Abstract
Aims
Angiopoietin‐like protein 4 (ANGPTL‐4) had been reported to be associated with the risk of ischemic stroke, but its prognostic value remained unclear. The aim of this study was to investigate the association between plasma ANGPTL‐4 concentrations and prognosis of ischemic stroke.
Methods
Baseline plasma ANGPTL‐4 concentrations were measured in 3379 acute ischemic stroke patients. The primary outcome was a combination of death or major disability (modified Rankin Scale score, ≥3) at 3 months after ischemic stroke.
Results
At 3 months after ischemic stroke, 850 (26.16%) participants experienced major disability or died (750 major disabilities and 100 deaths). After adjusting for important covariates, odds ratios for the highest tertile of plasma ANGPTL‐4 concentrations were 1.59 (1.22–2.06) for primary outcome, 1.53 (1.18–1.97) for major disability, and 2.03 (1.03–4.00) for death when compared with the lowest tertile of plasma ANGPTL‐4 concentrations. For 1‐SD increase in log‐ANGPTL‐4 concentrations (0.44 ng/mL), the adjusted odds ratios were 1.24 (1.11–1.38), 1.14 (1.03–1.27), and 1.72 (1.32–2.23), respectively. Adding ANGPTL‐4 to a model containing conventional risk factors improved risk prediction for composite outcome of death and major disability.
Conclusion
Higher plasma ANGPTL‐4 concentration was associated with poor prognosis in acute ischemic stroke patients, suggesting that ANGPTL‐4 might be a prognostic marker for ischemic stroke.
Introduction
Stroke is one of the main causes of death and long‐term disability for adult worldwide, accounting for estimated 6.5 million deaths and 113 million disability‐adjusted life‐years in 2013. 1 Several primary and secondary prevention strategies had been implemented in past decades. 2 , 3 , 4 but the global burden and prevalence of stroke continued to increase. 5 Thus, measurements of novel biomarkers may be an efficient tool for early risk stratification and improvement of therapeutic strategies.
Angiopoietin‐like proteins‐4 (ANGPTL‐4), a member of ANGPTL family, has been shown to regulate angiogenesis and to be involved in lipid, glucose, and energy metabolisms. 6 , 7 Several studies reported that increased ANGPTL‐4 was associated with increased risk of hypertension, 8 atherosclerosis, 9 coronary artery disease, 10 and stroke. 11 Moreover, circulating levels of ANGPTL‐4 had a positive correlation with severity of stroke and ischemic volume. 12 Nevertheless, there is little about the knowledge of prognostic value of ANGPTL‐4 for clinical outcomes in ischemic stroke patients. Therefore, we investigated the associations between plasma ANGPTL‐4 concentrations and clinical outcomes after ischemic stroke.
Study Participants and Methods
Study participants
This study was performed based on the data from the China Antihypertensive Trial in Acute Ischemic Stroke (CATIS), a multicenter, single‐blind, blinded end points, randomized clinical trial conducted in 26 hospitals across China to test whether moderate lowering of blood pressure (BP) within the first 48 h after acute ischemic stroke onset would reduce death and major disability at 14 days or hospital discharge. Details about the CATIS design, methods and main results have been previously reported. 13 Briefly, a total of 4071 ischemic stroke patients aged 22 years or older confirmed by CT or MRI of the brain within 48 h of symptom onset with an elevated systolic BP between 140 and <220 mmHg were recruited in this trial. Patients with a BP ≥220/120 mmHg, severe heart failure, acute myocardial infarction or unstable angina, atrial fibrillation, aortic dissection, cerebrovascular stenosis, or resistant hypertension, in a deep coma, or those treated with IV thrombolytic therapy were excluded from the CATIS trial. Stroke subtypes for the included ischemic stroke patients included large artery atherosclerosis (thrombotic), cardiac embolism (embolic), and small artery occlusion lacunae (lacunar) according to TOAST criteria. In this study, 692 participants were further excluded because they did not offer blood samples, or some collected samples were hemolyzed in collections of blood samples, or we failed to determine plasma ANGPTL‐4 concentrations.
The CATIS was registered at clinicaltrials.gov (NCT01840072) and approved by the ethical committee at Soochow University in China and institutional review board at Tulane University in the USA, as well as ethical committees at the 26 participating hospitals. Written consent was obtained from all study participants or their immediate family members.
Data collection
Baseline data including demographic characteristics, clinical characteristics, medical histories, and lifestyles were collected at the time of enrollment using a standard questionnaire. Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) by trained neurologists at baseline. 14 Three BP measurements were obtained at baseline by trained nurses according to a common protocol adapted from procedures recommended by the American Heart Association. 15 BP was measured with the participant in a supine position using a standard mercury sphygmomanometer based on arm circumference of participant. The mean of three BP measurements was used in analyses. Fasting blood samples were collected within 24 h of hospital admission after at last 8 h of fasting. Routine laboratory analyses (fasting blood glucose, blood lipids, blood creatinine, etc.) were performed for all enrolled patients, in each participating hospital at admission. All plasma and serum samples were frozen at −80°C in the Central Laboratory of School of Public Health in Soochow University until laboratory testing.
ANGPTL‐4 detection
Plasma ANGPTL‐4 concentrations were measured centrally at Soochow University with a commercially available DuoSet ELISA kit (R&D Systems, Minneapolis, MN). Intra‐assay and interassay coefficients of variation were 3.3% and 3.9%, respectively. Laboratory technicians who performed these measurements were blind to the clinical characteristics and outcomes of the study participants.
Study outcomes
Participants were followed up in person at 3 months after ischemic stroke by trained neurologists. The primary outcome of this study was a combination of death and major disability (modified Rankin Scale [mRS] score ≥3). Secondary outcomes were separately those of death and major disability (modified Rankin scale score of 6 and 3–5, respectively), stroke recurrence, and vascular disease events (i.e., recurrent nonfatal stroke, nonfatal myocardial infarction, hospitalized and treated angina, hospitalized and treated congestive heart failure, and hospitalized and treated peripheral arterial disease).
Statistical analysis
All participants were divided into three groups according to tertiles of plasma ANGPTL‐4 concentrations and baseline characteristics were compared across three groups. The generalized linear regression analysis was used to test for trend across the tertiles of ANGPTL‐4 for continuous variables, and the Cochran‐Armitage trend χ2 test was used for categorical variables. Multivariate logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs) of primary and secondary outcomes for the highest tertile (≥112.9 ng/mL) of ANGPTL‐4 concentrations compared to the lowest tertile (<78.7 ng/mL) and for 1‐SD increment of log‐transformed ANGPTL‐4 concentrations (0.44 ng/mL). Important conventional covariates like age, sex, antihypertensive treatment, hospital area, time from onset to hospitalization, current smoking, current alcohol drinking, body mass index, dyslipidemia, fasting plasma glucose, history of hypertension, history of diabetes mellitus, family history of stroke, SBP at baseline, ischemic stroke subtypes, and baseline NIHSS score were included in the multivariable models. Restricted cubic splines were performed to explore the shapes of the associations between plasma ANGPTL‐4 concentrations and adverse clinical outcomes with four knots (at the 5th, 35th, 65th, and 95th percentiles). Furthermore, C statistics, net reclassification index (NRI), and integrated discrimination improvement (IDI) were used to evaluate the incremental prognostic value of plasma ANGPTL‐4 concentrations beyond conventional risk factors (covariates in multivariable models). 16 , 17
It was reported that circulating ANGPTL‐4 concentrations might be associated with stroke‐related risk factors (i.e., obesity, metabolic syndrome, hypertension, and diabetes mellitus). Thus, we performed subgroup analyses to determine the potential effect modification stratified by sex, age, baseline systolic BP, body mass index, dyslipidemia, current smoking, alcohol consumption, fasting plasma glucose, and receiving immediate BP reduction in multivariate adjusted logistic regression models. Interactions between circulating ANGPTL‐4 concentrations and covariates on primary outcome were tested by the likelihood ratio test of models with interaction terms. Two‐tailed P < 0.05 was considered to be statistically significant. All statistical analyses were conducted using SAS statistical software (version 9.4, Cary, NC, USA).
Results
Baseline characteristics
A total of 3379 participants were included in this study and the baseline characteristics were well balanced between enrolled and all participants in CATIS (Table S1), indicating that those enrolled basically represented the total participants of CATIS. Among 3379 patients, 2154 were male and the average age was 62.3 ± 10.9 years. The median plasma ANGPTL‐4 levels were 95.3 ng/mL (interquartile range 71.4–126.5 ng/mL). Compared to patients with low plasma ANGPTL‐4 concentrations, patients with higher ANGPTL‐4 concentrations tended to be older, male, have higher admission NIHSS score, systolic BP, fasting plasma glucose, total cholesterol, low‐density lipoprotein cholesterol, and higher prevalence of history of diabetes mellitus (Table 1).
Table 1.
Baseline characteristics of the study participants according to plasma ANGPTL‐4 levels.
| Characteristics | Total | ANGPTL‐4, ng/mL | P value | ||
|---|---|---|---|---|---|
| <78.7 | 78.7–112.9 | ≥112.9 | |||
| No. of subjects | 3379 | 1073 | 1159 | 1147 | |
| Demographics | |||||
| Age, years | 62.3 (10.9) | 59.6 (9.7) | 61.8 (10.7) | 65.39 (11.2) | <0.001 |
| Male | 2154 (63.8) | 639 (59.6) | 725 (62.6) | 790 (68.9) | <0.001 |
| Current cigarette smoking | 1237 (36.6) | 396 (36.9) | 437 (37.7) | 404 (35.2) | 0.400 |
| Current alcohol drinking | 1063 (31.5) | 346 (32.3) | 367 (31.7) | 350 (30.5) | 0.378 |
| Medical history | |||||
| History of hypertension | 2657 (78.6) | 826 (77.0) | 917 (79.1) | 914 (79.7) | 0.123 |
| History of hyperlipidemia | 243 (7.2) | 73 (6.8) | 87 (7.5) | 83 (7.2) | 0.701 |
| History of diabetes mellitus | 599 (17.7) | 150 (14.0) | 215 (18.6) | 234 (20.4) | <0.001 |
| Family history of stroke | 637 (18.9) | 199 (18.6) | 237 (20.5) | 201 (17.5) | 0.517 |
| Clinical features | |||||
| NIHSS score | 4 (2–8) | 4 (2–6) | 4 (2–7) | 6 (3–10) | <0.001 |
| SBP, mmHg | 166 (17) | 164 (16) | 166 (17) | 168 (18) | <0.001 |
| DBP, mmHg | 97 (11.01) | 97 (10) | 97 (11) | 97 (11) | 0.819 |
| BMI, kg/m2 | 24.9 (3.1) | 24.8 (3.1) | 25.1 (3.1) | 24.9 (3.1) | 0.834 |
| Fasting blood glucose, mmol/L | 5.8 (5.1–7.2) | 5.5 (4.9–6.7) | 5.8 (5.0–7.3) | 6.0 (5.3–7.7) | <0.001 |
| TG, mmol/L | 1.5 (1.0–2.2) | 1.5 (1.1–2.3) | 1.5 (1.1–2.2 | 1.4 (1.0–2.0) | 0.156 |
| TC, mmol/L | 5.0 (4.3–5.7) | 4.9 (4.2–5.6) | 5.0 (4.3–5.7) | 5.1 (4.3–5.9) | <0.001 |
| LDL‐C, mmol/L | 2.9 (2.3–3.5) | 2.8 (2.2–3.3) | 2.9 (2.3–3.5) | 2.9 (2.3–3.6) | 0.002 |
| HDL‐C, mmol/L | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) | 0.807 |
| Ischemic stroke subtype | |||||
| Thrombotic | 2642 (78.2) | 663 (77.7) | 644 (75.5) | 685 (76.6) | 0.303 |
| Embolic | 164 (4.9) | 38 (4.5) | 44 (5.1) | 44 (5.1) | 0.280 |
| Lacunar | 655 (19.4) | 173 (20.3) | 190 (22.3) | 146 (17.1) | 0.221 |
Continuous variables are expressed as mean (standard deviation), or as median (interquartile range). Categorical variables are expressed as frequency (percent). ANGPTL‐4: 95.3 (71.4–126.5) ng/mL. ANGPTL‐4, angiopoietin‐like protein 4; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; TG, triglycerides; TC, total cholesterol; LDL‐C, low‐density lipoprotein cholesterol; HDL‐C, high‐density lipoprotein cholesterol; NIHSS, National Institute of Health Stroke Scale.
Association between plasma ANGPTL‐4 concentrations and clinical outcomes of ischemic stroke
During 3 months’ follow‐up, 850 (26.2%) participants experienced major disability or died (750 major disabilities and 100 deaths). After adjusted for age, sex, admission NIHSS score and other covariates, the ORs for the highest tertile of plasma ANGPTL‐4 concentrations were 1.59 (95% CI 1.22–2.06), 1.53 (95% CI 1.18–1.97), and 2.03 (95% CI 1.03–4.00) for the composite outcome of death and major disability, major disability alone and death alone, respectively, compared with the lowest tertile. Each increased 1‐SD of log‐ANGPTL‐4 concentration was associated with 24% (OR 1.24, 95% CI 1.11–1.38), 14% (OR 1.14, 95% CI 1.03–1.27), and 72% (OR 1.72, 95% CI 1.32–2.23) increased risk of for the composite outcome of death and major disability, major disability alone and death alone, respectively (Table 2). Multivariable‐adjusted spline regression models showed a linear association between plasma ANGPTL‐4 concentrations and the primary outcome (P for linearity = 0.001) and death (P for linearity = 0.001) (Fig. 1). Furthermore, Multivariable ordinal logistic regression analyses showed a significant shift in mRS distributions with plasma ANGPTL‐4 concentrations at 3 months after ischemic stroke (P value for trend <0.001; Fig. 2; Table S2). However, we did not find any significant association between plasma ANGPTL‐4 concentration and risk of stroke recurrence and vascular events.
Table 2.
Odds ratios and 95% confidence interval of 3‐month outcomes according to ANGPTL‐4 levels at baseline.
| Characteristics | ANGPTL‐4, ng/mL | Each SD increase in log‐ANGPTL‐4 | |||
|---|---|---|---|---|---|
| <78.7 | 78.7–112.9 | ≥112.9 | P trend | ||
| Primary outcome: death or major disability (mRS 3–6) | |||||
| Case, n (%) | 154 (14.4) | 280 (24.2) | 416 (36.3) | ||
| Age‐ and sex‐adjusted | 1.00 (reference) | 1.63 (1.30–2.05) | 2.72 (2.18–3.40) | <0.001 | 1.59 (1.46–1.73) |
| Multiple‐adjusted | 1.00 (reference) | 1.51 (1.16–1.97) | 1.64 (1.25–2.14) | <0.001 | 1.23 (1.11–1.37) |
| Secondary outcomes | |||||
| Major disability (mRS score 3–5) | |||||
| Case, n (%) | 141 (13.1) | 262 (22.6) | 347 (30.3) | ||
| Age‐ and sex‐adjusted | 1.00 (reference) | 1.70 (1.36–2.12) | 2.35 (1.88–2.92) | <0.001 | 1.43 (1.31–1.56) |
| Multiple‐adjusted | 1.00 (reference) | 1.52 (1.16–1.98) | 1.61 (1.24–2.09) | <0.001 | 1.14 (1.02–1.26) |
| Death | |||||
| Case, n (%) | 13 (1.2) | 18 (1.6) | 69 (6.0) | ||
| Age‐ and sex‐adjusted | 1.00 (reference) | 1.11 (0.54–2.27) | 4.05 (2.23–7.36) | <0.001 | 2.23 (1.79–2.78) |
| Multiple‐adjusted | 1.00 (reference) | 0.79 (0.35–1.759) | 2.10 (1.06–4.16) | <0.001 | 1.73 (1.33–2.24) |
| Stroke recurrence | |||||
| Case, n (%) | 19 (1.8) | 20 (1.7) | 25 (2.2) | ||
| Age‐ and sex‐adjusted | 1.00 (reference) | 0.92 (0.49–1.74) | 1.06 (0.57–1.98) | 0.838 | 0.95 (0.74–1.23) |
| Multiple‐adjusted | 1.00 (reference) | 0.85 (0.43–1.65) | 0.99 (0.50–1.94) | 0.972 | 0.93 (0.70–1.23) |
| Vascular events | |||||
| Case, n (%) | 22 (2.1) | 26 (2.2) | 50 (4.4) | ||
| Age‐ and sex‐adjusted | 1.00 (reference) | 1.01 (0.56–1.80) | 1.77 (1.04–2.99) | 0.020 | 1.22 (0.99–1.51) |
| Multiple‐adjusted | 1.00 (reference) | 0.88 (0.48–1.61) | 1.33 (0.75–2.35) | 0.280 | 1.09 (0.86–1.37) |
Multiple‐adjusted for age, sex, hospital location (by province), antihypertensive treatment, time from onset to hospitalization, current smoking, current alcohol drinking, body mass index, dyslipidemia, fasting blood glucose, history of hypertension, history of diabetes mellitus, family history of stroke, SBP at baseline, ischemic stroke subtypes and baseline NIHSS score. ANGPTL‐4, angiopoietin‐like protein 4; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale.
Figure 1.
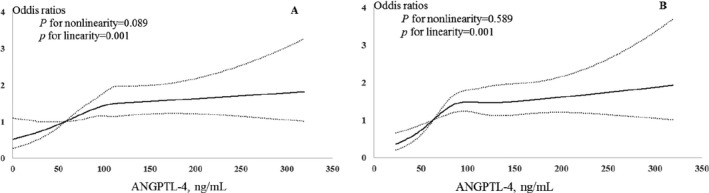
Relationship of plasma ANGPTL‐4 with study outcomes in patients with acute ischemic stroke. Odds ratios or hazard ratios and 95% confidence intervals derived from restricted cubic spline regression, with knots placed at the 5th, 35th, 65th, and 95th percentiles of the distribution of plasma ANGPTL‐4. The reference point is the midpoint of the reference group from categorical analysis. Odds ratios were adjusted for covariates shown in Table 2. (A) Primary outcome of death or major disability. (B) Death. ANGPTL‐4, angiopoietin‐like protein 4.
Figure 2.
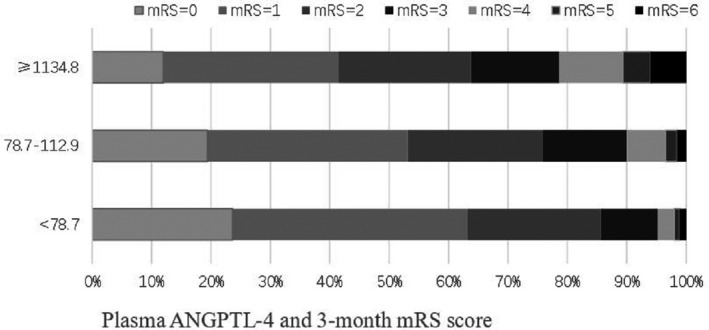
Plasma ANGPTL‐4 and mRS score at 3 months. ANGPTL‐4, angiopoietin‐like protein 4; mRS, modified Rankin Scale.
We further examined whether adding plasma ANGPTL‐4 to a model with conventional risk factors might improve the risk prediction of poor clinical outcomes after ischemic stroke. As seen in the Table 4, adding ANGPTL‐4 to the conventional risk factors improved continuous NRI of 11.36% (95% CI 2.31–20.41%; P = 0.014) and IDI of 0.23% (95% CI 0.11–0.44%; P = 0.033) for the composite outcome of death or major disability (Table 3).
Table 4.
Subgroup analysis of adjusted ORs (95% CI) of primary outcome according to plasma ANGPTL‐4 tertiles.
| Characteristics | ANGPTL‐4, ng/mL | Each SD increase in log‐ANGPTL‐4 | P interaction | |||
|---|---|---|---|---|---|---|
| <78.7 | 78.7–112.9 | ≥112.9 | P trend | |||
| Sex | ||||||
| Male | 1.00 | 1.58 (1.12–2.23) | 1.86 (1.33–2.60) | 0.011 | 1.29 (1.13–1.48) | 0.413 |
| Female | 1.00 | 1.69 (1.13–2.54) | 2.10 (1.38–3.21) | 0.010 | 1.41 (1.20–1.67) | |
| Age, years | ||||||
| <65 | 1.00 | 1.69 (1.18–2.42) | 1.72 (1.23–2.41) | 0.014 | 1.29 (1.11–1.49) | 0.100 |
| ≥65 | 1.00 | 1.39 (0.92–2.09) | 1.87 (1.26–2.76) | <0.001 | 1.28 (1.10–1.49) | |
| Baseline SBP, mmHg | ||||||
| <160 | 1.00 | 1.54 (0.99–2.41) | 1.86 (1.18–2.94) | 0.008 | 1.23 (1.03–1.47) | 0.054 |
| ≥160 | 1.00 | 1.53 (1.10–2.12) | 1.55 (1.11–2.16) | 0.013 | 1.24 (1.09–1.42) | |
| Body mass index, kg/m2 | ||||||
| <24 | 1.00 | 1.87 (1.24–2.81) | 2.29 (1.53–3.44) | <0.001 | 1.30 (1.12–1.52) | 0.250 |
| ≥24 | 1.00 | 1.26 (0.87–1.81) | 1.29 (1.01–1.83) | 0.034 | 1.17 (1.01–1.37) | |
| Dyslipidemia | ||||||
| No | 1.00 | 1.78 (1.20–2.64) | 2.02 (1.37–2.96) | 0.007 | 1.25 (1.08–1.45) | 0.224 |
| Yes | 1.00 | 1.15 (0.80–1.67) | 1.50 (1.04–2.17) | 0.020 | 1.22 (1.05–1.43) | |
| Cigarette smoking | ||||||
| No | 1.00 | 1.38 (1.01–1.91) | 1.69 (1.22–2.34) | 0.001 | 1.27 (1.11–1.45) | 0.088 |
| Yes | 1.00 | 1.48 (0.91–2.40) | 1.77 (1.11–2.83) | 0.034 | 1.18 (0.98–1.42) | |
| Current alcohol drinking | ||||||
| No | 1.00 | 1.37 (1.01–1.87) | 1.68 (1.22–2.30) | 0.001 | 1.25 (1.10–1.41) | 0.792 |
| Yes | 1.00 | 1.45 (0.86–2.44) | 2.03 (1.23–3.37) | 0.019 | 1.18 (0.95–1.45) | |
| Fasting plasma glucose, mmol/L | ||||||
| <7 | 1.00 | 1.63 (1.19–2.24) | 1.69 (1.25–2.29) | 0.004 | 1.25 (1.10–1.41) | 0.524 |
| ≥7 | 1.00 | 1.23 (0.74–2.05) | 1.58 (0.96–2.58) | 0.046 | 1.18 (0.97–1.47) | |
| Receiving immediate BP reduction | ||||||
| No | 1.00 | 1.24 (0.85–1.80) | 1.42 (0.96–2.08) | 0.075 | 1.17 (1.01–1.36) | 0.190 |
| Yes | 1.00 | 1.82 (1.25–2.65) | 1.84 (1.26–2.68) | 0.002 | 1.30 (1.12–1.51) | |
In the multivariate models, confounding factors such as age, sex, hospital location (by province), antihypertensive treatment, time from onset to hospitalization, current smoking, current alcohol drinking, body mass index, dyslipidemia, fasting blood glucose, history of hypertension, history of diabetes mellitus, family history of stroke, SBP at baseline, ischemic stroke subtypes and baseline NIHSS score were included unless the variable was used as a subgroup variable. ORs, odds ratios; CI, confidence interval; ANGPTL‐4, angiopoietin‐like protein 4; SBP, systolic blood pressure.
Table 3.
Reclassification and discrimination statistics for 3‐month clinical outcomes by plasma ANGPTL‐4 among patients with acute ischemic stroke.
| Characteristics | C statistic | NRI (continuous), % | IDI, % | |||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | Estimate (95% CI) | P value | |
| Death or major disability (mRS score 3–6) | ||||||
| Conventional model | 0.822 (0.806–0.836) | Reference | Reference | |||
| Conventional model + ANGPTL‐4 | 0.823 (0.808–0.838) | 0.209 | 11.36 (2.31–20.41) | 0.014 | 0.23 (0.11–0.44) | 0.033 |
| Major disability (mRS score 3–5) | ||||||
| Conventional model | 0.804 (0.789–0.819) | Reference | Reference | |||
| Conventional model + ANGPTL‐4 | 0.805 (0.789–0.820) | 0.464 | 1.11 (−8.30 to 10.53) | 0.817 | 0.11 (−0.21 to 0.17) | 0.292 |
| Death | ||||||
| Conventional model | 0.819 (0.804–0.833) | Reference | Reference | |||
| Conventional model + ANGPTL‐4 | 0.830 (0.816–0.845) | 0.204 | 43.11 (19.35–66.87) | <0.001 | 0.85 (−0.64 to 2.33) | 0.26 |
Conventional model included age, sex, hospital location (by province), antihypertensive treatment, time from onset to hospitalization, current smoking, current alcohol drinking, body mass index, dyslipidemia, fasting blood glucose, history of hypertension, history of diabetes mellitus, family history of stroke, SBP at baseline, ischemic stroke subtypes and baseline NIHSS score. ANGPTL‐4, angiopoietin‐like protein 4; NRI, net reclassification improvement; IDI, integrated discrimination index; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; SBP, systolic blood pressure.
In subgroup analyses, the significant associations of ANGPTL‐4 concentrations with risk of primary outcome were observed in almost all subgroups, whereas no significant interactions between plasma ANGPTL‐4 and any of these interested variables on primary outcome were observed (Table 4).
Discussion
Up to now, the reports on the effect of circulating ANGPTL‐4 concentrations on prognosis after ischemic stroke were rarely seen, although there had been some studies suggesting that increased ANGPTL‐4 concentration increase risks of hypertension, 10 atherosclerosis, 11 coronary artery disease, 10 cardiovascular events, 10 and stroke 11 in general population. In a case‐control study, ANGPTL‐4 concentrations were significantly correlated with increased NIHSS scores (r = 0.172, P = 0.003) and large volumes of brain lesion (r = 0.124, P = 0.031) among the cases with ischemic stroke, 18 indicating that ANGPTL‐4 concentrations may be associated with prognosis of ischemic stroke. We investigated the associations between plasma ANGPTL‐4 concentrations and clinical outcomes after ischemic stroke in the patients from the CATIS trial with large sample size and strict data collection. In the analysis, we adjusted for almost all important established risk factors including lifestyle risk factors, medical history, clinical characteristics, and admission NIHSS score. Our findings showed positive associations of increased plasma ANGPTL‐4 concentrations with composite outcome of death and major disability, major disability and death at 3 months after ischemic stroke. Furthermore, subgroup analyses also showed significant associations in almost all subgroups. In addition, adding plasma ANGPTL‐4 to conventional risk factors significantly improved risk‐predictive ability for composite outcome of death and major disability. Our study suggested that increased plasma ANGPTL‐4 concentrations may be an independent risk factor for poor prognosis of ischemic stroke and could improve risk prediction ability for the combined outcome of death and major disability.
Some studies had found that ANGPTL‐4 loss‐of‐function mutations were substantially associated with lower triglyceride levels, and a lower risk of coronary artery disease and ischemic stroke. 10 , 11 , 19 ANGPTL‐4 might be involved in lipid metabolisms. 6 , 7 Accumulating evidences suggested that ANGPTL‐4 regulated triglyceride in circulation by inhibiting lipoprotein lipase and modulate the uptake of free fatty acids. 20 , 21 Muendlein et al. reported that plasma ANGPTL‐4 concentrations significantly predicted future cardiovascular events in patients with coronary artery disease. 10 Furthermore, a case‐control study found that serum ANGPTL‐4 levels were significantly higher in patients with large artery atherosclerotic stroke than in healthy controls, and ANGPTL‐4 concentrations were significantly correlated with increased NIHSS scores (r = 0.172, P = 0.003) and large volumes of brain lesion (r = 0.124, P = 0.031). 18
Previous observational studies and randomized controlled trials had demonstrated that circulating triglycerides were one of the most important modifiable and causal risk factors for cardiovascular disease and ischemic stroke. 22 , 23 , 24 Lipoprotein lipase is the main enzyme that hydrolyzes lipoprotein triglycerides and releases free fatty acids for tissue utilization and clearance. 25 Increasing lipoprotein lipase activity could decrease serum triglyceride concentrations, whereas decreased activity of lipoprotein lipase has been shown to increase serum triglyceride concentrations and risk of cardiovascular disease. 26 , 27 Our findings had some clinical implications. It is of clinical interest to evaluate whether inhibition of ANGPTL‐4 had therapeutic value in ischemic stroke patients. In fact, there had been evidence that supported ANGPTL‐4 as a therapeutic target for reducing the risk of cardiovascular disease in humans. 19 The mechanisms that association of increased ANGPTL‐4 level with increased risk of poor prognosis after stroke are not clear. Several potential pathophysiological mechanisms might explain the association, for example, ANGPTL‐4 might highly expressed under the pressure of stimuli hypoxia in endothelial cells, cardiomyocytes, and ischemic brain injury, 28 , 29 and increased ANGPTL‐4 level might play role in endothelial dysfunction, 30 atherosclerosis, 31 vascular permeability, 32 and angiogenesis. 31
Our study had some limitations. First, this study was a post hoc analysis of CATIS and some serious patients (BP ≥220/120 mmHg) or those treated with intravenous thrombolytic therapy at admission were excluded. Therefore, a selection bias may unavoidably be present. However, baseline characteristics of the participants were similar to those from the China National Stroke Registry, 33 indicating that the selection bias may be minimal. Second, plasma ANGPTL‐4 concentrations were tested only once for each patient at baseline, and changes over time were not considered. Third, this study was limited to Chinese individuals, thus, the results may not be extrapolated to other race/ethnic groups. More evidences are needed to determine the validity of present findings through independent replications in more race/ethnic populations. Finally, relatively few number of stroke recurrence and vascular events (maybe because some fatal stroke recurrences and vascular events were classified into death events) were observed during 3‐month follow‐up of relatively short time, which might limit our power to detect significant association between plasma ANGPTL‐4 and stroke recurrences and vascular events.
In conclusion, this study indicated that increased plasma ANGPTL‐4 concentrations at admission were associated with poor prognosis in ischemic stroke patients and adding plasma ANGPTL‐4 to conventional risk factors significantly improved predictive ability for risk for composite outcome of death and major disability, indicating that ANGPTL‐4 may be a potential prognostic biomarker for ischemic stroke.
Conflict of Interest
The authors declare that they have no competing interests.
Author’s Contributions
Tan Xu, Jing Chen, YonghongZhang, and Jiang He conceived and designed the study. Tan Xu, Yanbo Peng, and Yonghong Zhang coordinated the study. Zhengbao Zhu, Chongke Zhong, Daoxia Guo, Aili Wang, Tan Xu, Hao Peng, Suwen Shen, Zhong Ju, Deqin Geng, Yonghong Zhang, and Jiang He oversaw subjects’ recruitment and monitored gathering of clinical data. Yonghong Zhang and Jiang He revised the paper.
Supporting information
Tables S1. Baseline characteristics between the assayed and total patients’ groups.
Table S2. Baseline characteristics between the assayed and not‐assayed groups.
Table S3. Odds ratios and 95% confidence interval of 3‐months outcomes according to ANGPTL‐4 levels at baseline.
Acknowledgments
We thank the study participants and their relatives and the clinical staff at all participating hospitals for their support and contribution to this project.
This manuscript was uploaded to preprint server and the link was https://www.researchsquare.com/article/rs‐31690/v1.
Funding Information
This study was supported by the National Key Research and Development Program of China (grant: 2016YFC1307300), the National Natural Science Foundation of China (grant: 81673263), and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Funding Statement
This work was funded by National Natural Science Foundation of China grant 81673263; National Key Research and Development Program of China grant 2016YFC1307300; Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions grant .
References
- 1. Feigin VL, Krishnamurthi RV. 13 – global burden of stroke[J]. Stroke 2016:165–206. [Google Scholar]
- 2. Meschia JF, Bushnell C, Boden‐Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta‐analysis. Lancet 2007;369:1876–1882. [DOI] [PubMed] [Google Scholar]
- 4. Furie KL, Kasner SE, Adams RJ. Guidelines for the prevention of stroke in patient with stroke or transient ischemic attack. Stroke 2011;42:1–50. [DOI] [PubMed] [Google Scholar]
- 5. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tai H, Tabata M, Oike Y. The role of angiopoietin‐like proteins in angiogenesis and metabolism. Trends Cardiovasc Med 2008;18:6–14. [DOI] [PubMed] [Google Scholar]
- 7. Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin‐like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med 2005;11:473–479. [DOI] [PubMed] [Google Scholar]
- 8. Abu‐Farha M, Cherian P, Qaddoumi MG, et al. Increased plasma and adipose tissue levels of ANGPTL8/Betatrophin and ANGPTL4 in people with hypertension. Lipids Health Dis 2018;17:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adachi H, Fujiwara Y, Kondo T, et al. Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochem Biophys Res Commun 2009;379:806–811. [DOI] [PubMed] [Google Scholar]
- 10. Muendlein A, Saely CH, Leiherer A, et al. Angiopoietin‐like protein 4 significantly predicts future cardiovascular events in coronary patients. Atherosclerosis 2014;237:632–638. [DOI] [PubMed] [Google Scholar]
- 11. Yang Q, Yin RX, Cao XL, et al. ANGPTL4 variants and their haplotypes are associated with serum lipid levels, the risk of coronary artery disease and ischemic stroke and atorvastatin cholesterol‐lowering responses. Nutr Metab (Lond) 2018;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019;18:394–405. [DOI] [PubMed] [Google Scholar]
- 13. He J, Zhang Y, Xu T, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA 2014;311:479–489. [DOI] [PubMed] [Google Scholar]
- 14. Brott TG, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 15. Kurtz TW, Griffin KA, Bidani AK, et al. Recommendations for blood pressure measurement in animals: summary of an AHA scientific statement from the Council on High Blood Pressure Research, Professional and Public Education Subcommittee. Arterioscler Thromb Vasc Biol 2005;25:478–479. [DOI] [PubMed] [Google Scholar]
- 16. Delong ER, Delong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 17. Kharitonenkov A, Wroblewski VJ, Koester A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor‐21. Endocrinology 2007;148:774–781. [DOI] [PubMed] [Google Scholar]
- 18. He X‐W, Shen Y‐G, Zhu M, et al. Angiopoietin‐like protein 4 serum levels and gene polymorphisms are associated with large artery atherosclerotic stroke. J Neurol Sci 2016;362:333–338. [DOI] [PubMed] [Google Scholar]
- 19. Dewey FE, Gusarova V, O'Dushlaine C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med 2016;374:1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lafferty MJ, Bradford KC, Erie DA, Neher SB. Angiopoietin‐like protein 4 inhibition of lipoprotein lipase: evidence for reversible complex formation. J Biol Chem 2013;288:28524–28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin‐like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA 2006;103:17450–17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collaboration* TERF . Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willey JZ, Qiang X, Boden‐Albala B, et al. Lipid profile components and risk of ischemic stroke the Northern Manhattan Study (NOMAS). Arch Neurol 2009;66:1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 2009;297:E271–E288. [DOI] [PubMed] [Google Scholar]
- 26. Jensen MK, Rimm EB, Rader D, et al. S447X variant of the lipoprotein lipase gene, lipids, and risk of coronary heart disease in 3 prospective cohort studies. Am Heart J 2009;157:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rip J. Lipoprotein lipase S447X: a naturally occurring gain‐of‐function mutation. Arterioscler Thromb Vasc Biol 2006;26:1236–1245. [DOI] [PubMed] [Google Scholar]
- 28. Galaup A, Gomez E, Souktani R, et al. Protection against myocardial infarction and no‐reflow through preservation of vascular integrity by angiopoietin‐like 4. Circulation 2012;125:140–149. [DOI] [PubMed] [Google Scholar]
- 29. Wiesner G, Brown RE, Robertson GS, et al. Increased expression of the adipokine genes resistin and fasting‐induced adipose factor in hypoxic/ischaemic mouse brain. Neuroreport 2006;17:1195–1198. [DOI] [PubMed] [Google Scholar]
- 30. Huang RL, Teo Z, Han CC, et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE‐cadherin and claudin‐5 clusters. Blood 2011;118:3990–4002. [DOI] [PubMed] [Google Scholar]
- 31. Katano H, Yamada K. Upregulation of ANGPTL4 messenger RNA and protein in severely calcified carotid plaques. J Stroke Cerebrovasc Dis 2014;23:933–947. [DOI] [PubMed] [Google Scholar]
- 32. Guo L, Li SY, Ji FY, et al. Role of Angptl4 in vascular permeability and inflammation. Inflamm Res 2013;63:13–22. [DOI] [PubMed] [Google Scholar]
- 33. Luo Y, Wang X, Matsushita K, et al. Associations between estimated glomerular filtration rate and stroke outcomes in diabetic versus nondiabetic patients. Stroke 2014;45:2887–2893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1. Baseline characteristics between the assayed and total patients’ groups.
Table S2. Baseline characteristics between the assayed and not‐assayed groups.
Table S3. Odds ratios and 95% confidence interval of 3‐months outcomes according to ANGPTL‐4 levels at baseline.


