Abstract
Ever since the role of endogenous nitric oxide (NO) in controlling a wide variety of biological functions was discovered approximately three decades back, multiple NO releasing polymeric materials have been developed. However, most of these materials are typically short lived due to the inefficient incorporation of the NO donor molecules within the polymer matrix. In the present study, S-nitroso-N-acetylpenicillamine (SNAP) is covalently attached to polydimethylsiloxane (PDMS) to create a highly stable nitric oxide (NO) releasing material for biomedical applications. By tethering SNAP to the crosslinker of PDMS, the NO donor is unable to be leached into the surrounding environment. This is the first time that a sustainable NO release and bacterial inhibition for over 125 days has been achieved by any NO releasing polymer with supporting evidence of potential long-term hemocompatibility and biocompatibility. The material proves to have very high antibacterial efficacy against Staphylococcus aureus by demonstrating a 99.99% reduction in the first 3 days in a continuous flow CDC bioreactor while a similar inhibitory potential of 99.50% was maintained by the end of one month. Hemocompatibility of SNAP-PDMS was tested using a rabbit extracorporeal circuit (ECC) model over a 4 h period. Thrombus formation was greatly reduced within the SNAP-PDMS coated ECCs compared to the control circuits, observing a 78% reduction in overall thrombus mass accumulation. These results demonstrate the potential of utilizing this material for blood and tissue contacting biomedical devices in long-term clinical applications where infection and unwanted clotting are major issues.
Keywords: nitric oxide, hemocompatibility, antimicrobial, bioreactor, S-nitroso-N-acetyl penicillamine, extracorporeal circulation
Graphical Abstract
1. Introduction
When a foreign material or medical device comes in contact with blood, proteins such as albumin and fibrinogen immediately adsorb to the surface to facilitate the attachment of platelets. Once attached, platelets directly interact with the adsorbed proteins, exposing the glycoprotein GPIIb/IIIa integrin receptor that allows platelet binding to fibrinogen.1 This facilitates more platelet activation and aggregation to form a clot. In addition to reducing the efficiency of the implant, these clots also have a risk of breaking off and causing embolism further down the vasculature. Infection is another complication that occurs with medical devices in a clinical setting. The longer devices such as catheters and endotracheal tubes stay within a patient, the higher the risk of an infection.2 This leads to constant removal and replacement of these devices, requiring invasive surgeries and overall discomfort to the patient.
A strategy to improve the biocompatibility in these scenarios can be through the utilization of nitric oxide (NO). Nitric oxide is a free radical molecule produced in the body with a wide range of biological signaling functions. Some of the most notable physiological functions are the prevention of platelet adhesion to vasculature, regulation of blood pressure through vasodilation, and as a method for macrophages to eliminate pathogens via nitrosative stress.3–4 Knowing these mechanisms has led to the development of NO releasing materials that are capable of mimicking vital endogenous effects under certain conditions. S-nitrosothiols (RSNOs) are a popular class of NO releasing molecules that are produced by the body. Some RSNOs such as S-nitroso-N-acetyl penicillamine (SNAP), S-nitrosoglutathione (GSNO), and S-nitrosocysteine have been incorporated into polymer matrices to create environments with localized and controlled NO release.5–7 The release mechanism of NO from RSNOs is done by the cleavage of the sulfur-nitrogen bond and is facilitated by either thermal degradation, metal ion catalysis, and/or light. Thermal degradation is one of those most commonly used methods for initiating this release for RSNO containing materials as in vivo temperatures are able to promote a passive, steady NO release. Diazeniumdiolates are another class of NO-donating compounds that use physiological temperature and pH to passively release large quantities of NO over short periods of time.8
The role of NO releasing polymers as a method to prevent thrombus formation has been thoroughly investigated in multiple studies in vivo using both extracorporeal circuit (ECC) and catheter models.9–14 Since NO has a short half-life, its mode of action demonstrates a more localized effect when suppressing platelet activation. Blood thinners such as heparin have systemic effects within a patient which can lead to low platelet counts, unwanted internal bleeding, and thrombocytopenia.15–16 Polymers capable of generating NO are a possible solution to this as they demonstrate a drastic reduction in thrombus formation and platelet adhesion on both ECC and catheter surfaces without having this detrimental systemic effect.
Another common problem with tissue contacting medical devices is the ongoing risk of infection. A large number of antimicrobial surfaces have been developed with strategies such as incorporating metal or metal oxide nanoparticles like silver17–19 and copper20–22 as well as polymeric materials that facilitate the diffusion of certain antibiotics.23–25 However, these metallic nanoparticles can be cytotoxic to the surrounding tissue in addition to the bacteria being targeted if used in high concentration. Drug-resistant bacteria have also been on the rise, limiting the potential application of antibiotics.26–27 Antibiotics have also been shown to be ineffective against biofilms, which provide a protective matrix containing proteins and polysaccharides for the bacteria.28 Controlled levels of nitric oxide from a wide range of nitric oxide donors have been shown to be able to eliminate numerous types of bacterial strains while still demonstrating noncytotoxic effects to surrounding cells.29–32 This strategy utilizes NO as an antimicrobial agent in a similar manner the way macrophages employ it to eliminate bacteria and has proven to be effective against antibiotic-resistant strains of bacteria and to disperse biofilms.33–35 Most NO releasing materials are generally short-lived and cannot be applied to materials or devices that require a long implantation time. The most efficient way to have a long-term NO releasing antimicrobial material is to ensure the covalent attachment of an RSNO to a material. This strategy prevents leaching out of the polymer matrix, which usually yields high bursts of NO over a short period of time and depletion of its NO reservoir. High molecular weight dendrimers have also been synthesized with covalently bound NO donors like RSNO’s and diazeniumdiolates and have shown to have a large, controlled NO releasing capacity.36–37 Using this strategy, synthetic have shown the potential to be stable in vitro platforms for delivering NO to cells over a monitored period of time.38–39
Herein, we investigated the effectiveness of covalently attaching SNAP to PDMS (SNAP-PDMS) as a biocompatible polymer to ward off unwanted thrombus formation and as a long-term antimicrobial agent. By covalently attaching SNAP to the aminosilane crosslinker in PDMS, its in vitro longevity release capabilities were proven by demonstrating the ability to maintain a stable, unprecedented NO release for over 125 days under physiological conditions. The long-term NO release opens a large array of possibilities such as the fabrication and modification of a variety of silicone rubber based biomedical devices such as blood and urinary catheters, PICC lines, and feeding tubes. In terms of hemocompatibility, SNAP-PDMS was coated on the inner lumen of PDMS tubing used for 4 h ECC experiments to observe thrombus formation and overall platelet count using a rabbit model. In a separate experiment, SNAP-PDMS films were tested for 28 days using a CDC biofilm reactor to demonstrate the polymer’s antimicrobial capabilities.
2. Materials and Methods
2.1. Materials.
N-Acetyl-D-penicillamine (NAP), hydroxy terminated poly(dimethylsiloxane) 2550–3570 cSt (PDMS), (3-aminopropyl) trimethoxysilane, dibutyltin dilaurate, toluene, chloroform, pyridine, tert-butyl nitrite, acetic anhydride, ethylenediaminetetraacetic acid (EDTA), concentrated hydrochloric acid (HCl), 1,4,8,11-tetraazacyclotetradecane (cyclam), anhydrous magnesium sulfate, and hexanes were purchased from Sigma Aldrich (St. Louis, MO). Trypsin-EDTA and Cell Counting Kit-8 (CCK-8) was purchased from Sigma-Aldrich (St. Louis, MO). The antibiotic Penicillin-Streptomycin (Pen-Strep) and fetal bovine serum (FBS) were obtained from Gibco-Life Technologies (Grand Island, NY). The bacterial strain Staphylococcus aureus (ATCC 5538) and mouse 3T3 cells (ATCC 1658) were originally obtained from American Type Culture Collection.
2.1.1. Synthesis of NAP-thiolactone.
Thiolactone self-protected NAP was synthesized using the established protocol developed by Moynihan and Robert.40 Briefly, 5 g of NAP was dissolved in 10 mL of pyridine in a round bottom flask while a separate vial containing 10 mL of pyridine and 10 mL of acetic anhydride was made. Both solutions were allowed to chill in an ice bath for 1 h before being combined and allowed to stir for 24 hrs. The solution was then rotary evaporated at 60 °C until all of the pyridine is evaporated and only small amount of a viscous, orange solution remains. The remaining solution was then dissolved in 20 mL of chloroform and washed and extracted three times with equal volume quantities of 1M HCl. The organic layer was then dried over anhydrous magnesium sulfate and filtered. The chloroform is then removed under vacuum at room temperature and the resulting solid is washed and filtered with hexanes. The collected pale, white-yellow solid is then dried overnight at room temperature before being stored at 5 °C (1.16 g).
2.1.2. Synthesis of SNAP-PDMS.
SNAP-PDMS was synthesized by slightly modifying a protocol by Frost et al.41 A schematic is shown in Figure 1, where initially 1.6 g of hydroxy terminated PDMS was dissolved in 8 mL of toluene. In a separate vial, 0.3 g of (3-aminopropyl) trimethoxysilane (1.67 mmol) and 2.4 mg of dibutyltin dilaurate were dissolved in 2 mL of toluene. The two solutions were then combined and thoroughly mixed and allowed to stir overnight. A slight excess (300 mg, 1.73 mmol) of NAP-thiolactone with respect to the crosslinking agent was dissolved in the crosslinked PDMS solution and then allowed to stir for 24 hrs. Nitrosation of the formed NAP-PDMS was done by adding t-butyl nitrite. T-butyl nitrite was first chelated of any copper contaminants by vortexing it with an equal volume amount of 20 mM cyclam solution and repeated three times. The organic t-butyl nitrite layer is then separated into an amber vial and stored at 5 °C. 300 μL of t-butyl nitrite is then added to 3 mL of NAP-PDMS to form a dark green, SNAP-PDMS solution. The nitrosated solution was then placed into a 2.54 cm diameter Teflon ring, protected from light, and left to air dry overnight.
Figure 1.
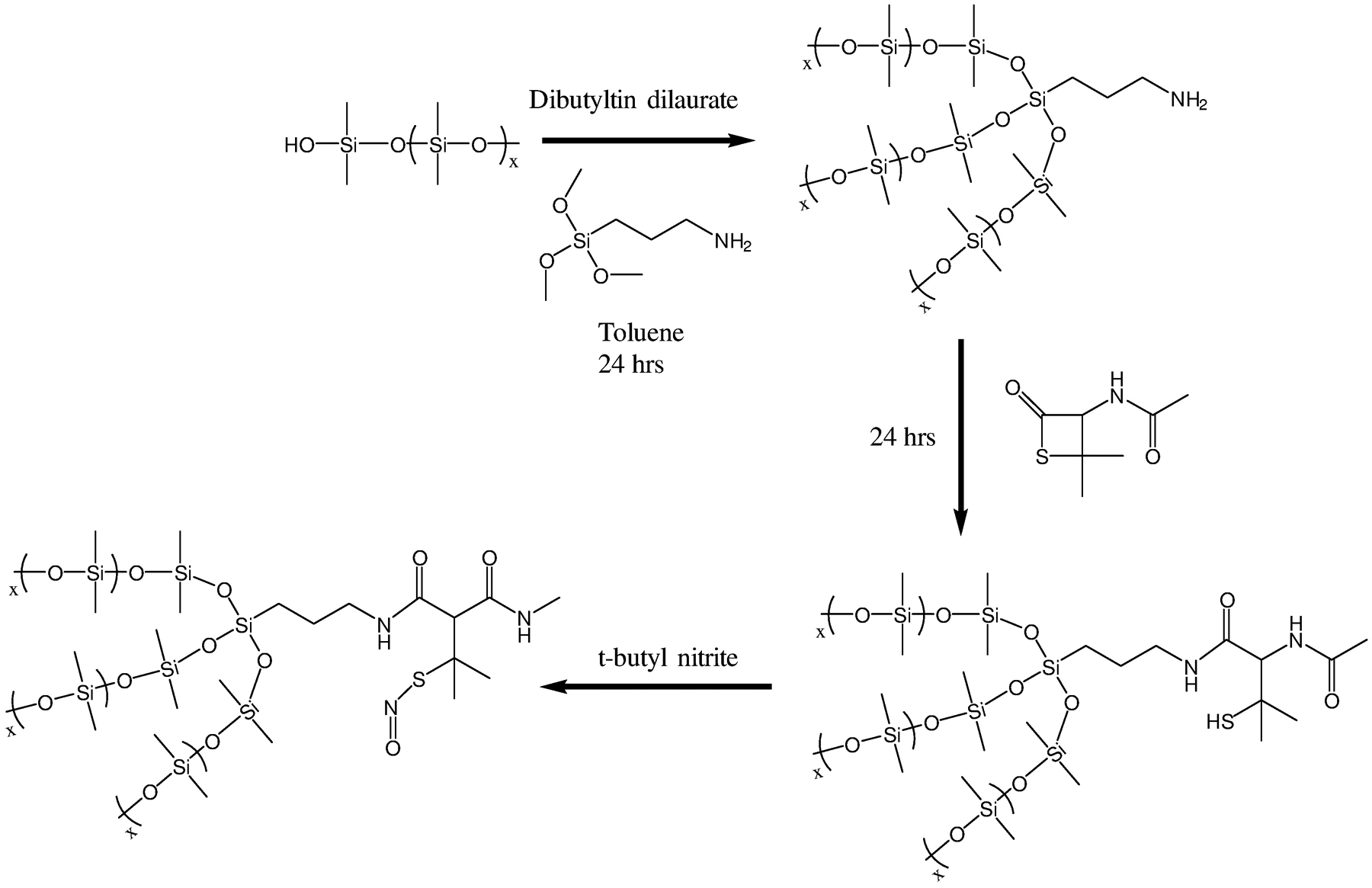
Synthesis route for covalently binding the SNAP molecule to hydroxy terminated PDMS polymer.
2.1.3. Nitric oxide detection.
NO release from the SNAP-PDMS polymers was directly measured in real time via chemiluminescence using a Sievers Nitric Oxide Analyzer (NOA) model 280i (Boulder, CO). Films were tested by immersing them in 0.01M PBS containing EDTA at 37 °C inside of an amber reaction chamber. A nitrogen bubbler was then placed in the solution containing the polymer at a flow rate of 200 mL min−1 to carry any NO being emitted to the NOA.
2.1.4. SNAP leaching assay.
Comparison of leaching between covalent bound SNAP-PDMS and blended SNAP PDMS was quantified by a Genesys 10S UV-Vis spectrophotometer (ThermoFisher, Waltham, MA). The characteristic S-nitroso bond seen in RSNO’s shows absorbance maxima at 340 and 590 nm.42–43 Diffusion of SNAP from the PDMS was done by soaking SNAP-PDMS and SNAP blended PDMS films for varying amounts of time in 0.01M PBS (pH = 7.4) with 100 μm EDTA to ensure there is little catalytic metal ion interaction in the buffer. Blended SNAP in PDMS and covalent bound SNAP-PDMS were both tested with and without a topcoat of PDMS. Film were measured and incubated in PBS with EDTA at room temperature while being protected from light to preserve the SNAP leaching values over the course of the study. Measured aliquots of the PBS solution were placed into cuvettes to be measured at 340 nm.
2.1.5. Bacterial adhesion assay.
The ability of SNAP-PDMS to prevent bacterial binding and growth on the polymeric surface was tested in vitro for 3, 14, and 28 days in a continuous flow CDC bioreactor (BioSurface Technologies) against Staphylococcus aureus (S. aureus). The use of CDC bioreactor provides a highly favorable environment for bacterial growth through a continuous supply of nutrients for the formation of a biofilm on the polymeric surface to test the long-term performance of the polymer. A single isolated colony of the bacterial strain (S. aureus) from a pregrown culture was incubated overnight in LB medium for 14 h at 150 rpm at 37 °C. After 14 h, optical density (O.D) of the liquid suspension of bacteria was measured at 600 nm (OD600) using a UV-vis spectrophotometer as recommended by earlier reports.44 Before being tested, the sample films (SNAP-PDMS and control PDMS, n=3 for each time point) were sterilized by UV irradiation for 30 mins under a Biosafety Cabinet (Thermo Scientific 1300 A2) and fitted inside the CDC bioreactor. Prior to use, the CDC bioreactor was sterilized using high pressure saturated steam for 30 min at 121 °C in an autoclave. The CDC bioreactor (working volume 1000 mL) with 400 mL of LB medium (2 g L−1) was inoculated with the bacterial culture in a manner that the final OD600 falls in the range of 107–109 CFU mL−1 to simulate chronic infection conditions. The CDC bioreactor on one end was connected to a feed bottle having a continuous supply of sterile LB medium (2 g L−1) and to a sealed container to collect the wash out in a sterile manner on the other end. After 3, 14, and 28 days, the films (controls and tests) were removed and gently rinsed with phosphate buffer saline, pH 7.4 (PBS) to get rid of any loosely bound bacteria. The rinsed films were then transferred to a 15 mL tube with 2 mL sterile PBS and homogenized for 60 sec using an OmniTip homogenizer. The shear force from the homogenizer tip ensured the transfer of the bound bacterial strains from the tubing to the PBS solution. Thereafter, serial dilution (10−1 to 10−5) were made using sterile PBS and bacterial strains were plated on different Petri-dishes solid LB-agar medium using an L-spreader. After adjusting the dilution factor, the volume of bacteria culture plate, starting culture volume, and other variables, the antimicrobial efficacy of the SNAP-PDMS films was compared to the control films as follows.
2.1.6. In vitro cell cytotoxicity.
Cell cytotoxicity was performed on SNAP-PDMS films on 3T3 mouse fibroblast cells (ATCC-1658) per the ISO 10993 standard. The Cell Counting Kit-8 (CCK-8) protocol was followed which uses WST-8 dye [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt]. WST-8 is enzymatically reduced in live cells to produce formazan, which is detectable at 450 nm. This measurement is then used to directly quantify the number of living cells while not requiring the killing of the cells.
Mouse fibroblast cells were cultured on 75 cm2 T-flasks in DMEM containing 4.5 g L−1 glucose and L-glutamine, 10% FBS, and 1% penicillin-streptomycin at 37 °C under a humidified atmosphere with 5% CO2. Once confluency reached 90%, cells were trypsinized (0.18% trypsin and 5 mM EDTA) and seeded in 96-well plates at a concentration of 5000 cells mL−1. Simultaneously, leachates from control PDMS and SNAP-PDMS films were obtained by soaking the films (n=3) in DMEM medium (1 mL of medium per 1 mg of polymer film) and incubated for 24 h at 37 °C. The films were then removed from the solution and discarded while the DMEM containing extracts was kept at 4 °C prior to being used in the cell culture experiments.
The previously made suspension of cells (5000 cells mL−1) were inoculated in a 96 well plate (100 μL per well) to be used for cytotoxicity studies. The 96 well plate was then incubated at 37 °C, 5% CO2 for 24 h before the leachates were added (10 μL) to each well. The plate was then incubated for another 24 h to allow the possible toxic leachates to impact the cells. Each well then had 10 μL of CCK-8 solution added and was incubated for another 3 h. The absorbance was measured at 450 nm and a comparison was made between the cells with and without leachate to calculate relative cell viability. Results were reported as a percentage of cell viability using the following equation.
2.1.7. Extracorporeal circuit preparation.
The ECC loop configuration was used as previously described.45–46 Briefly, the fully constructed ECC loops consisted of 16-gauge and 14-gauge IV polyurethane angiocatheters (Kendall Monoject Tyco Healthcare Mansfield, MA), two 16 cm lengths of 1/4 inch inner diameter (ID) silicone rubber (SR) tubing, and one 8 cm length of 3/8 inch SR tubing to create a thrombogenicity chamber to promote stagnant and recirculating regions of blood. The angiocatheters were coated only a single time with a more dilute solution of SNAP-PDMS (80 mg mL−1). The SR control ECC loops consisted of the SR tubing (no SNAP) and angiocatheters coated with PDMS at the same concentrations as the SNAP-PDMS ECC loops. All ECC loops pieces were assembled together using a solution of 80 mg mL−1 PDMS in toluene. The tubing and coating solutions were protected from light throughout this process to minimize the loss of NO. The ECC loops dried under ambient conditions for 48 h followed by vacuum drying for 24 h. The ECC loops were soaked in saline solution for 1 h and this solution was discarded immediately prior to the rabbit experiment. Small sections of the tubing were used to examine the NO release before and after the study.
2.1.8. Rabbit ECC Model.
A previously used rabbit ECC model was used to evaluate the hemocompatibility of the SNAP-PDMS coated tubing.45–47 All animal handling and surgical procedures were approved by the University of Georgia Institutional Animal Care and Use Committee. Over the course of the study, 8 New Zealand white male rabbits (2.5–3.5 kg, Charles River) were used. All rabbits were anesthetized using intramuscular injections of ketamine (7 mg kg−1), acepromazine (0.01 mg kg−1), midazolam (0.1 mg kg−1), and buprenorphine (0.03 mg kg−1). Isoflurane gas was used as a maintenance anesthesia in 100% oxygen, delivered via tracheotomy at an inhalation rate of 1–3%. Anesthesia was then maintained through mechanical ventilation of isoflurane in 100% oxygen (from 0.5–1.5%) at a rate of 12 breaths per minute and a tidal volume of 10–15 mL kg−1 (Hallowell EMC, Pittsfield, MA 01201). Blood pressure was carefully monitored using a Doppler ultrasonic flow probe (Parks Medical, Las Vegas, NV 89119). Continuous ECG and heart rate were carefully monitored using a multiparameter monitor (Grady Medical, Los Angeles, CA). To aid in the maintenance of blood pressure stability, Lactated Ringer’s were administered at a rate of 10 mL kg−1 h−1 via catheterization of the ear vein. Body temperature was measured via a rectal probe and maintained at 38 °C using a warm water heating blanket and forced air heater. Before the ECC experiment began, blood samples were collected to obtain baseline measurements.
The ECC was primed with 0.9% NaCl and then clamped and placed into position by cannulating the right carotid artery and left jugular vein. Flow through the ECC was then initiated by unclamping both ends to allow blood to move freely through the loop and monitored using an ultrasonic flow probe and flow meter (Transonic 400 Ithaca, NY). Clotting of the ECC loop was defined as when the flow rate reached 0 mL min−1 and remained at no flow for 5 min. After clotting occurred or after the 4 h time period had been reached, the ECC loop was clamped, removed from the animal, and rinsed with 60 mL of saline to observe any clotting. Any clots that were formed in the ECC loop were collected, weighed, and stored in formalin. All animals were not systemically anticoagulated during the experiments.
Whole blood samples were drawn from the femoral artery by direct catheterization and collected for analysis of complete blood count using a Heska Element HT5 Hematology Analyzer (CBC; including platelet count). Blood samples were collected every hour for 4 h following the initiation of flow through the ECC, where 1 mL of blood was drawn before each sample was collected. Complete blood count was performed using an impedance counter (CBC-Diff, Heska Corp. Loveland, CO).
2.1.9. Statistical analysis.
Data taken were expressed as mean ± standard deviation. Statistical analysis was carried out using a student’s t-test with SAS JMP software. P value < 0.05 was considered statistically significant for all data throughout the study.
3. Results and Discussion
3.1. Nitric Oxide Release Measurements and Characterization
Release of NO from SNAP-PDMS films was measured in real time using a chemiluminescence nitric oxide analyzer. Samples were tested in amber reaction vessels containing 0.01M PBS with EDTA at 37 °C using a nitrogen bubbler and sweep gas at a combined flowrate of 200 mL min−1. Up to 125 days of NO testing was done on SNAP-PDMS films while being incubated under the same testing conditions for the entire duration. By the end of the study, the films still possessed the characteristic green color seen with tertiary RSNO modified materials, indicating there is still a reservoir of NO to be emitted. Figure 2 shows a general overview of the NO release kinetics of the films during the study. A summary of the flux being emitted at designated time points is shown in Figure 2A, where the PBS used to incubate the films is changed regularly. By the end of the testing period, the films were not completely exhausted and still releasing an NO flux of 0.1 × 10−10 mol cm−2 min−1. Figure 2B shows the cumulative NO release that was calculated over this 125-day testing period and demonstrates the overall large NO storage ability the material holds while Figure 2C gives the initial release profile when first placed in PBS on day 0. Films were weighed and measured before testing and found to have a SNAP loading capacity of 0.379 ± 0.016 μmol mg−1 using UV-VIS. After day 125, there is still 18% of the remaining covalently attached SNAP as the cumulative NO release was normalized to 0.311 ± 0.009 μmol mg−1. This duration of release under physiological conditions exceeds expectations when compared to other popular NO releasing polymers that contain non-tethered RSNO’s, which normally only release NO under these conditions for a matter of hours or days. This is often due to leaching of the NO donor out of the polymer matrix. Leaching is a challenge for non-covalently attached drugs, and a topcoat of polymer is often used as a preventative measure. However, this method only delays the release of blended components for a few days, limiting the potential applications for materials that require long-lasting NO release.
Figure 2.
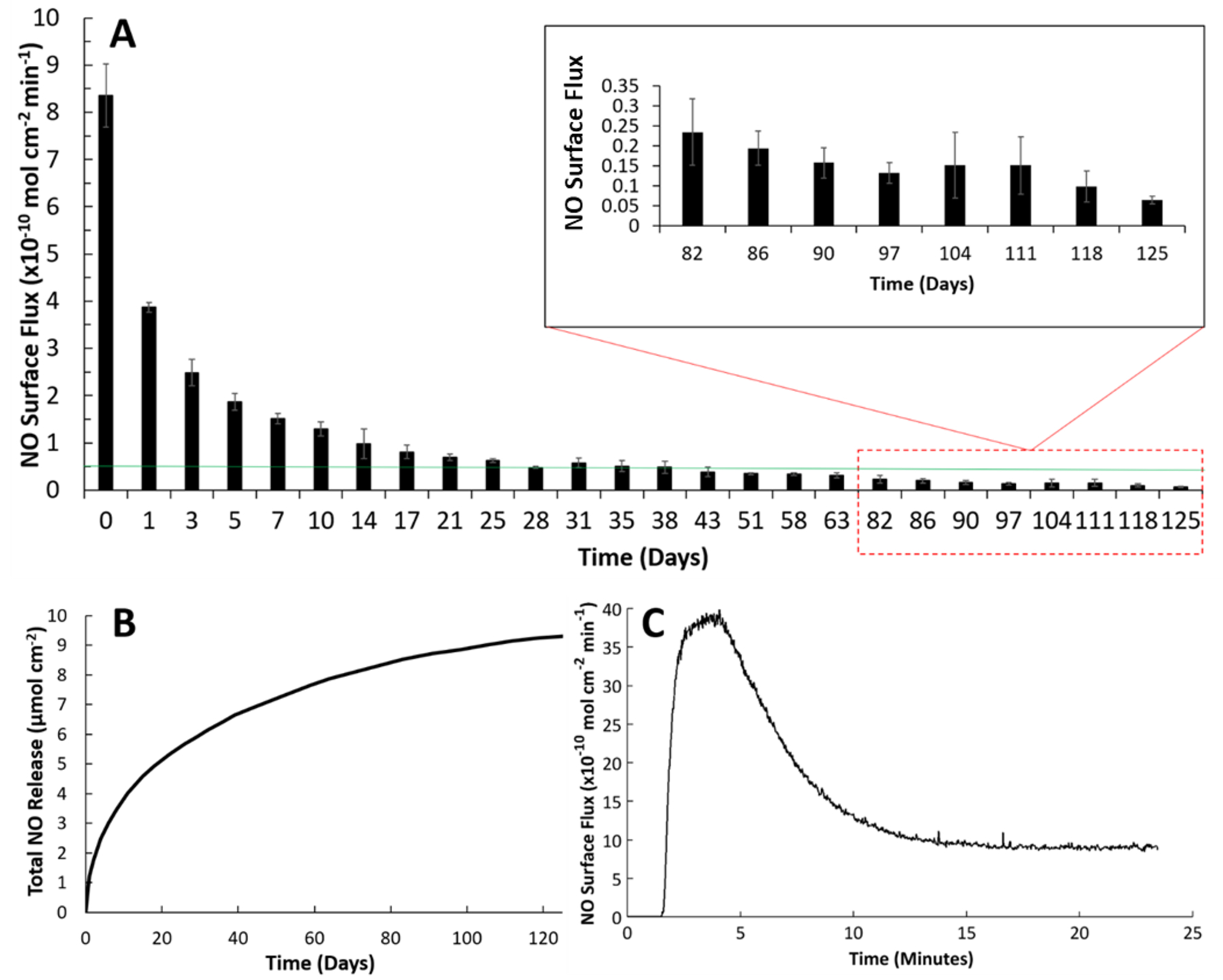
Nitric oxide releasing kinetics of SNAP-PDMS films where (A) continuous NO release flux measurements were taken on specified days while storing the films in PBS with EDTA at 37 °C (n=4). The green line represents the minimum physiological level of NO flux (0.5 × 10−10 mol cm−2 min−1). (B) Cumulative NO release over the 125-day testing period was measured and normalized per cm2 of SNAP-PDMS. (C) Representative NO release profile on day 0 from SNAP-PDMS films when placed in PBS with EDTA at 37 °C. Error bars represent standard deviation.
Silicone rubber tubing containing coats of SNAP-PDMS was also tested for NO release for 4 h to simulate the release seen during the in vivo ECC tests. Three coats of SNAP-PDMS in toluene (160 mg mL−1) were cast on the inner lumen of the SR tubing by completely filling the tubing with the solution and draining it, then allowing 1 h to dry between each coat. It is important that between each drying step that most of the excess SNAP-PDMS solution is removed from the tubing. This is primarily done to minimize the unevenness of each polymer layer within the lumen of the tubing to give a uniform NO release throughout the entire circuit during testing. Before the ECC experiments, the tubing was left to vacuum dry at room temperature for 24 h to ensure any remaining solvent was properly evaporated. Nitric oxide release was also measured after in vivo ECC testing to ensure the flux was consistent with the previous in vitro NOA testing, releasing an NO flux of 8.15 ± 1.68 × 10−10 mol cm−2 min−1, while 8.35 ± 0.666 × 10−10 mol cm−2 min−1 was measured before testing.
3.2. Measurements of SNAP Leachates
An important distinctive trait with SNAP-PDMS is not having the need for a topcoat to prevent any leaching when placing it in any aqueous environments, cutting down the processing time and allowing the application of extremely thin coats of polymer to a surface. However, topcoats of silicone rubber based materials can further decrease the amount of water uptake into the films along with preventing diffusion of certain ions. This could further extend its longevity by only allowing heat as the method for NO release but lowering the overall NO flux as a result. As shown in the initial NO release profile seen in Figure 2C, NO release from SNAP-PDMS was able to stabilize in under 15 minutes without the presence of a topcoat. Materials containing loosely bound NO donors often have an initial release that is extremely high to the point where it can be cytotoxic towards surrounding cells. There can also be occurrences where it takes a long time for the materials to maintain a level flux due to this constant leaching of the NO donor. This can be difficult when trying to pinpoint an exact flux of NO being emitted from a material over the duration of a study. When comparing the initial NO releasing trend of SNAP-PDMS to these types of polymers, this burst release is much lower in duration. Most of these materials require a pre-incubation period in PBS for 24 hr to ensure this burst effect is not as severe while this is not needed for SNAP-PDMS.
The key factor in determining the longevity of RSNO’s under physiological conditions is how quickly the donor is leached into the surrounding environment. By covalently attaching SNAP directly to the PDMS, the amount of leaching is significantly reduced when compared to blending it within the polymer. In the past, a common preventative measure to keep blended SNAP within the polymer matrix is to apply a hydrophobic topcoat.12 However, even though a noticeable reduction in SNAP leaching is seen in the films using this strategy, the leaching is still much greater than what is seen with the covalently bound SNAP-PDMS.
Quantification of the amount of leaching seen by the films was observed using UV-Vis absorbance spectroscopy at 340 nm. Over the course of a 48 h period, while being kept at room temperature and protected from light, absorbance was periodically checked from the solutions containing the possible leached SNAP and the trends for each material is shown in Figure 3. The same solution was used for each testing period so that the cumulative leaching could be calculated for the initial 24 h and a new batch of PBS was used for the following 24 h. After 48 h of soaking the SNAP-PDMS films in PBS at room temperature, little leaching (< 0.015 mg cm−1) was detected from the SNAP-PDMS films while continuous leaching was seen from the films with the blended SNAP. Even with a protective topcoat, there is still an increasing trend in the amount of leached SNAP from the blended films. This is also visibly verified over the course of the study, as the characteristic green color seen with non-covalent bound SNAP based materials was quickly diminishing. Virtually no difference was detected in the amount of leachates from the SNAP-PDMS with and without a topcoat. Due to the chemistry from the NAP-thiolactone attachment to the aminosilane crosslinker within the PDMS, the only free thiol groups capable of being nitrosated in the PDMS will be covalently bound as any unreacted NAP-thiolactone will remain in its ring structure, preventing it from forming unbound SNAP. There was only a minor increase seen in the initial 6 h of measuring for the SNAP-PDMS films, while the amount of cumulative leachates beyond this timepoint remains almost constant for the duration of the study.
Figure 3.
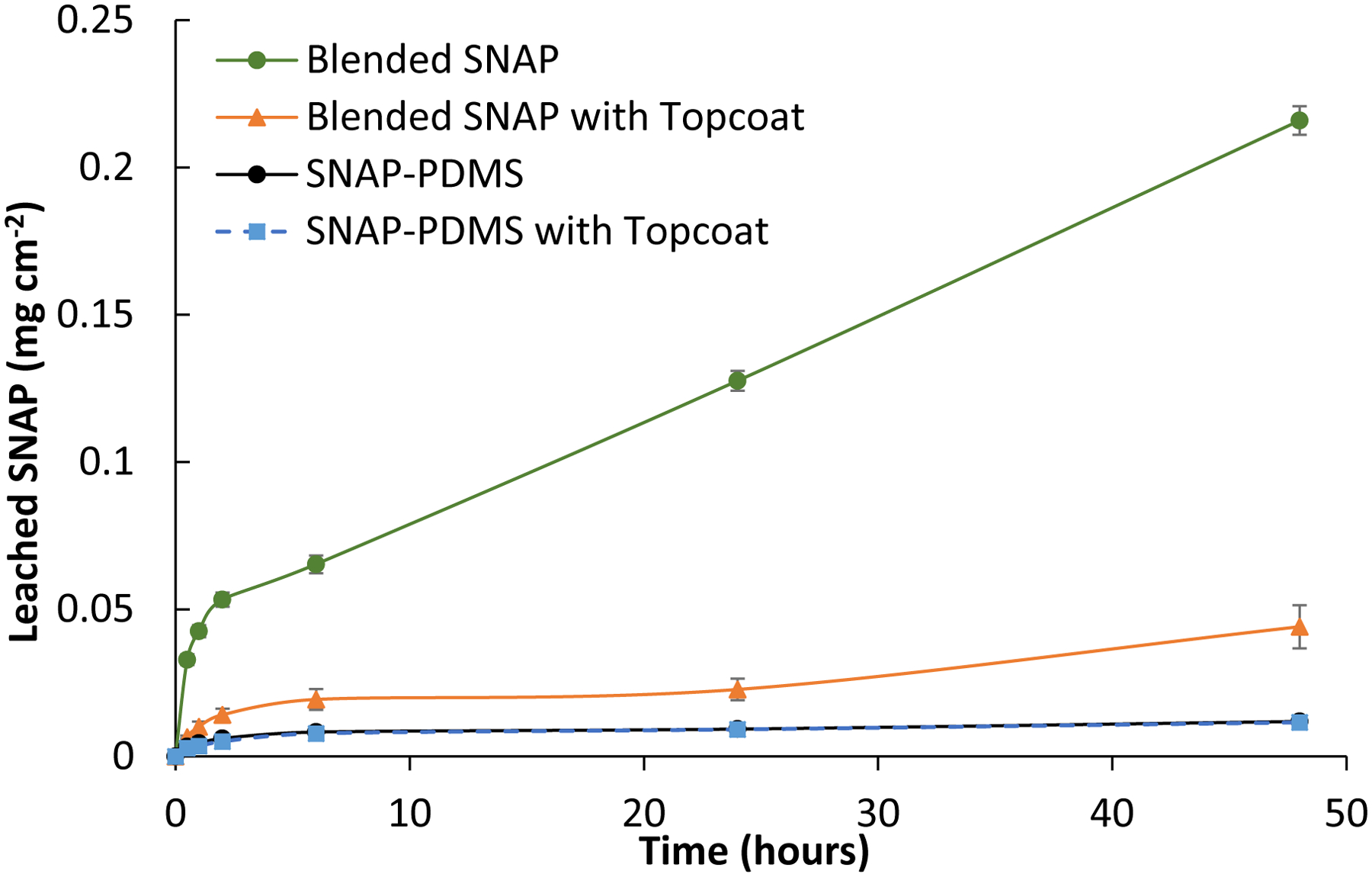
Cumulative leaching of SNAP into PBS from SNAP blended PDMS and covalent bound SNAP-PDMS films with and without a topcoat over the course of 48 hours (n=3). P<0.05 were used for comparison. Error bars represent standard deviation.
3.3. Long Term Inhibition of Bacteria on SNAP-PDMS
Implants are prone to infection due to their surface characteristics along with failure to maintain sterile conditions during medical practices. For instance, catheters are susceptible to infection as they stay implanted for long periods of time. These infections reduce the life time of the device and often need to be replaced before it becomes life threatening. Thus, biomedical device related infections not only add to the suffering of the patient but also increases the overall cost of the healthcare due to prolonged hospital stay. It is important to have long-term antimicrobial strategies so that these types of medical complications can be prevented. The two main parameters by which bacterial adhesion and growth are supported is by the surface roughness of the material and the efficacy of the bactericidal agent being released. Nitric oxide-releasing materials have been proven to greatly reduce bacterial activity, but most of these tests are only done for very short time frames.44, 48–51 In this study, SNAP-PDMS films were incubated with S. aureus in a CDC bioreactor and their antibacterial potential was observed at day 3, 14, and 28 while a separate 24 h study was performed on films that had previously been releasing NO for 125 days continuously. Staphylococcus aureus is among one of the major players of hospital-acquired infection which causes biofilm formation on the polymeric surface which renders antibiotics ineffective against it.
The bioreactor used is able to provide a shear force to the films with a constant supply of nutrients to the bacteria to simulate a highly favorable infectious environment seen in vivo.52 SNAP-PDMS and control PDMS films were placed in CDC bioreactors containing S. aureus over a period of 28 days. A constant supply of nutrients (LB Broth, 0.5 g L−1) was fed into the bioreactors at a flow rate of 100 mL hr−1 while keeping the bioreactor at 37 °C with an agitator speed of 100 rpm. Films (n=3 for each time point) were then taken out at their designated time point: on days 3, 14, and 28. After taking out the films from the bioreactors, they were gently rinsed of any loosely bound bacteria and homogenized in sterile PBS solution with the help of a sonicator (Omni International TH) to detach the bound bacteria from the film. Serial dilutions (10−1 to 10−5) were made and the bacterial suspension in PBS was plated in pre-made LB agar plates (40 mg mL−1). The colony forming units per surface area of the SNAP-PDMS films were counted and compared with the control samples and are shown in Figure 4. A significant reduction in the amount of viable S. aureus attachment to the SNAP-PDMS films was observed at all time points. The maximum reduction was observed at the initial time points of 3 and 14 days due to the high flux of NO being emitted from the SNAP-PDMS at these times, demonstrated over 4-log reduction at day 3 and 3-log reduction by day 14. Even after 28 days when the NO release begins to diminish, there is still a 2-log reduction in viable bacteria to the SNAP-PDMS films. Exact values over the course of the bioreactor study are shown in Table 1.
Figure 4.
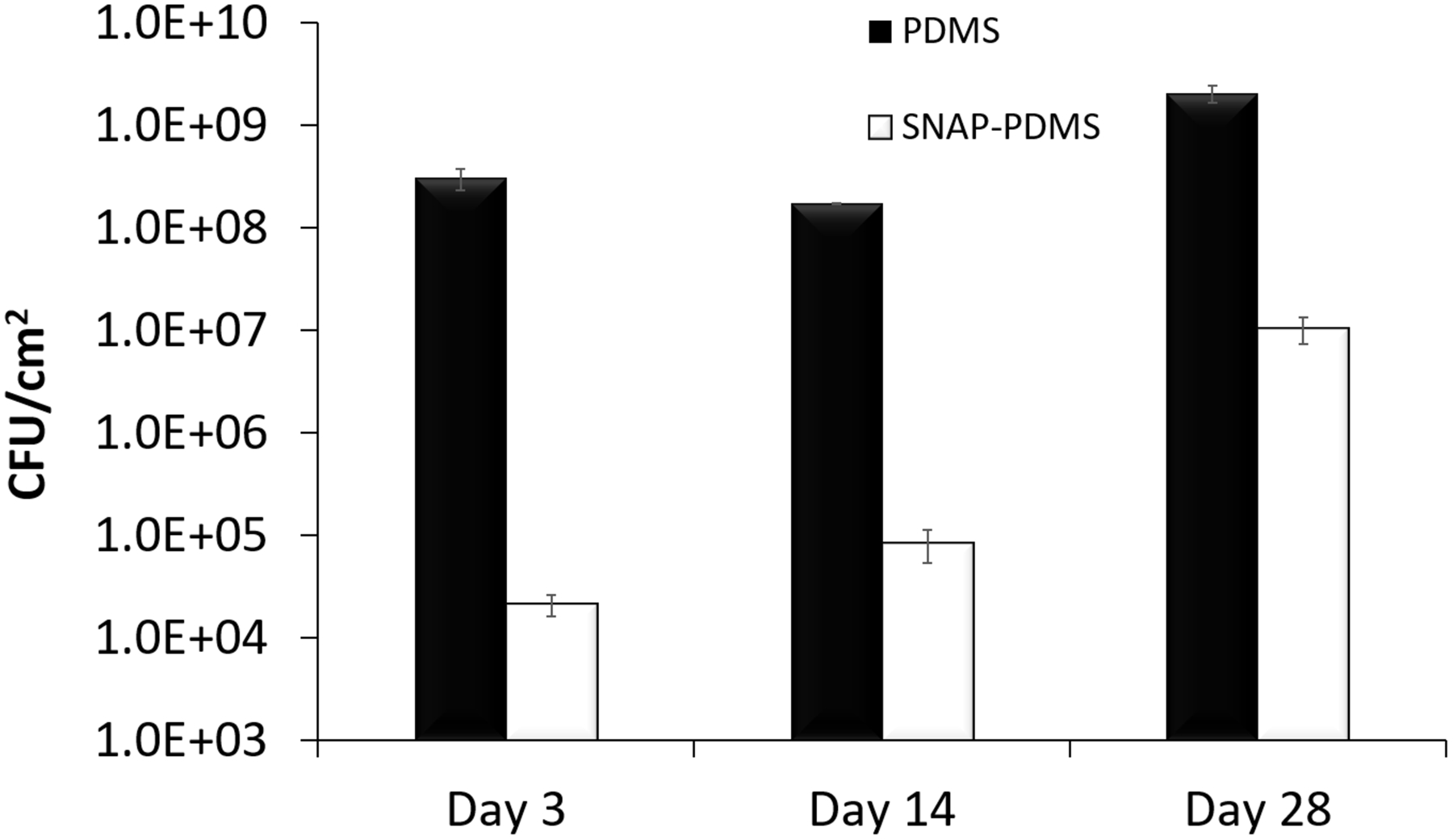
Long term antimicrobial ability of S. aureus adhesion to SNAP-PDMS. (A) Bacterial adhesion in 28-day bioreactor study on control PDMS and SNAP-PDMS films (n=3 per timepoint), showing approximately a 4, 3, and 2 log reduction in bacterial adhesion by days 3, 14, and 28 respectively. P<0.05 was used for comparison between groups. Error bars represent standard deviation.
Table 1.
Nitric oxide flux before and after bioreactor study with colony forming unit (CFU) counts at specified time points.
| NO Flux(initial) (x10−10 mol cm−2 sec−1) | NO Flux(28d) (x10−10 mol cm−2 sec−1) | CFU(3d) | CFU(14d) | CFU(28d) | |
|---|---|---|---|---|---|
| PDMS | - | - | 3.05 × 108 ± 7.31 × 107 | 1.72 × 108 ± 1.20 × 106 | 2.05 × 109 ± 3.90 × 108 |
| SNAP-PDMS | 8.35 ± 0.666 | 1.01 ± 0.120 | 2.11 × 104 ± 5.09 × 103 | 8.38 × 104 ± 3.01 × 104 | 1.04 × 107 ± 3.05 × 106 |
Finally, the residual NO flux after the bioreactor study was observed to extrapolate how effective the films would be beyond the 28-day testing period. It was found that even after 28 days of bacteria killing, SNAP-PDMS films were still releasing an NO flux of 1.01 ± 0.120 × 10−10 mol min−1 cm−2, proving that there were still potential antimicrobial properties to be utilized. This level of NO is higher than what was seen with in vitro testing for the films in PBS after 28 days shown previously in Figure 2A where only approximately 0.5 × 10−10 mol min−1 cm−2 was still being emitted. Theoretically, this discrepancy could be due to a few factors that differ between keeping the films in a bioreactor versus PBS. Layers of dead bacteria could be forming on the surface of the polymer films, artificially creating a biological “topcoat” that slows the release of NO after a period of time. Since the films were homogenized and sonicated after testing to remove any remaining bacteria biofilm, it was not possible to prove this theory after the bioreactor study. Another possibility is that the salinity content in the broth was much lower than the PBS. One of the main methods for facilitating NO release for RSNO’s is through catalytic based ionic interaction to break the sulfur-nitrogen bond.42 Lower ion content would then cause a slower release profile over the 28 days, eventually leveling off at a higher flux by day 28 as seen in the data.
The SNAP-PDMS films were also tested for their potential antimicrobial abilities after 125 days of sustained release in PBS at 37 °C in a 24 h bacterial adhesion study. Although the end recorded flux of the films were emitting an NO flux of approximately 0.1 × 10−10 mol cm−2 min−1, past studies have demonstrated that these levels of NO can still have antimicrobial effects.53–54 The SNAP-PDMS films were still able to inhibit the adhesion of S. aureus by 58.6% (Figure 5), giving insight into how even NO fluxes below the normal physiological levels from exogenous NO donating sources can still possess antimicrobial properties.
Figure 5.
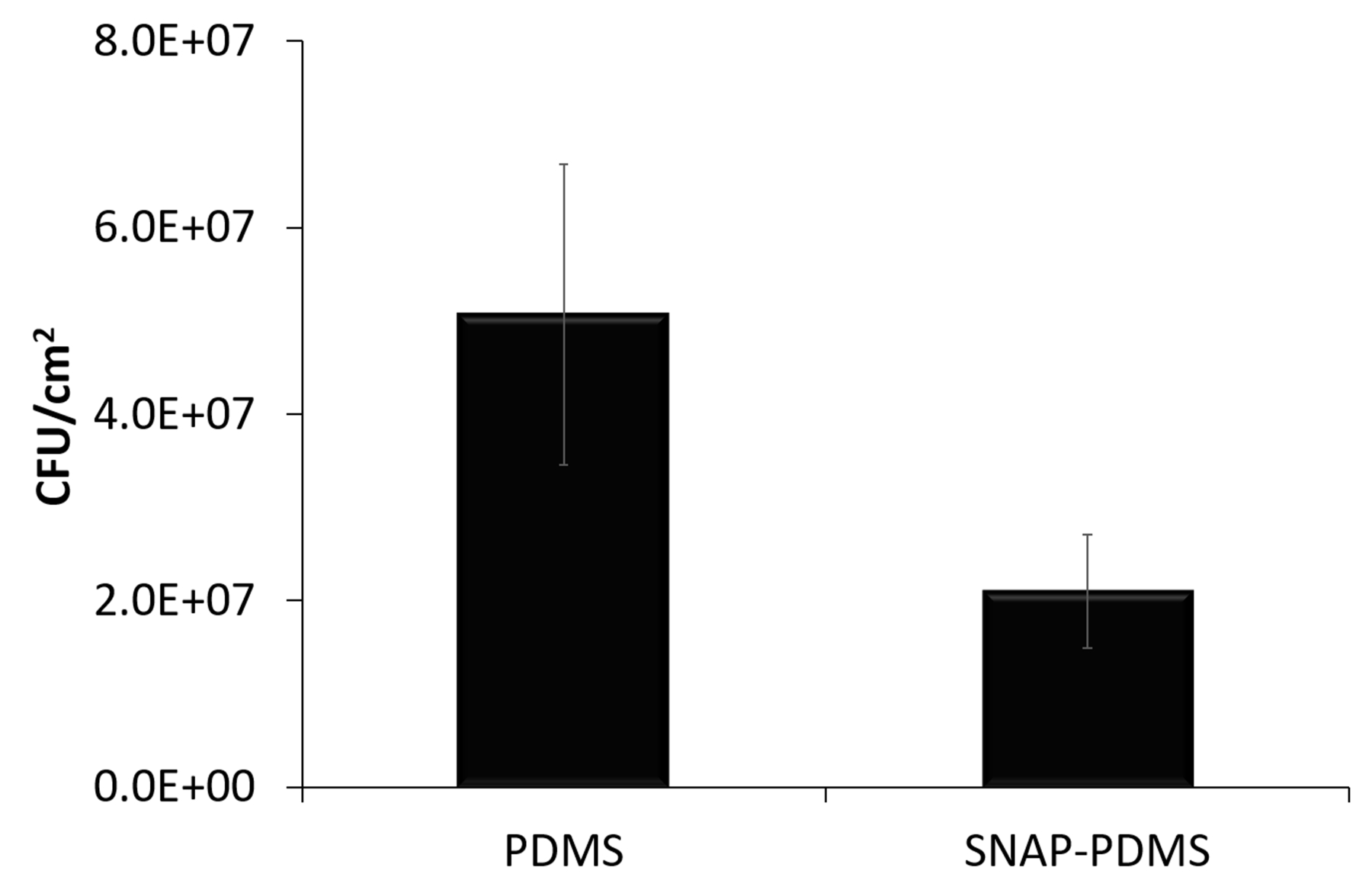
Films previously tested for 125 days under physiological conditions were also examined (n=3), still demonstrating a 58.6% reduction in viable bacteria. Error bars represent standard deviation.
3.4. Cytotoxicity of SNAP-PDMS
Materials that can have any potential leachates will be detrimental to the surrounding cells, so it is important to investigate any possible toxic effects. Using a standard protocol, SNAP-PDMS films were tested for any cytotoxic leachates to cultured mouse fibroblast cells.44 The SNAP-PDMS films were submerged in DMEM at 37 °C in amber vials for 24 h to allow any leachates from the films to diffuse into the medium. After 24 hrs, the fibroblast cells that were grown in parallel were exposed to the leachates and incubated for the next 24 hrs. The CCK-8 kit based cytotoxicity assay showed that compared with the control PDMS films, more than 96% of fibroblast cells were found to be viable when exposed to the leachates from SNAP-PDMS films. Thus, the cytotoxicity study provides supportive evidence for the potential biocompatibility of SNAP-PDMS films towards mouse fibroblast cells. In the past, different NO releasing materials are shown to be highly effective in inhibiting bacterial growth as well as platelet activation. Having high antibacterial potential is a great advantage for biomedical device fabrication but not at the cost of toxic side-effects on host mammalian cells. Other studies have shown very high doses of antibacterial agents: antibiotics, silver nanoparticles, or NO donors, without validating optimal therapeutic dose which is noncytotoxic to mammalian cells.23, 55–56 Thus the present study is significant in proving the antibacterial efficacy and antithrombotic potential of NO releasing PDMS films without causing undesired cytotoxic responses. In addition, the morphology of the mouse fibroblast cells was maintained at the end of the study further supporting that there was no alternation in cellular metabolism.
This result was expected as NAP, the precursor of SNAP, is FDA approved and is often used to control heavy metal poisoning.57–58 Treatments using NAP have also been used to treat cystinuria at levels as high as 2–4 g/day over the course of 155 days.59 Similar results in the past have been shown where more than 90% of cell viability was exhibited by different NO releasing polymeric composites.60–62 Further testing in animal models would be helpful to establish in vivo data to reaffirm the efficacy of these materials in pre-clinical settings.
3.5. SNAP-PDMS Extracorporeal Circuit Hemocompatibility
Comparison between control PDMS and SNAP-PDMS coated ECC loops were performed in a rabbit model for 4 h (Figure 6). The two major parameters that were investigated for this study were platelet count and thrombus formation. Quantification of these parameters gives insight into how the release of NO from SNAP-PDMS coatings can be used as a method for improving hemocompatibility of blood contacting devices. Platelet count was recorded every hour for the duration of the ECC test and compared to the measured baseline taken before the experiment and corrected for hemodilution. Although SNAP-PDMS has been proven to release for several months, only the first 4 h of hemocompatibility is to be observed with this study to demonstrate its initial effectiveness in an extracorporeal setting.
Figure 6.
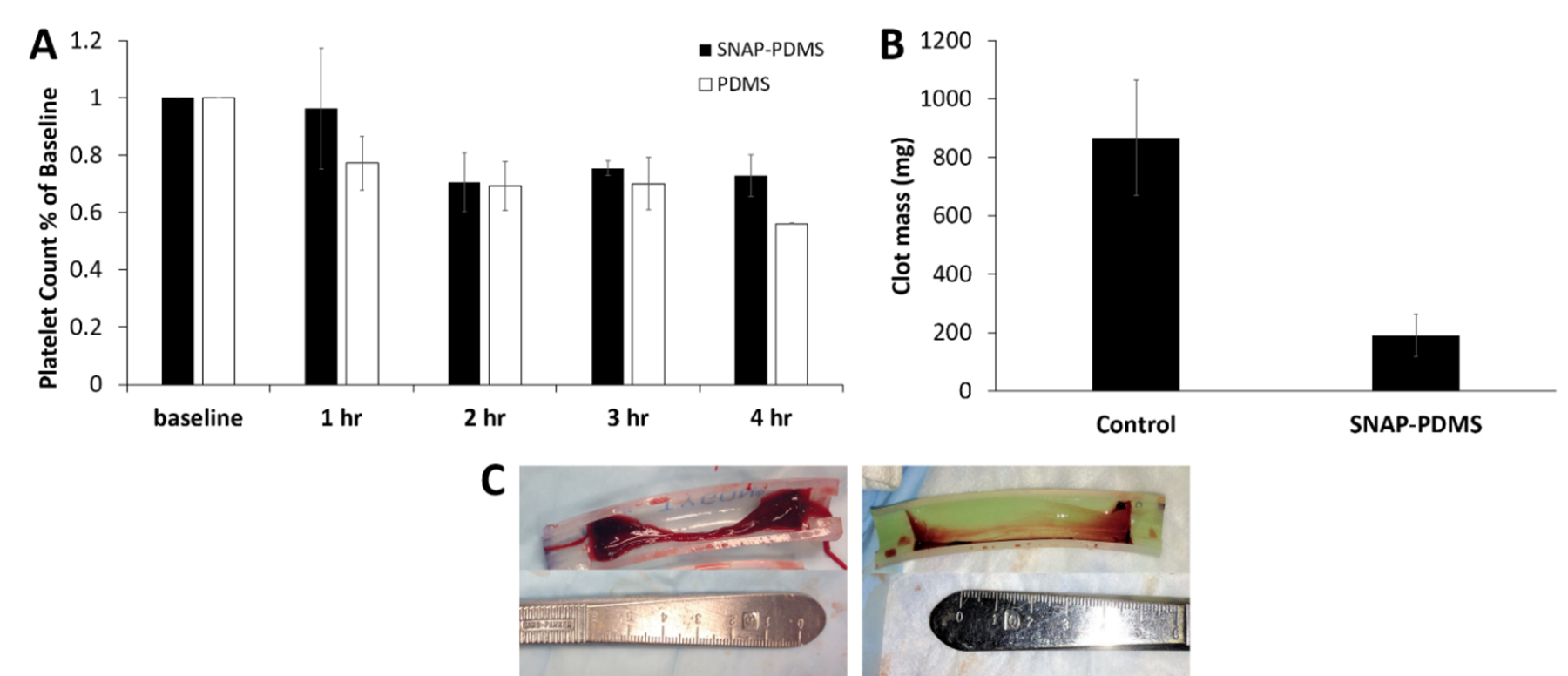
Hemocompatibility measurements of SNAP-PDMS coated tubing for ECC testing. (A) Time-dependent effects of NO release from the ECC on platelet count over the course of the 4 h study (n=3). (B) Quantification of clot mass obtained from the thrombogenicity chamber. (C) Visual representation of the clotting that occurred in PDMS coated controls (left) and SNAP-PDMS coated circuits (right). P<0.05 was used for comparison. Error bars represent standard deviation.
For silicone rubber coated controls, platelet concentration dropped to ~55% of the baseline levels (n=3), where one control loop clotted prior to the 4 h (Figure 6A). All NO releasing circuits maintained flow ca. the initial flow through the ECC loop (100 mL min−1), with platelet levels at ~75% of the baseline after 4 hr. A previous study where SNAP was swelled into PDMS tubing, platelet count reached as low as 12% of the baseline for control samples over the 4 h period while the NO releasing tubing maintained 62%.29 The SNAP-PDMS coating demonstrated to perform even better than this strategy of incorporating SNAP due to the high flux of NO that was able to be maintained during the experiment. However, the control PDMS coating used in this study proved to preserve a significant amount of the platelet count compared to pure silicone rubber. It has been shown in the past that both hydroxy-terminated PDMS and the aminosilane crosslinker used have some hemocompatible properties to attribute to this effect.63–64 Since SNAP is directly added to the aminosilane crosslinker for the NO releasing loops, the aminosilane has very little functionality compared to control loops.
After the study, the amount of thrombus formation inside the loops was analyzed. Thrombus formation was measured by cutting open the thrombogenicity chamber of the ECC and removing any visible clots. Control loops had large amounts of clotting occur during the duration of the study, where the chamber was covered with a thick, dense layer of loosely bound thrombus formation. The SNAP-PDMS coated circuits showed much less clotting with only a thin layer of thrombus that was much more tightly bound to the surface (Figure 6C). The SNAP-PDMS coated circuits showed a significant reduction (78% less) in overall thrombus mass compared to control PDMS coated circuits (Figure 6B).
4. Conclusion
In this study, the covalent attachment of SNAP to PDMS demonstrated to be an effective long term (> 4 months) NO releasing material. By eliminating the potential of unwanted leaching of the NO donor into the surrounding environment, the sustained and passive NO release was suitable as both a long duration antimicrobial and short term antithrombotic surface in addition to being non-cytotoxic to mammalian cells. Compared to the traditional method of blending RSNOs into a polymer, the covalent attachment allows no potential leaching of the NO donor into the surrounding environment which is key to its longevity and noncytotoxic effects. The SNAP-PDMS material demonstrated that it was able to significantly inhibit bacterial adhesion of S. aureus even after constant exposure for a month in a CDC bioreactor. Even after 125 days of physiological release, the films were able to reduce nearly 60% of the overall bacterial adhesion, showing that the films still retained antimicrobial efficacy with low levels of sustained NO release. The multifunctional ability of NO was further proven as thrombus formation on the inner lumen of ECC loops coated with SNAP-PDMS was also greatly reduced due to the NO releasing SNAP-PDMS, indicating the flexibility of the polymer as a both a hemocompatible surface as well as antimicrobial.
Acknowledgements
Funding for this work was supported by the National Institutes of Health, USA grants K25HL111213 and R01HL134899.
References
- (1).Gorbet MB; Sefton MV, Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. Biomaterials 2004, 25 (26), 5681–5703. [DOI] [PubMed] [Google Scholar]
- (2).Wu H; Moser C; Wang H-Z; Høiby N; Song Z-J, Strategies for Combating Bacterial Biofilm Infections. International journal of oral science 2015, 7 (1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).MacMicking J; Xie; Nathan C, Nitric Oxide and Macrophage Function. Annu. Rev. Immunol 1997, 15 (1), 323–350. [DOI] [PubMed] [Google Scholar]
- (4).Ignarro LJ; Buga GM; Wood KS; Byrns RE; Chaudhuri G, Endothelium-Derived Relaxing Factor Produced and Released from Artery and Vein Is Nitric Oxide. Proceedings of the National Academy of Sciences 1987, 84 (24), 9265–9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Frost MC; Reynolds MM; Meyerhoff ME, Polymers Incorporating Nitric Oxide Releasing/Generating Substances for Improved Biocompatibility of Blood-Contacting Medical Devices. Biomaterials 2005, 26 (14), 1685–1693. [DOI] [PubMed] [Google Scholar]
- (6).Joslin JM; Lantvit SM; Reynolds MM, Nitric Oxide Releasing Tygon Materials: Studies in Donor Leaching and Localized Nitric Oxide Release at a Polymer-Buffer Interface. ACS Appl. Mater. Interfaces 2013, 5 (19), 9285–9294. [DOI] [PubMed] [Google Scholar]
- (7).Bohl Masters KS; Lipke EA; Rice EE; Liel MS; Myler HA; Zygourakis C; Tulis DA; West JL, Nitric Oxide-Generating Hydrogels Inhibit Neointima Formation. J. Biomater. Sci. Polym. Ed 2005, 16 (5), 659–672. [DOI] [PubMed] [Google Scholar]
- (8).Hrabie JA; Keefer LK, Chemistry of the Nitric Oxide-Releasing Diazeniumdiolate (“Nitrosohydroxylamine”) Functional Group and Its Oxygen-Substituted Derivatives. Chem. Rev 2002, 102 (4), 1135–1154. [DOI] [PubMed] [Google Scholar]
- (9).Annich GM; Meinhardt JP; Mowery KA; Ashton BA; Merz SI; Hirschl RB; Meyerhoff ME; Bartlett RH, Reduced Platelet Activation and Thrombosis in Extracorporeal Circuits Coated with Nitric Oxide Release Polymers. Crit. Care Med 2000, 28 (4), 915–920. [DOI] [PubMed] [Google Scholar]
- (10).Handa H; Major TC; Brisbois EJ; Amoako KA; Meyerhoff ME; Bartlett RH, Hemocompatibility Comparison of Biomedical Grade Polymers Using Rabbit Thrombogenicity Model for Preparing Nonthrombogenic Nitric Oxide Releasing Surfaces. Journal of Materials Chemistry B 2014, 2 (8), 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Major TC; Brisbois EJ; Jones AM; Zanetti ME; Annich GM; Bartlett RH; Handa H, The Effect of a Polyurethane Coating Incorporating Both a Thrombin Inhibitor and Nitric Oxide on Hemocompatibility in Extracorporeal Circulation. Biomaterials 2014, 35 (26), 7271–7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Amoako KA; Archangeli C; Handa H; Major T; Meyerhoff ME; Annich GM; Bartlett RH, Thromboresistance Characterization of Extruded Nitric Oxide-Releasing Silicone Catheters. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2012, 58 (3), 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ren H; Coughlin MA; Major TC; Aiello S; Rojas Pena A; Bartlett RH; Meyerhoff ME, Improved in Vivo Performance of Amperometric Oxygen (P O2) Sensing Catheters Via Electrochemical Nitric Oxide Generation/Release. Anal. Chem 2015, 87 (16), 8067–8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Brisbois EJ; Major TC; Goudie MJ; Meyerhoff ME; Bartlett RH; Handa H, Attenuation of Thrombosis and Bacterial Infection Using Dual Function Nitric Oxide Releasing Central Venous Catheters in a 9day Rabbit Model. Acta Biomater. 2016, 44, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Warkentin TE, Heparin-Induced Thrombocytopenia. Critical Decisions in Thrombosis and Haemostasis. BC Decker Inc. Hamilton,. Pagg 1998, 100–108. [Google Scholar]
- (16).Fidlar E; Jaques L, The Effect of Commercial Heparin on the Platelet Count. The Journal of laboratory and clinical medicine 1948, 33 (11), 1410–1423. [PubMed] [Google Scholar]
- (17).Monteiro DR; Gorup LF; Takamiya AS; Ruvollo-Filho AC; de Camargo ER; Barbosa DB, The Growing Importance of Materials That Prevent Microbial Adhesion: Antimicrobial Effect of Medical Devices Containing Silver. Int. J. Antimicrob. Agents 2009, 34 (2), 103–110. [DOI] [PubMed] [Google Scholar]
- (18).Maneerung T; Tokura S; Rujiravanit R, Impregnation of Silver Nanoparticles into Bacterial Cellulose for Antimicrobial Wound Dressing. Carbohydr. Polym 2008, 72 (1), 43–51. [Google Scholar]
- (19).Kim JS; Kuk E; Yu KN; Kim J-H; Park SJ; Lee HJ; Kim SH; Park YK; Park YH; Hwang C-Y, Antimicrobial Effects of Silver Nanoparticles. Nanomed. Nanotechnol. Biol. Med 2007, 3 (1), 95–101. [DOI] [PubMed] [Google Scholar]
- (20).Ruparelia JP; Chatterjee AK; Duttagupta SP; Mukherji S, Strain Specificity in Antimicrobial Activity of Silver and Copper Nanoparticles. Acta Biomater. 2008, 4 (3), 707–716. [DOI] [PubMed] [Google Scholar]
- (21).Ramyadevi J; Jeyasubramanian K; Marikani A; Rajakumar G; Rahuman AA, Synthesis and Antimicrobial Activity of Copper Nanoparticles. Mater. Lett 2012, 71, 114–116. [Google Scholar]
- (22).Bagchi B; Dey S; Bhandary S; Das S; Bhattacharya A; Basu R; Nandy P, Antimicrobial Efficacy and Biocompatibility Study of Copper Nanoparticle Adsorbed Mullite Aggregates. Materials Science and Engineering: C 2012, 32 (7), 1897–1905. [DOI] [PubMed] [Google Scholar]
- (23).Stigter M; Bezemer J; De Groot K; Layrolle P, Incorporation of Different Antibiotics into Carbonated Hydroxyapatite Coatings on Titanium Implants, Release and Antibiotic Efficacy. J. Controlled Release 2004, 99 (1), 127–137. [DOI] [PubMed] [Google Scholar]
- (24).Mader JT; Calhoun J; Cobos J, In Vitro Evaluation of Antibiotic Diffusion from Antibiotic-Impregnated Biodegradable Beads and Polymethylmethacrylate Beads. Antimicrob. Agents Chemother 1997, 41 (2), 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ungaro F; d’Angelo I; Coletta C; di Villa Bianca R.d. E.; Sorrentino R; Perfetto B; Tufano MA; Miro A; La Rotonda MI; Quaglia F, Dry Powders Based on Plga Nanoparticles for Pulmonary Delivery of Antibiotics: Modulation of Encapsulation Efficiency, Release Rate and Lung Deposition Pattern by Hydrophilic Polymers. J. Controlled Release 2012, 157 (1), 149–159. [DOI] [PubMed] [Google Scholar]
- (26).Fischbach MA; Walsh CT, Antibiotics for Emerging Pathogens. Science 2009, 325 (5944), 1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Taubes G, The Bacteria Fight Back. Science 2008, 321 (5887), 356–361. [DOI] [PubMed] [Google Scholar]
- (28).Stewart PS; Costerton JW, Antibiotic Resistance of Bacteria in Biofilms. The lancet 2001, 358 (9276), 135–138. [DOI] [PubMed] [Google Scholar]
- (29).Brisbois EJ; Major TC; Goudie MJ; Bartlett RH; Meyerhoff ME; Handa H, Improved Hemocompatibility of Silicone Rubber Extracorporeal Tubing Via Solvent Swelling-Impregnation of S-Nitroso-N-Acetylpenicillamine (Snap) and Evaluation in Rabbit Thrombogenicity Model. Acta Biomater. 2016, 37, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Lu Y; Slomberg DL; Schoenfisch MH, Nitric Oxide-Releasing Chitosan Oligosaccharides as Antibacterial Agents. Biomaterials 2014, 35 (5), 1716–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Lutzke A; Pegalajar-Jurado A; Neufeld BH; Reynolds MM, Nitric Oxide-Releasing S-Nitrosated Derivatives of Chitin and Chitosan for Biomedical Applications. Journal of Materials Chemistry B 2014, 2 (42), 7449–7458. [DOI] [PubMed] [Google Scholar]
- (32).Pant J; Goudie MJ; Hopkins SP; Brisbois EJ; Handa H, Tunable Nitric Oxide Release from S-Nitroso-N-Acetylpenicillamine Via Catalytic Copper Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Hetrick EM; Schoenfisch MH, Antibacterial Nitric Oxide-Releasing Xerogels: Cell Viability and Parallel Plate Flow Cell Adhesion Studies. Biomaterials 2007, 28 (11), 1948–1956. [DOI] [PubMed] [Google Scholar]
- (34).Barraud N; Hassett DJ; Hwang S-H; Rice SA; Kjelleberg S; Webb JS, Involvement of Nitric Oxide in Biofilm Dispersal of Pseudomonas Aeruginosa. J. Bacteriol 2006, 188 (21), 7344–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Hetrick EM; Shin JH; Paul HS; Schoenfisch MH, Anti-Biofilm Efficacy of Nitric Oxide-Releasing Silica Nanoparticles. Biomaterials 2009, 30 (14), 2782–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Stasko NA; Fischer TH; Schoenfisch MH, S-Nitrosothiol-Modified Dendrimers as Nitric Oxide Delivery Vehicles. Biomacromolecules 2008, 9 (3), 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Lu Y; Slomberg DL; Shah A; Schoenfisch MH, Nitric Oxide-Releasing Amphiphilic Poly (Amidoamine)(Pamam) Dendrimers as Antibacterial Agents. Biomacromolecules 2013, 14 (10), 3589–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).He W; Frost MC, Direct Measurement of Actual Levels of Nitric Oxide (No) in Cell Culture Conditions Using Soluble No Donors. Redox biology 2016, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).He W; Frost MC, Cellno Trap: Novel Device for Quantitative, Real-Time, Direct Measurement of Nitric Oxide from Cultured Raw 267.4 Macrophages. Redox biology 2016, 8, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Moynihan HA; Roberts SM, Preparation of Some Novel S-Nitroso Compounds as Potential Slow-Release Agents of Nitric Oxide in Vivo. J. Chem. Soc., Perkin Trans. 1 1994, (7), 797–805. [Google Scholar]
- (41).Gierke GE; Nielsen M; Frost MC, S-Nitroso-N-Acetyl-D-Penicillamine Covalently Linked to Polydimethylsiloxane (Snap–Pdms) for Use as a Controlled Photoinitiated Nitric Oxide Release Polymer. Science and technology of advanced materials 2011, 12 (5), 055007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Williams DLH, The Chemistry of S-Nitrosothiols. Acc. Chem. Res 1999, 32 (10), 869–876. [Google Scholar]
- (43).Bartberger MD; Houk K; Powell SC; Mannion JD; Lo KY; Stamler JS; Toone EJ, Theory, Spectroscopy, and Crystallographic Analysis of S-Nitrosothiols: Conformational Distribution Dictates Spectroscopic Behavior. J. Am. Chem. Soc 2000, 122 (24), 5889–5890. [Google Scholar]
- (44).Pant J; Goudie MJ; Hopkins SP; Brisbois EJ; Handa H, Tunable Nitric Oxide Release from S-Nitroso-N-Acetylpenicillamine Via Catalytic Copper Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2017, 9 (18), 15254–15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Brisbois EJ; Handa H; Major TC; Bartlett RH; Meyerhoff ME, Long-Term Nitric Oxide Release and Elevated Temperature Stability with S-Nitroso-N-Acetylpenicillamine (Snap)-Doped Elast-Eon E2as Polymer. Biomaterials 2013, 34 (28), 6957–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Handa H; Brisbois EJ; Major TC; Refahiyat L; Amoako KA; Annich GM; Bartlett RH; Meyerhoff ME, In Vitro and in Vivo Study of Sustained Nitric Oxide Release Coating Using Diazeniumdiolate-Doped Poly (Vinyl Chloride) Matrix with Poly (Lactide-Co-Glycolide) Additive. Journal of Materials Chemistry B 2013, 1 (29), 3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Major TC; Brant DO; Reynolds MM; Bartlett RH; Meyerhoff ME; Handa H; Annich GM, The Attenuation of Platelet and Monocyte Activation in a Rabbit Model of Extracorporeal Circulation by a Nitric Oxide Releasing Polymer. Biomaterials 2010, 31 (10), 2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Lu Y; Shah A; Hunter RA; Soto RJ; Schoenfisch MH, S-Nitrosothiol-Modified Nitric Oxide-Releasing Chitosan Oligosaccharides as Antibacterial Agents. Acta Biomater. 2015, 12, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Nablo BJ; Rothrock AR; Schoenfisch MH, Nitric Oxide-Releasing Sol–Gels as Antibacterial Coatings for Orthopedic Implants. Biomaterials 2005, 26 (8), 917–924. [DOI] [PubMed] [Google Scholar]
- (50).Pant J; Gao J; Goudie MJ; Hopkins S; Locklin J; Handa H, A Multi-Defense Strategy: Enhancing Bactericidal Activity of a Medical Grade Polymer with a Nitric Oxide Donor and Surface-Immobilized Quaternary Ammonium Compound. Acta Biomater. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Colletta A; Wu J; Wo Y; Kappler M; Chen H; Xi C; Meyerhoff ME, S-Nitroso-N-Acetylpenicillamine (Snap) Impregnated Silicone Foley Catheters: A Potential Biomaterial/Device to Prevent Catheter-Associated Urinary Tract Infections. ACS biomaterials science & engineering 2015, 1 (6), 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Williams DL; Bloebaum RD, Observing the Biofilm Matrix of Staphylococcus Epidermidis Atcc 35984 Grown Using the Cdc Biofilm Reactor. Microsc. Microanal 2010, 16 (02), 143–152. [DOI] [PubMed] [Google Scholar]
- (53).Sundaram J; Pant J; Goudie MJ; Mani S; Handa H, Antimicrobial and Physicochemical Characterization of Biodegradable, Nitric Oxide-Releasing Nanocellulose–Chitosan Packaging Membranes. J. Agric. Food Chem 2016, 64 (25), 5260–5266. [DOI] [PubMed] [Google Scholar]
- (54).Xu L-C; Wo Y; Meyerhoff ME; Siedlecki CA, Inhibition of Bacterial Adhesion and Biofilm Formation by Dual Functional Textured and Nitric Oxide Releasing Surfaces. Acta Biomater. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Hetrick EM; Schoenfisch MH, Reducing Implant-Related Infections: Active Release Strategies. Chem. Soc. Rev 2006, 35 (9), 780–789. [DOI] [PubMed] [Google Scholar]
- (56).AshaRani P; Low Kah Mun G; Hande MP; Valiyaveettil S, Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS nano 2008, 3 (2), 279–290. [DOI] [PubMed] [Google Scholar]
- (57).Kark RP; Poskanzer DC; Bullock JD; Boylen G, Mercury Poisoning and Its Treatment with N-Acetyl-D, L-Penicillamine. New Engl. J. Med 1971, 285 (1), 10–16. [DOI] [PubMed] [Google Scholar]
- (58).Stephens A; Watts R, The Treatment of Cystinuria with N-Acetyl-D-Penicillamine, a Comparison with the Results of D-Penicillamine Treatment. QJM 1971, 40 (3), 355–370. [PubMed] [Google Scholar]
- (59).Stokes G; Potts J; Lotz M; Bartter F, New Agent in the Treatment of Cystinuria: N-Acetyl-D-Penicillamine. Br. Med. J 1968, 1 (5587), 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Hetrick EM; Shin JH; Stasko NA; Johnson CB; Wespe DA; Holmuhamedov E; Schoenfisch MH, Bactericidal Efficacy of Nitric Oxide-Releasing Silica Nanoparticles. Acs Nano 2008, 2 (2), 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Masters KSB; Leibovich SJ; Belem P; West JL; Poole-Warren LA, Effects of Nitric Oxide Releasing Poly (Vinyl Alcohol) Hydrogel Dressings on Dermal Wound Healing in Diabetic Mice. Wound Repair Regen. 2002, 10 (5), 286–294. [DOI] [PubMed] [Google Scholar]
- (62).Coneski PN; Rao KS; Schoenfisch MH, Degradable Nitric Oxide-Releasing Biomaterials Via Post-Polymerization Functionalization of Cross-Linked Polyesters. Biomacromolecules 2010, 11 (11), 3208–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Zhu R; Wang X; Yang J; Wang Y; Zhang Z; Hou Y; Lin F, Influence of Hydroxyl-Terminated Polydimethylsiloxane on High-Strength Biocompatible Polycarbonate Urethane Films. Biomedical Materials 2016, 12 (1), 015011. [DOI] [PubMed] [Google Scholar]
- (64).Marxer SM; Schoenfisch MH, Sol− Gel Derived Potentiometric Ph Sensors. Anal. Chem 2005, 77 (3), 848–853. [DOI] [PubMed] [Google Scholar]


