there was a false‐negative case. Moreover, adhering to the principle of pooling those with similar rather than disparate experiences, the risk should definitely be assessed separately for the U.S. versus Europe (Fig. 1, Table 1).
Naturally, one might wonder why the incidence of natalizumab‐associated PML in Europe is 2.5–3 times that in the U.S., even after adjusting for the continental variation in the established risk factors. 2 , 4 However, low bodyweight seems to be strongly connected with a higher risk of PML on natalizumab (Fig. 4): in a case–control study by Foley, 6 among subjects with a weight <60 kg, the odds of developing PML were 8.6‐fold higher than among those ≥75 kg (P < 0.0001). Additionally, in that (U.S.) cohort, the median weight was 78 kg, whereas in the REFINE trial—which had European enrollment only—, the median was 67 kg. 31 And this probably explains most, if not all, of the incidence gap between Europe and the U.S.; since Americans are heavier on average, Europeans effectively receive a higher dose, which in turn induces a greater α4‐integrin receptor saturation, thus presumably more potently suppressing CNS immunosurveillance. 6 Conversely, a lesser receptor saturation has been suggested to be responsible for the reduced risk of PML with extended‐interval dosing. 32 Two other relevant cellular attributes that are influenced by natalizumab treatment and which (partially) recover on longer infusion schedules are expression of α4‐integrin and L‐selectin. 33 , 34 The corollary is that the lower risk on a 5‐ or 6‐week infusion schedule, 5 and the higher incidence in Europe are, in all likelihood, simply two sides of the same coin.
In point of fact, the question of whether or not a patient’s bodyweight correlates with their risk is a current one. Not long ago, two case series of patients with natalizumab‐associated PML were published, one from the U.S. and one from Germany, 35 , 36 and, surprisingly, both papers asserted that subjects’ BMIs did not appear to be lower. However, the former cohort was quite small (n = 17), and the mean weight was still just 67.9 kg, which is actually lower than the mean of 69.6 kg in Foley’s study. 6 As for the German cohort, although larger (n = 142), the BMI was recorded for fewer than half of subjects and is not an appropriate measure here anyhow; for example, among women—making up roughly three quarters of natalizumab users with multiple sclerosis 4 , 7 —, even obese individuals tend to have fairly normal plasma volumes. 37 The bottom line is that if the risk of PML on natalizumab was not higher with a lower weight, that would make it difficult to explain how extended‐interval dosing should bring the risk down. 5 It is worth emphasizing that at first, a weight difference of just 10–12 kg 6 , 31 would perhaps not be expected to meaningfully impact a pharmacological therapy’s risk profile. Nonetheless, that is really well in line with the sharp PML incidence reduction observed in TOUCH, where the treatment cycles were also lengthened by only a week on average—once again, a priori, one might not think that an extension of what is barely half of natalizumab’s half‐life 38 should cut the risk by 80–90%. 9 We remark that other parameters that vary geographically and which could conceivably alter the risk include population genetics, JC‐viral subtypes, and co‐infections. Notably, one factor that does not contribute to the discrepancy between Europe and the U.S. is immunosenescence: in TYGRIS anyway, American patients were on average 7 years older. 7
Regarding the stratification of the PML risk by JCV antibody titer, the boundary thresholds were chosen suboptimally; those with an index in the range of 0.6–0.9 do not have an annual risk <1 per 1000 in the longer term (Table 2), and should therefore undergo high‐frequency brain MRI. 19 , 39 Incidentally, in late 2018, there was a fatal case of PML reported in a JCV‐seropositive natalizumab user in Australia having had no prior IS exposure and an index of 0.7, after 48 months of therapy. 40 This case illustrates what can go wrong when applying the updated algorithm 4 in practice, for according to the latter, that individual’s cumulative risk was 0.6 per 1000 at the time of PML diagnosis and thus considered “low”, 40 when in reality it was 1.7 per 1000 (Table 2). On the other hand, with respect to Ho et al. including all available index measurements per patient in their analysis—as opposed to just the highest ones—, that has possibly led to the risk being overstated in low‐index patients, and at any rate contradicts the (right) decision to consider anyone with a single positive JCV assay to henceforward always have been positive. 4 Interestingly, with a recent evaluation of the distribution of the JCV index in TOUCH, also funded by the drugmaker, 41 for each patient, only their highest‐ever index was included, and the cautious handling of this situation should improve the sensitivities of the lower thresholds as well. Further progress is achievable by dispensing with the dogma that JCV antibody titers had no significance in patients with prior IS exposure, compatible with the notion that the concept of IS exposure is itself intrinsically ill‐defined. 19 As an aside, so far, the JCV index has been unequivocally replicated purely qualitatively: a higher antibody response does confer a higher risk of PML. However, the precise cut‐offs vary in different populations. For instance, in the second aforementioned German index cohort, the risk only increased substantially from a value of 3.0, 13 whereas with Ho et al.’s dataset, 11 the greatest risk already starts at 1.7. 4 Another circumstance to be aware of is that the test–retest correlation of the screening step (which gives the index) of the STRATIFY antibody assay is very good alright, but not perfect. 12
What is relieving, as far as the alleged raising of JCV antibody titers goes, 12 , 20 Biogen’s agent can likely be exonerated. Certainly according to our analyses, the phenomenon of natalizumab‐induced JCV seroconversion is unreal, plainly due to natural fluctuations and because seronegative patients on the drug are normally retested 2–4 times per year. Intriguingly, an independent study that specifically assessed the longitudinal evolution of the JCV index before and during therapy with commonly‐used immunomodulators found no association either. 42 To date, just one publication has provided convincing evidence that natalizumab exposure is indeed causally linked to a higher conversion rate, 43 in a patient population treated for 4 years on average. More research exploring JCV seroconversion and the effects of natalizumab on antibody indexes particularly over longer periods is merited.
Speaking of longer periods, although the incidence curves from TOUCH indicate that the annual risk of PML starts to decline after about 5 years of natalizumab therapy (Fig. 2) and as appeared to be the case in Ho et al.’s pooled cohort, too, 8 in TYGRIS, the incidence with more than 72 infusions literally exploded—among just 13 patients, three were diagnosed with PML after receiving 73–77 infusions. 7 Of course, the clustering of those three cases might well be due to chance alone, but better data would surely be helpful in this context. A new risk assessment seems in order anyway, given that Ho et al.’s cohort is as of July 2015; 4 the one used for extrapolating the distribution of the JCV antibody index is actually as of March 2014, 11 and the recent evaluation in U.S. natalizumab users 41 suggests that the specificity of the lowest index threshold is higher than hitherto assumed.
Author Contributions
Both authors contributed to the conception and design of the study, and jointly drafted the manuscript. B.T. performed data analysis.
Conflicts of Interest
In the last 36 months, Dr. Berger has received grant support from Biogen and Genentech. He has received consultant fees from Amgen, Biogen, Celgene, Encycle, Excision‐Bio, Genentech/Roche, Genzyme, Inhibikase, Millennium/Takeda, Novartis, Sanofi/Genzyme, Shire, and Serono. Mr. Tugemann has nothing to report.
Supporting information
Data S1. The regionally stratified Kaplan–Meier curves from Figure 1.
Data S2. Properly computing the incidence of PML in TYGRIS.
Acknowledgments
We found the comments of the anonymous reviewers most helpful in improving this manuscript.
Funding Information
This study had no financial or non‐financial support.
References
- 1. Plavina T, Subramanyam M, Bloomgren G, et al. Anti‐JC virus antibody levels in serum or plasma further define risk of natalizumab‐associated progressive multifocal leukoencephalopathy. Ann Neurol 2014;76:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab‐associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–1880. [DOI] [PubMed] [Google Scholar]
- 3. Koendgen H, Chang I, Sperling B, et al. New algorithm to estimate risk of natalizumab‐associated progressive multifocal leukoencephalopathy (PML) in anti‐JCV antibody positive patients: analyses of clinical trial data to provide further temporal precision and inform clinical practice. Mult Scler 2016;22(suppl 3):659–660 (abstr P1249).26362896 [Google Scholar]
- 4. Ho P‐R, Koendgen H, Campbell N, et al. Risk of natalizumab‐associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol 2017;16:925–933. [DOI] [PubMed] [Google Scholar]
- 5. Zhovtis Ryerson L, Foley J, Chang I, et al. Risk of natalizumab‐associated PML in patients with MS is reduced with extended interval dosing. Neurology 2019;93:e1452–e1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foley J. Natalizumab related PML: an evolving risk stratification paradigm. Neurology 2013;80(suppl):S30.002 (abstr). [Google Scholar]
- 7. Foley J, Carrillo‐Infante C, Smith J, et al. The 5‐year Tysabri global observational program in safety (TYGRIS) study confirms the long‐term safety profile of natalizumab treatment in multiple sclerosis. Mult Scler Relat Disord 2020;39:101863. [DOI] [PubMed] [Google Scholar]
- 8. Ho P‐R, Campbell N, Chang I. The risk of PML from natalizumab – Authors’ reply. Lancet Neurol 2019;18:230–231. [DOI] [PubMed] [Google Scholar]
- 9. Zhovtis Ryerson L, Foley J, Chang I, et al. Reduced risk of progressive multifocal leukoencephalopathy (PML) associated with natalizumab extended interval dosing (EID): updated analysis of the TOUCH prescribing program database. Neurology 2019;92(suppl):S26.006 (abstr). [Google Scholar]
- 10. Diener‐West M, Kanchanaraksa S. Life tables. Johns Hopkins Bloomberg School of Public Health, 2008. https://ocw.jhsph.edu/courses/FundEpi/PDFs/Lecture8.pdf (accessed 13 May 2020). [Google Scholar]
- 11. Kuesters G, Plavina T, Lee S, et al. Anti‐JC virus (JCV) antibody index differentiates risk of progressive multifocal leukoencephalopathy (PML) in natalizumab‐treated multiple sclerosis (MS) patients with no prior immunosuppressant (IS) use: an updated analysis. Neurology 2015;84(suppl):P4.031 (abstr). [Google Scholar]
- 12. Schwab N, Schneider‐Hohendorf T, Pignolet B, et al. Therapy with natalizumab is associated with high JCV seroconversion and rising JCV index values. Neurol Neuroimmunol Neuroinflamm 2016;3:e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salmen A, von Ahsen N, Trampe AK, et al. Longitudinal analyses of anti‐JCV antibody index for risk assessment of progressive multifocal leukoencephalopathy. Mult Scler J Exp Transl Clin 2016;2:2055217316630008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vennegoor A, van Rossum JA, Polman CH, et al. Longitudinal JCV serology in multiple sclerosis patients preceding natalizumab‐associated progressive multifocal leukoencephalopathy. Mult Scler 2015;21:1600–1603. [DOI] [PubMed] [Google Scholar]
- 15. European Medicines Agency . Assessment report of the Pharmacovigilance Risk Assessment Committee (PRAC) – Procedure under article 20 of regulation (EC) no 726/2004 resulting from pharmacovigilance data (invented name: Tysabri; EMA/PRAC/171485/2016), 11 February 2016. https://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Tysabri_20/Opinion_provided_by_Committee_for_Medicinal_Products_for_Human_Use/WC500203426.pdf (accessed 13 May 2020).
- 16. O'Connor P, Goodman A, Kappos L, et al. Long‐term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS Study. Neurology 2014;83:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scarpazza C, Signori A, Prosperini L, et al. Early diagnosis of progressive multifocal leucoencephalopathy: longitudinal lesion evolution. J Neurol Neurosurg Psychiatry 2019;90:261–267. [DOI] [PubMed] [Google Scholar]
- 18. Fox RJ, Cree BAC, De Sèze J, et al. MS disease activity in RESTORE: a randomized 24‐week natalizumab treatment interruption study. Neurology 2014;82:1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. Lancet Neurol 2018;17:467–480. [DOI] [PubMed] [Google Scholar]
- 20. Schwab N, Schneider‐Hohendorf T, Hoyt T, et al. Anti‐JCV serology during natalizumab treatment: review and meta‐analysis of 17 independent patient cohorts analyzing anti‐John Cunningham polyoma virus seroconversion rates under natalizumab treatment and differences between technical and biological sero‐converters. Mult Scler 2018;24:563–573. [DOI] [PubMed] [Google Scholar]
- 21. Bozic C, Richman S, Plavina T, et al. Anti‐John Cunnigham virus antibody prevalence in multiple sclerosis patients: baseline results of STRATIFY‐1. Ann Neurol 2011;70:742–750. [DOI] [PubMed] [Google Scholar]
- 22. Campagnolo D, Ho P‐R, Patel RC, et al. Four‐year longitudinal index stability data from STRATIFY‐2 support the clinical utility of index for risk stratification of natalizumab‐associated progressive multifocal leukoencephalopathy. Neurology 2016;87:e25 (abstr P5.407). [Google Scholar]
- 23. Mason L, Berger T, Kapoor R, et al. Longitudinal stability of anti‐JC virus antibody index over 2 years in multiple sclerosis patients treated with natalizumab in the ASCEND study. Neurology 2019;92(suppl):P4.2‐009 (abstr). [Google Scholar]
- 24. Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong‐Si T, Richman S, Wattjes MP, et al. Outcome and survival of asymptomatic PML in natalizumab‐treated MS patients. Ann Clin Transl Neurol 2014;1:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dong‐Si T, Weber T, Richert N, et al. Classification of natalizumab case reports with progressive multifocal leukoencephalopathy. Neurology 2012;78(suppl):P7.058 (abstr). [Google Scholar]
- 27. Mowry EM, McArthur JC. PML in natalizumab‐treated multiple sclerosis: modeling errors and risk miscalculations. Neurology 2017;88:1110–1111. [DOI] [PubMed] [Google Scholar]
- 28. Giovannoni G, Kappos L, Berger J, et al. Incidence of natalizumab‐associated progressive multifocal leucoencephalopathy and its relationship with the pattern of natalizumab exposure over time. Mult Scler 2018;24(suppl 2):296 (abstr P604). [Google Scholar]
- 29. Vukusic S, Rollot F, Casey R, et al. Progressive multifocal leukoencephalopathy incidence and risk stratification among natalizumab users in France. JAMA Neurol 2020;77:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butzkueven H, Kappos L, Wiendl H, et al. Long‐term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real‐world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry 2020;91:660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trojano M, Ramió‐Torrentà L, Grimaldi LME, et al. Subcutaneous natalizumab 300mg every 4 weeks is comparable to standard intravenous dosing in REFINE: a study exploring the safety, tolerability, and efficacy of multiple natalizumab treatment regimens in patients with relapsing multiple sclerosis. Neurology 2015;85:e46 (abstr). [Google Scholar]
- 32. Foley JF, Goelz S, Hoyt T, et al. Evaluation of natalizumab pharmacokinetics and pharmacodynamics with standard and extended interval dosing. Mult Scler Relat Disord 2019;31:65–71. [DOI] [PubMed] [Google Scholar]
- 33. Puñet‐Ortiz J, Hervás‐García JV, Teniente‐Serra A , et al. Monitoring CD49d receptor occupancy: a method to optimize and personalize natalizumab therapy in multiple sclerosis patients. Cytometry Part B 2018;94B:327–333. [DOI] [PubMed] [Google Scholar]
- 34. Schwab N, Schneider‐Hohendorf T, Pignolet B, et al. Prospective validation of the PML risk biomarker L‐selectin and influence of natalizumab extended intervals. Neurology 2019;93:550–554. [DOI] [PubMed] [Google Scholar]
- 35. Stefoski D, Balabanov R, Waheed R, et al. Treatment of natalizumab‐associated PML with filgrastim. Ann Clin Transl Neurol 2019;6:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blankenbach K, Schwab N, Hofner B, et al. Natalizumab‐associated progressive multifocal leukoencephalopathy in Germany. Neurology 2019;92:e2232–e2239. [DOI] [PubMed] [Google Scholar]
- 37. Retzlaff JA, Tauxe WN, Kiely JM, Stroebel CF. Erythrocyte volume, plasma volume, and lean body mass in adult men and women. Blood 1969;33:649–667. [PubMed] [Google Scholar]
- 38. Tysabri (natalizumab) summary of product characteristics. Biogen (Cambridge, MA), 2019. https://www.ema.europa.eu/en/documents/product‐information/tysabri‐epar‐product‐information_en.pdf (accessed 13 May 2020).
- 39. Scarpazza C, Signori A, Cosottini M, et al. Should frequent MRI monitoring be performed in natalizumab‐treated MS patients? A contribution to a recent debate. Mult Scler 2019;26:1227–1236 [DOI] [PubMed] [Google Scholar]
- 40. Kelly E, Kurunawai C, Slee M. Natalizumab related PML in a low‐risk patient: when “low risk” is not “no risk”. Mult Scler 2019;25:484 (abstr P‐111). [Google Scholar]
- 41. Foley J, Zhovtis Ryerson L, Chang I, et al. Evaluation of anti‐JC virus index values in multiple sclerosis patients on natalizumab extended interval dosing and standard interval dosing: an analysis of the TOUCH prescribing program database. Mult Scler 2019;25(suppl 2):329–330 (abstr P666). [Google Scholar]
- 42. Hegen H, Walde J, Bsteh G, et al. Impact of disease‐modifying treatments on the longitudinal evolution of anti‐JCV antibody index in multiple sclerosis. Front Immunol 2018;9:2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peters J, Williamson E. Natalizumab therapy is associated with changes in serum JC virus antibody indices over time. J Neurol 2017;264:2409–2412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. The regionally stratified Kaplan–Meier curves from Figure 1.
Data S2. Properly computing the incidence of PML in TYGRIS.


