Abstract
Background
The incidence of secondary pulmonary infections is not well described in hospitalized COVID-19 patients. Understanding the incidence of secondary pulmonary infections and the associated bacterial and fungal microorganisms identified can improve patient outcomes.
Objective
This narrative review aims to determine the incidence of secondary bacterial and fungal pulmonary infections in hospitalized COVID-19 patients, and describe the bacterial and fungal microorganisms identified.
Method
We perform a literature search and select articles with confirmed diagnoses of secondary bacterial and fungal pulmonary infections that occur 48 h after admission, using respiratory tract cultures in hospitalized adult COVID-19 patients. We exclude articles involving co-infections defined as infections diagnosed at the time of admission by non-SARS-CoV-2 viruses, bacteria, and fungal microorganisms.
Results
The incidence of secondary pulmonary infections is low at 16% (4.8–42.8%) for bacterial infections and lower for fungal infections at 6.3% (0.9–33.3%) in hospitalized COVID-19 patients. Secondary pulmonary infections are predominantly seen in critically ill hospitalized COVID-19 patients. The most common bacterial microorganisms identified in the respiratory tract cultures are Pseudomonas aeruginosa, Klebsiella species, Staphylococcus aureus, Escherichia coli, and Stenotrophomonas maltophilia. Aspergillus fumigatus is the most common microorganism identified to cause secondary fungal pulmonary infections. Other rare opportunistic infection reported such as PJP is mostly confined to small case series and case reports. The overall time to diagnose secondary bacterial and fungal pulmonary infections is 10 days (2–21 days) from initial hospitalization and 9 days (4–18 days) after ICU admission. The use of antibiotics is high at 60–100% involving the studies included in our review.
Conclusion
The widespread use of empirical antibiotics during the current pandemic may contribute to the development of multidrug-resistant microorganisms, and antimicrobial stewardship programs are required for minimizing and de-escalating antibiotics. Due to the variation in definition across most studies, a large, well-designed study is required to determine the incidence, risk factors, and outcomes of secondary pulmonary infections in hospitalized COVID-19 patients.
Keywords: Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, Coronavirus disease 2019, COVID-19, Secondary infection, Superimposed infection, Superinfection, Bacterial infection, Fungal infection
Introduction
Since coronavirus disease 2019 (COVID-19) was first recognized in December 2019, it has resulted in the ongoing worldwide pandemic. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an enveloped RNA beta-coronavirus. SARS-CoV-2 shares a similar genetic identity with severe acute respiratory syndrome coronavirus (SARS-CoV) and belongs to the sarbecovirus subgenus of the Coronaviridae family [1]. COVID-19 primarily presents as a respiratory tract infection with symptoms varying from mild flu-like illness to acute respiratory distress syndrome (ARDS) [2, 3]. Viral-related respiratory infections belonging to the same family of coronaviruses such as SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) have been reported to be associated with secondary bacterial and fungal infections [4–7]. However, secondary pulmonary infections in COVID-19 patients are not well described and raised an important knowledge gap. Furthermore, other infectious and non-infectious complications have been described in hospitalized COVID-19 patients strongly associated with underlying COVID-19 infection such as pneumothorax, myocarditis, and even device-related secondary infections (e.g., central venous catheter, foley catheter).[8–10]. The aim of this review is to explore the incidence of secondary bacterial and fungal pulmonary infections in hospitalized patients with COVID-19 infection. We also discuss the bacterial and fungal microorganisms identified, the time to diagnose secondary pulmonary infections, and the frequency of antibiotic use in hospitalized COVID-19 patients with suspected or confirmed secondary pulmonary infections. There is a lack of data in terms of well-defined risk factors or predictors, and associated outcomes of secondary pulmonary infections in hospitalized patients with COVID-19 infection and, therefore, will not be a major focus of this review.
Method
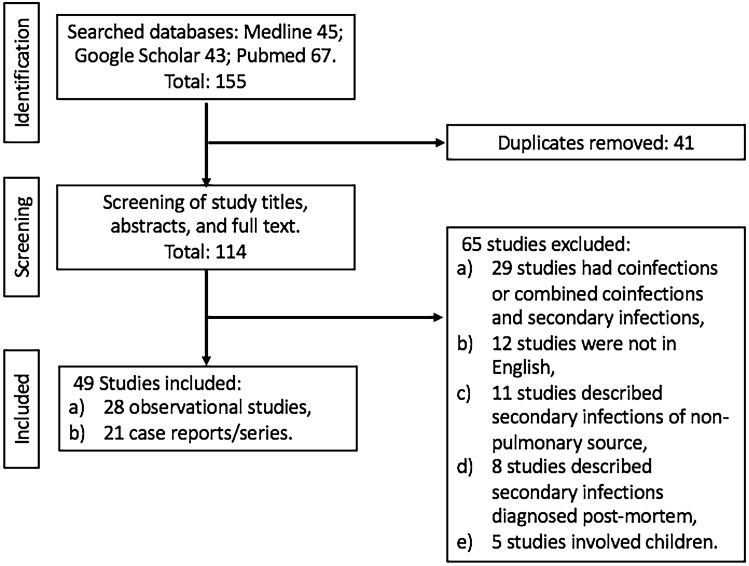
A literature search was performed through MEDLINE, Pubmed, and Google Scholar using keywords of “coronavirus disease 2019 (COVID-19),” “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),” “secondary infection,” “superimposed infection,” “superinfection,” “bacterial infection,” “fungal infection,” “bacterial pneumonia,” “fungal pneumonia,” “bacteremia,” “fungemia,” “hospital-acquired pneumonia (HAP),” and “ventilator-associated pneumonia (VAP)” from January 1st, 2020 to December 31st, 2020. Our selection criteria comprised of articles with confirmed diagnoses of secondary bacterial and fungal pulmonary infections (defined as new microorganisms identified 48 h after admission) using respiratory tracts with corresponding blood cultures for similar microorganisms thought to be respiratory in origin in hospitalized adult COVID-19 patients. Respiratory tract cultures were defined as cultures obtained from sputum, endotracheal aspirates, and bronchoalveolar lavage (BAL). We also included articles in which the diagnoses of secondary pulmonary infections were suspected based on the description of cultures obtained that were respiratory in nature or microorganisms that are recognized to be respiratory in origin. Articles published in the English language were selected, and any cited references were reviewed to identify relevant literature in the English language that comprised of observational studies, case reports, and series that met our selection criteria that described secondary pulmonary infections in hospitalized COVID-19 patients. We excluded articles involving COVID-19 infections in children and pregnant women; non-hospitalized COVID-19 patients; patients with pulmonary co-infections (defined as infections diagnosed at the time of admission) by non-SARS-CoV-2 viruses, bacteria, and fungal microorganisms; secondary pulmonary infections from microorganisms that were known to be colonizers such as candida; studies that included both secondary infections and co-infections from a non-pulmonary source in hospitalized COVID-19 patients; and the diagnosis of secondary pulmonary infections made during the post-mortem examination of deceased COVID-19 patients. We screened 114 studies and included 49 studies that described secondary pulmonary infections in hospitalized adult COVID-19 patients that met our criteria (Fig. 1). Of the 12 studies published in the non-English language (defined as non-English language articles and had no English translation versions/options) that described secondary pulmonary infections in hospitalized COVID-19 patients, 6 were published in Mandarin, 4 were published in Spanish, and the remaining 2 were published in French. The diagnosis of COVID-19 was made by reverse transcriptase-polymerase chain reaction (RT-PCR) in all cases from respiratory tract specimens that include nasal and pharyngeal swabs, sputum, endotracheal aspirates, and bronchoalveolar lavage (BAL).
Fig. 1.
Flowchart for Studies Selected in Review of Hospitalized COVID-19 Patients With Secondary Pulmonary Infections
Results
Incidence of secondary pulmonary infections
Among the 49 studies identified (Table 1), 28 (57%) studies were observational studies done on hospitalized COVID-19 patients; the remainder, 21 (43%), were small case series and case reports. Of the 28 observational studies, 78.6% were retrospective and 21.4% were prospective in nature. The majority of observational studies originated from China in 25% (7/28) of cases followed by 17.9% (5/28) in Spain, 14.3% (4/28) in France, 7.1% in Netherlands and USA, respectively, and the remainder in Belgium, Denmark, England, Germany, Italy, Mexico, Pakistan, and Switzerland. A total of 5,047 hospitalized patients with COVID-19-related pneumonia were identified in the 28 observational studies included in our review (Table 1). The incidence of secondary bacterial pulmonary infections in hospitalized COVID-19 patients reported was 16% (580/3,633) and ranged between 4.8–42.8% in 14 observational studies, whereas the incidence of secondary fungal infections in hospitalized COVID-19 patients was 6.3% (171/2,703) and ranged between 0.9 and 33.3% according to 18 observational studies (Table 1). The majority of hospitalized COVID-19 patients who developed secondary bacterial and fungal infections were critically ill where they required ICU admission and invasive mechanical ventilation (IMV). Studies by Chang et al. , Rouze et al. , and Torrego et al. were the only observational studies that examined the incidence of secondary bacterial pulmonary infection based solely on BAL findings in COVID-19 patients requiring IMV with moderate to severe ARDS [11–13]. However, for examining the incidence of secondary fungal pulmonary infections, respiratory cultures obtained from BAL were only used in observational studies by Bartoletti et al. , Rutsaert et al. , and Van Biesen et al. [14–16].
Table 1.
Characteristics of Secondary Pulmonary Infections Studies in Hospitalized COVID-19 Patients With Incidence of Secondary Bacterial and Fungal Infections
| Study | Region | Study Type | Total COVID-19 Patients (N) | Age | ICU (%) | IMV (%) | Hospital Mortality* (%) | Secondary bacterial infections (%) | Secondary fungal infections (%) |
|---|---|---|---|---|---|---|---|---|---|
| Observational Studies | |||||||||
| Alanio et al. [43] | France | Retrospective, ICU | 27 | 63 | 100 | 100 | 44.4 | NR | 33.3 |
| Barrasa et al. [25] | Spain | Retrospective, ICU | 48 | 63 (mean) | 100 | 93.7 | 35.4 | 12.5 | NR |
| Bartoletti et al. [14] | Italy | Prospective, ICU | 108 | 63* (median) | 100 | 100 | 44.0 | NR | 27.7 |
| Chang et al. [12] | USA | Retrospective, ICU | 412 | 62 (median) | 100 | 100 | NR | 15.5 | NR |
| Du et al. [56] | China | Prospective, Hospital | 179 | 58 (median) | NR | NR | 11.7 | 5.6 | NR |
| Dupont et al. [69] | France | Prospective, ICU | 106 | 69* (median) | 100 | 100 | 35.3 | NR | 17.9 |
| Fekkar et al. [17] | France | Retrospective, ICU | 145 | 55 (median) | 100 | 100 | 57.1 | NR | 4.8 |
| Feng et al. [32] | China | Retrospective, Hospital/ICU | 410 | 53 (median) | 14.8 | 8.0 | 8.0 | 9.0 | NR |
| Fu et al. [23] | China | Retrospective, Hospital/ICU | 101 | 69* (mean) | 35.6 | 100 | NR | 5.0 | 0.9 |
| Gangneux et al. [70] | France | Prospective, ICU | 45 | 70* (mean) | 100 | 100 | 28.6 | NR | 15.6 |
| Garcia-Vidal et al. [22] | Spain | Retrospective, Hospital/ICU | 989 | 62 (median) | 11.9 | NR | 9.8 | 4.3 | 0.7 |
| Helleberg et al. [71] | Denmark | Retrospective, ICU | 25 | 58 | 100 | 100 | 100 | NR | 8.0 |
| Huang et al. [30] | China | Retrospective, Hospital/ICU | 41 | 49 (median) | 46.0 | 5.0 | 15.0 | 10.0 | NR |
| Karmen-Tuohy et al. [58] | USA | Retrospective, Hospital | 63 | 60 (median) | 20.6 | 15.8 | 25.3 | 6.3 | NR |
| Lamoth et al. [72] | Switzerland | Retrospective, ICU | 118 | 62* (mean) | 100 | 100 | 33.3 | NR | 3.8 |
| Machado et al. [35] | Spain | Prospective, ICU | 239 | 63* (mean) | 100 | 100 | 100 | NR | 2.5 |
| Nasir et al. [26] | Pakistan | Retrospective, Hospital/ICU | 147 | 71 (median) | 15.6 | 40 | 60.0 | 4.8 | 6.1 |
| Roman-Montes et al. [73] | Mexico | Retrospective, ICU | 144 | 48* (mean) | 100 | NR | 57.1 | NR | 9.7 |
| Rouze et al. [13] | Germany | Retrospective, ICU | 568 | 64 (mean) | 100 | 100 | NR | 36.1 | NR |
| Rutsaert et al. [15] | Belgium | Retrospective, ICU | 34 | 66 | 100 | 100 | 58.8 | NR | 20.5 |
| Segrelles-Calvo et al. [74] | Spain | Retrospective, ICU | 215 | 60 (median) | 100* | 100* | 71.4 | NR | 3.3 |
| Torrego et al. [11] | Spain | Retrospective, ICU | 93 | NR | 100 | NR | NR | 19.3 | NR |
| Van Arkel et al. [75] | Netherlands | Retrospective, ICU | 31 | 64 | 100 | 83.9 | 67.7 | NR | 19.4 |
| Van Biesen et al. [16] | Netherlands | Retrospective, ICU | 42 | 68 (mean) | 100 | 100 | 22.2 | NR | 21.4 |
| Wang et al. [57] | China | Retrospective, Hospital/ICU | 339 | 71 (median) | NR | 23.6 | 19.1 | 42.8 | NR |
| White et al. [34] | England | Prospective, ICU | 135 | 57 (median) | 100 | 72.0 | 52.0 | NR | 18.5 |
| Yang et al. [24] | China | Retrospective, ICU | 52 | 60 (mean) | 100 | 71.0 | 61.5 | 7.7 | 3.8 |
| Zhou et al. [31] | China | Retrospective, Hospital/ICU | 191 | 56 (median) | 26.0 | 17.0 | 28.2 | 15.0 | NR |
| Case Series/Reports | Age* (mean) | ICU* (%) | IMV* (%) | Death* (%) | Secondary bacterial infections (%) | Secondary fungal infections (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Abdalla et al. [76] | Qatar | Case Series, ICU | 2 | 66 | 100 | 100 | 100 | NR | 100 |
| Blanco et al. [19] | Spain | Case Series, Hospital | 5 | 38 | 40.0 | 20.0 | 0.0 | NR | 20.0 |
| Falces-Romero et al. [77] | Spain | Case Series, Hospital | 10 | 70 | 70.0 | 70.0 | 70.0 | NR | 100 |
| Koehler et al. [78] | Germany | Case Series, ICU | 19 | 63 | 100 | 100 | 57.8 | NR | 26.3 |
| Lahmer et al. [79] | Germany | Case Series, ICU | 2 | 75 | 100 | 100 | 100 | NR | 100 |
| Lescure et al. [80] | France | Case Series, Hospital | 5 | 47 | 60.0 | 40.0 | 40.0 | 20.0 | 20.0 |
| Sharifipour et al. [81] | Iran | Case Series, ICU | 19 | 67 | 100 | 100 | 94.7 | 100 | NR |
| Antinori et al. [82] | Italy | Case Report, ICU | 1 | 56 | 100 | 100 | 100 | NR | 100 |
| Blaize et al. [83] | France | Case Report, ICU | 1 | 74 | 100 | 100 | 100 | NR | NR |
| Duployez et al. [84] | France | Case Report, ICU | 1 | 30 s | 100 | 100 | 100 | NR | NR |
| Fernandez et al. [85] | Argentina | Case Report, ICU | 1 | 85 | 100 | 100 | 100 | 100 | 100 |
| Ghelfenstein et al. [86] | France | Case Report, ICU | 1 | 56 | 100 | 100 | 100 | NR | 100 |
| Kelly et al. [21] | England | Case Report, ICU | 1 | 50 s | 100 | 100 | 100 | NR | 100 |
| Mang et al. [20] | Germany | Case Report, ICU | 1 | 52 | 100 | 100 | 0.0 | NR | 100 |
| Meijer et al. [87] | Netherland | Case Report, ICU | 1 | 74 | 100 | 100 | 100 | NR | 100 |
| Menon et al. [18] | USA | Case Report, ICU | 1 | 83 | 100 | 100 | 0.0 | NR | NR |
| Mohamed et al. [47] | Ireland | Case Report, ICU | 1 | 66 | 100 | 100 | 100 | 100 | 100 |
| Nasri et al. [88] | Iran | Case Report, ICU | 1 | 42 | 100 | 100 | 100 | NR | 100 |
| Prattes et al. [89] | Austria | Case Report, ICU | 1 | 70 | 100 | 100 | 100 | NR | 100 |
| Schein et al. [90] | France | Case Report, Hospital | 1 | 87 | 0 | 0 | 100 | NR | 100 |
| Sharma et al. [91] | Australia | Case Report, ICU | 1 | 66 | 100 | 100 | 0 | NR | 100 |
*Among those with secondary infections
ICU = intensive care unit, IMV = invasive mechanical ventilation, NR = not reported
Microbiology of secondary pulmonary infections
Out of the 28 observational studies, 14.3% (4/28) of studies had no descriptions of the specific bacterial or fungal microorganisms identified (Table 2). The most common bacterial microorganisms identified in the respiratory tract cultures among the nine observational studies (Table 2) that reported the type and frequency of secondary bacterial infection were 21.1% (75/355) Pseudomonas aeruginosa, 17.2% (61/355) Klebsiella species, 13.5% (48/355) Staphylococcus aureus, 10.4% (37/355) Escherichia coli, and 3.1% (11/355) Stenotrophomonas maltophilia. However, the fungal microorganisms identified in 18 observational studies included in our review were predominantly Aspergillus species in which Aspergillus fumigatus was most frequently isolated in all studies. Other less common Aspergillus species identified were Aspergillus flavus, Aspergillus calidoustus, Aspergillus citrinoterreus, Aspergillus niger, Aspergillus terreus, and Aspergillus versicolor. One observational study by Fekkar et al. reported an uncommon finding of Mucor species and Fusarium proliferatum in respiratory tract cultures of critically ill COVID-19 patients [17]. Other rare opportunistic fungal infections such as Pneumocystis jirovecii (PJP) had been observed in four case reports/series included in our review [18–21].
Table 2.
Studies in Hospitalized COVID-19 Patients With Types of Microorganisms Reported, Rate of Antibiotic Use, and Time to Diagnosis of Secondary Pulmonary Infections
| Study | Top Five Bacterial Microorganisms From Respiratory Tract Culture | Fungal Microorganisms From Respiratory Tract Culture | Empirical Antibiotic Therapy (%) | Median Day to Secondary Pulmonary Infection Diagnosis From Admission |
|---|---|---|---|---|
| Observational Studies | ||||
| Alanio et al. [43] | NR | 100% Aspergillus fumigatus | NR | NR |
| Barrasa et al. [25] | 50% Pseudomonas aeruginosa, 16% Enterococcus faecium, 16% Haemophilus influenzae, 16% Methicillin-Resistant Staphylococcus aureus (MRSA) | NR | 87.5 | NR |
| Bartoletti et al. [14] | NR | 78.9% Aspergillus fumigatus, 15.8% Aspergillus niger, 5.3% Aspergillus flavus | NR | 4 (ICU) |
| Chang et al. [12] | 36% Klebsiella spp., 23% MSSA, 11% Escherichia coli, 11% Serratia spp., 11% Stenotrophomonas spp. | NR | NR | NR |
| Du et al. [56] | Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Staphylococcus species** | NR | NR | NR |
| Dupont et al. [69] | NR | 87.5% Aspergillus fumigatus, 6.3% Aspergillus flavus, 6.3% Aspergillus calidoustus | NR | 11 (ICU) |
| Fekkar et al. [17] | NR | 66.7% Aspergillus fumigatus, 22.2% Mucor spp., 11.1% Fusarium proliferatum | NR | 18 (ICU) |
| Feng et al. [32] | NR | NR | 67.1 | NR |
| Fu et al. [23] | 29% Pseudomonas aeruginosa, 29% Burkholderia cepacia, 14% Escherichia coli, 14% Extended-spectrum beta-lactamase (ESBL) Klebsiella pneumoniae, 14% Stenotrophomonas maltophilia | 100% Aspergillus fumigatus | NR | 12 |
| Gangneux et al. [70] | NR | 100% Aspergillus fumigatus | NR | NR |
| Garcia-Vidal et al. [22] | 33% multidrug-resistant (MDR) Pseudomonas aeruginosa, 25% ESBL Escherichia coli, 21% ESBL Klebsiella pneumoniae, 21% Staphylococcus aureus | 100% Aspergillus fumigatus | 61.9 | 11 |
| Helleberg et al. [71] | NR | 100% Aspergillus fumigatus | NR | 3 (ICU) |
| Huang et al. [30] | NR | NR | 100 | > 2 |
| Karmen-Tuohy et al. [58] | 29% Stenotrophomonas maltophilia, 29% Pseudomonas aeruginosa, 14% Escherichia coli, 14% Klebsiella pneumoniae, 14% Staphylococcus aureus | NR | NR | > 6 |
| Lamoth et al. [72] | NR | 100% Aspergillus fumigatus | 100 | 6 (ICU) |
| Machado et al. [35] | NR | 66.6% Aspergillus fumigatus, 16.7% Aspergillus citrinoterreus, 16.7% Aspergillus lentulus | 100 | 15 (ICU) |
| Nasir et al. [26] | 25% Pseudomonas aeruginosa, 25% Acinetobacter spp., 25% Stenotrophomonas maltophilia, 12.5% Klebsiella pneumoniae, 12.5% MRSA | 60% Aspergillus flavus, 20% Aspergillus fumigatus, 20% Aspergillus terreus | 77.7 | 7 |
| Roman-Montes et al. [73] | NR | 54.5% Aspergillus fumigatus, 27.3% Aspergillus spp., 9.1% Aspergillus flavus, 9.1% Aspergillus niger | NR | 9 |
| Rouze et al. [13] | 22.3% Pseudomonas aeruginosa, 18.8% Enterobacter spp., 11.5% Klebsiella spp., 9.4% MSSA, 8.4% Escherichia coli | NR | 93.2 | NR |
| Rutsaert et al. [15] | NR | 83% Aspergillus fumigatus, 17% Aspergillus Flavus | NR | 6 |
| Segrelles-Calvo et al. [74] | NR | 42.8% Aspergillus fumigatus, 28.6% Aspergillus flavus, 28.6% Aspergillus niger | NR | NR |
| Torrego et al. [11] | 39% Pseudomonas aeruginosa, 11% Enterobacter cloacae, 11% Enterococcus faecalis, 11% Klebsiella aerogenes, 11% Staphylococcus aureus | NR | NR | < 21 |
| Van Arkel et al. [75] | NR | 100% Aspergillus fumigatus | NR | 12 |
| Van Biesen et al. [16] | NR | 71.4% Aspergillus fumigatus, 14.3% Aspergillus flavus, 14.3% Aspergillus terreus | NR | 5 (ICU) |
| Wang et al. [57] | NR | NR | NR | NR |
| White et al. [34] | NR | 91.6% Aspergillus fumigatus, 8.4% Aspergillus versicolor | 98.0 | 8 (ICU) |
| Yang et al. [24] | 25% ESBL Klebsiella pneumoniae, 25% Carbapenem-resistant Klebsiella pneumoniae, 25% MDR Pseudomonas aeruginosa, 25% Serratia marcescens | 50% Aspergillus fumigatus, 50% Aspergillus flavus | 94.2 | NR |
| Zhou et al. [31] | NR | NR | 94.8 | 17 |
| Case Series/Reports | Top Five Bacterial Microorganisms From Respiratory Tract Culture | Fungal Microorganisms From Respiratory Tract Culture | Antibiotic/ Antifungal Therapy* (%) |
Median Day to Secondary Pulmonary Infection Diagnosis From Admission |
|---|---|---|---|---|
| Abdalla et al. [76] | NR | 50% Aspergillus niger, 50% Aspergillus terreus | 100 | 12 (ICU) |
| Blanco et al. [19] | NR | 100% Pneumocystis jirovecii | 60.0 | 12 |
| Falces-Romero et al. [77] | NR | 87.5% Aspergillus fumigatus, 12.5% A. nidulans | 80.0 | 17.1 |
| Koehler et al. [78] | NR | 100% Aspergillus fumigatus | NR | NR |
| Lahmer et al. [79] | NR | 100% Aspergillus fumigatus | NR | 5.5 (ICU) |
| Lescure et al. [80] | 100% Acinetobacter baumannii | 100% Aspergillus fumigatus | NR | NR |
| Sharifipour et al. [81] | 89.4% Acinetobacter baumannii, 5.3% MSSA, 5.3% MRSA | NR | NR | NR |
| Antinori et al. [82] | NR | 100% Aspergillus fumigatus | 100 | 9 |
| Blaize et al. [83] | NR | 100% Aspergillus fumigatus | 100 | 4 |
| Duployez et al. [84] | 100% Panton-Valentine Leukocidin (PVL) Methicillin-Sensitive Staphylococcus aureus (MSSA) | N/A | 100 | 5 |
| Fernandez et al. [85] | 100% Enterococcus faecalis, Acinetobacter baumannii | 100% Aspergillus fumigatus | 100 | 10 |
| Ghelfenstein et al. [86] | NR | 100% Aspergillus fumigatus | 100 | 6 |
| Kelly et al. [21] | NR | 100% Pneumocystis jirovecii | 100 | 10 |
| Mang et al. [20] | NR | 100% Pneumocystis jirovecii | 100 | 20 |
| Meijer et al. [87] | NR | 100% Aspergillus fumigatus | 100 | 6 |
| Menon et al. [18] | NR | 100% Pneumocystis jirovecii | NR | NR |
| Mohamed et al. [47] | Klebsiella Varicola | 100% Aspergillus fumigatus | 100 | 7 |
| Nasri et al. [88] | NR | 100% Aspergillus spp. | 100 | 9 |
| Prattes et al. [89] | NR | 100% Aspergillus fumigatus | 100 | 3 (ICU) |
| Schein et al. [90] | NR | 100% Aspergillus spp. | 100 | 16 |
| Sharma et al. [91] | NR | 100% Aspergillus fumigatus | 100 | 16 |
*Among those with secondary infections
**Percentage frequency of bacterial or fungal microorganisms not reported
NR not reported, Spp. Species
The time to diagnosis of secondary pulmonary infections and use of antibiotics
The average time taken to diagnose secondary bacterial and fungal pulmonary infections from hospital and ICU admission among the 18 observational studies described was 10 days (ranged 2–21 days) and 9 days (ranged 4–18 days), respectively (Table 2). The reported use of empirical antibiotics was 60–100% during the current pandemic between 11 observational studies (Table 2). Furthermore, although specific data on antibiotic resistance patterns lacked in the majority of observational studies included in our review, limited observational studies had reported of detection of multidrug-resistant (MDR) microorganisms such as extended-spectrum beta-lactamase (ESBL) Klebsiella pneumoniae, ESBL Escherichia coli, MDR Pseudomonas aeruginosa, carbapenem-resistant Klebsiella pneumoniae, and Methicillin-resistant Staphylococcus aureus (MRSA) from the respiratory tract and blood cultures in critically ill COVID-19 patients [22–26].
Discussion
In hospitalized COVID-19 patients, the incidence of secondary pulmonary infections was low at 16% (4.8–42.8%) for bacterial infections and lower for fungal infections with an incidence of 6.3% (0.9–33.3%). However, the frequency of empirical antibiotic therapy was high at 60–100% among several observational studies included. The most common bacterial microorganisms identified in the respiratory tract cultures were 21.1% Pseudomonas aeruginosa, 17.2% Klebsiella species, 13.5% Staphylococcus aureus, 10.4% Escherichia coli, and 3.1% Stenotrophomonas maltophilia. Aspergillus fumigatus was the most common fungal microorganism identified to cause secondary pulmonary infections. Other rare opportunistic infection such as PJP was mostly confined to small case series and case reports. The overall time to diagnose secondary bacterial and fungal pulmonary infections was 10 days (2–21 days) and 9 days (4–18 days), respectively, from the time of hospital and ICU admission.
In contrast, the incidence of secondary bacterial pulmonary infections during the 2009 Influenza A pandemic is up to 7% in critically ill patients [27]. However, for secondary fungal pulmonary infections, the incidence is as high as 14% in critically ill patients with seasonal influenza [28], 29. A retrospective study by Rouze et al. reported that secondary bacterial pulmonary infections were 1.6 times more likely to occur in critically ill COVID-19 patients compared to influenza patients. Four observational studies did not report any specific type of microorganism identified [3, 30–32]. In these studies, secondary pulmonary infections were minor secondary outcomes identified while assessing the many characteristics, risk factors, and outcomes of hospitalized COVID-19 patients. Furthermore, although 18 observational studies described secondary fungal pulmonary infections predominantly Aspergillus fumigatus, there was an absence of a standardized definition with the heterogeneity of diagnostic criteria used to differentiate between true infection versus colonization [33–35]. The microorganisms identified to cause secondary bacterial pulmonary infections in hospitalized COVID-19 patients are similar to microorganisms isolated during seasonal/pandemic influenza and even during the 2003 SARS outbreak [13, 36, 37]. The identification of gram-negative microorganisms in hospitalized COVID-19 patients is consistent with the type of pathogens commonly associated with hospital-acquired pneumonia involving Pseudomonas aeruginosa, Klebsiella species, Stenotrophomonas maltophilia, and Acinetobacter baumannii that does not necessarily suggest a specific preference for gram-negative infections in COVID-19 [38–41]. The time taken for the diagnosis of secondary pulmonary infections is highly variable between 2 and 21 days from hospital admission and 4–18 days from ICU admission according to the 18 observational studies included in our review (Table 2). This differs in contrast to secondary bacterial infections that are diagnosed earlier in patients with influenza infection, which are 3–6 days from the initial presentation [36, 42]. For secondary invasive pulmonary aspergillosis in influenza patients, the median time to diagnosis is between 5 and 10 days after ICU admission [15, 29]. Although all observational studies included described respiratory tract cultures obtained more than 48 h after admission, the variability in time to diagnosis can be due to the inconsistency on when and a lack of information on why surveillance cultures are obtained.
Bronchoscopy may be a useful tool to obtain respiratory tract cultures of sufficient quantity to help diagnose and isolate microorganisms in secondary pulmonary infections while determining the antibiotic sensitivities in hospitalized COVID-19 patients. The routine use of bronchoscopy may even lead to over-diagnosis of secondary pulmonary infections from respiratory tract colonization. According to three observational studies, the incidence of secondary bacterial pulmonary infections was 15% and more when routine bronchoscopy with BAL was performed in critically ill COVID-19 patients requiring IMV [11–13]. Four observational studies reported that the incidence of secondary fungal pulmonary infections was 20% and more in critically ill COVID-19 patients when bronchoscopy with BAL was performed routinely post-intubation, in a serial fashion, or any change in clinical status due to atelectasis, new lung infiltrates on imaging, and thick secretions [14–16, 43]. Chang et al. described that respiratory tract cultures obtained from BAL have a higher positivity rate when compared to endotracheal aspirate and a greater tendency to detect different or second microorganisms as a cause of secondary pulmonary infections [12]. In the study by Torrego et al. , the microbiology findings on BAL resulted in a change in antibiotic prescribed in 83% of critically ill COVID-19 patients requiring IMV. However, the bacterial microorganisms identified such as Pseudomonas aeruginosa, Klebsiella species, Enterobacter cloacae, and Staphylococcus aureus was similar to bacterial microorganisms in mechanically ventilated non-COVID-19 patients [11]. However, bronchoscopy is often avoided as it is an aerosol-generating procedure that will predispose healthcare workers and patients to a substantial risk of further transmitting COVID-19 infection. The use of bronchoscopy in COVID-19 patients has been recommended when current respiratory samples from sputum and endotracheal aspirates are negative, in which an alternate diagnosis provided by BAL would significantly impact clinical management [44]. Nevertheless, two recent single-center retrospective studies showed no increase in the risk of COVID-19 transmission to healthcare providers when bronchoscopy is routinely performed while adhering to the proper infection control protocol [12, 45].
The current knowledge of the risk factors for secondary pulmonary infections in SARS-CoV-2 is continuously evolving but remains poorly understood. Although it is becoming apparent that secondary pulmonary infections that occur in hospitalized COVID-19 patients can be associated with worse outcomes, it remains unclear if critically ill COVID-19 patients are at a greater likelihood of developing secondary pulmonary infections. COVID-19 infection will trigger innate and adaptive immune responses, including local immune response, recruitment of macrophages and monocytes, the release of cytokines, and prime adaptive T- and B-cell in an effort to resolve underlying inflammation [46–49]. However, in some cases, a dysfunctional immune response occurs that renders COVID-19 patients vulnerable to secondary pulmonary infections. Lymphocyte count, specifically T-cells, is substantially decreased, whereas inflammatory mediators of interleukins- (IL-)2, IL-6, IL-8, IL-10, tumor necrosis factor-alpha (TNF-a), and interferon-gamma are markedly increased within a week from COVID-19 presentation before recovering to normal levels, two weeks later [30, 50–53]. This dysregulated immune response that is seen to a greater degree in those with severe COVID-19 infections has an immunosuppression stage following the proinflammatory phase characterized by a sustained and substantial reduction in peripheral lymphocyte count [48, 50, 54]. Similar immunological findings have been described in SARS-CoV patients during the 2003 epidemic and H1N1 influenza during the 2009 pandemic [53–55]. This state of lymphocytopenia-induced immunosuppression observed in many hospitalized COVID-19 patients may explain the time taken for secondary pulmonary infection diagnosis seen in studies included in our review [30–32, 56].
Furthermore, in a multi-center study involving 410 COVID-19 patients, secondary pulmonary infections were significantly associated with outcome severity. Critically ill patients had the highest percentage of secondary pulmonary infections (34.5%) compared to severely ill (8.3%) and moderately ill (3.9%) COVID-19 patients [32]. This high rate of secondary pulmonary infections occurs despite a majority of critically ill patients (92.9%) receiving antibiotics compared to 83.3% and 59.4% in the severely ill and moderately ill groups. Five observational studies reported that among critically ill COVID-19 patients, non-survivors/critically ill patients had a greater tendency to suffer from multi-organ dysfunction and develop secondary pulmonary infections despite up to 98% of them received antibiotics [24, 30, 31, 56, 57]. In all these studies, the degree of lymphocytopenia and corticosteroids administration was significantly higher in the critically ill/non-survivor group than in other groups. Furthermore, although the nadir CD4 + T-cell count was less than 200 cells/106L in the majority of case reports/series describing PJP among HIV patients co-infected with COVID-19 [19–21], a case report by Menon et al. described a hospitalized COVID-19 patient diagnosed with PJP despite the absence of HIV infection. Though her nadir CD4 + T-cell count was 291 cells/106L and she was on chronic oral budesonide for her ulcerative colitis, the improvement with trimethoprim-sulfamethoxazole supported the diagnosis of secondary PJP infection [18]. On the contrary, a retrospective study by Karmen-Tuohy et al. reported no increased incidence of secondary bacterial or even PJP pulmonary infections in HIV-positive COVID-19 patients who were compliant with antiretroviral therapy, regardless of their CD4 + T cell count [58]. There is no single study to the current date, which has formally assessed lymphocytopenia as a risk factor for secondary pulmonary infections. Moreover, corticosteroids are frequently used in COVID-19 patients to prevent and treat cytokine storm and ARDS, which are suspected to be partly caused by dysregulated host immune response [50, 53, 59]. Recent studies assessing the use of corticosteroids in hospitalized COVID-19 patients demonstrated that a short course of corticosteroids over ten days has shown to be beneficial in the setting of hypoxic respiratory failure requiring oxygen therapy and mechanical ventilation requirement [60, 61]. However, previous studies have demonstrated that corticosteroids may inadvertently increase the mortality and secondary infections in influenza patients, and prolong viral shedding and induce lymphocytopenia in SARS-CoV patients by down-regulating the innate and adaptive immune system.[29, 54, 62, 63] Currently, there is no formal study to assess the risk of secondary pulmonary infections associated with corticosteroids administration in COVID-19 patients. However, in our review, although the majority of patients were receiving corticosteroids, the timing of administration, duration, and the dose of corticosteroids were not clearly described.
Differentiating viral from secondary bacterial and fungal pulmonary infections remains a challenge for clinicians. This diagnostic uncertainty has contributed to the overuse of antibiotics in patients with COVID-19 viral illness. Although the incidence of secondary bacterial pulmonary infections in COVID-19 patients is low, the reported use of empirical antibiotics is 60–100% among the observational studies included in our review (Table 2). These findings vastly differed when compared to patients with seasonal/pandemic influenza, in which the reported use of empirical antibiotics was 12–50% [36]. It is essential to consider how the frequent use of empirical antibiotic therapy could affect the prevalence of multidrug-resistant bacteria. The rising number of antibiotic use may predispose COVID-19 patients, especially those who are critically ill, to sepsis from secondary multidrug-resistant bacterial infections. An observational study during the SARS outbreak in 2003 demonstrated that MRSA acquisition identified on screening using nasal swabs drastically increased from 2.2 to 3.5 cases per 100 ICU admissions (pre-SARS and post-SARS period) to 25.3% per 100 ICU admissions (during SARS period), despite extensive infection control precautions [64]. This finding coincides with the increased use of broad-spectrum empiric antibiotics (4th generation cephalosporins, fluoroquinolones, aminoglycosides, and carbapenems) during the SARS period, in which MRSA was responsible for up to 48% of microorganisms isolated in patients with VAP. Furthermore, common bacterial microorganisms identified on post-mortem examination of SARS patients were Pseudomonas aeruginosa, Klebsiella species, and Staphylococcus aureus, which are known for their high resistance to broad-spectrum antibiotics [65, 66]. In the studies that we reviewed, antibiotic sensitivities of microorganisms and treatment duration were not reported even though MDR microorganisms were observed. Based on the current microbiological data from our review, it remains imperative that empiric antibiotic therapy covers multidrug-resistant microorganisms such as MRSA and ESBL that are associated with a high fatality rate when concerns exist of possible secondary pulmonary infections in critically ill COVID-19 patients [39, 40]. Our review supports the notion of frequently obtaining surveillance cultures (from sputum, endotracheal aspirate, blood, and BAL if beneficial) and daily decision-making on antibiotic requirements to de-escalate and avoid prolonged therapy that will lead to the development of antibiotic resistance.
The considerable variability in the incidence of secondary bacterial (4.8–42.8%) and fungal (0.9–33.3%) pulmonary infections reported and time taken for the diagnosis can be due to several limitations across the various observational studies included in this review. (1) The majority of the studies examining the incidence of secondary pulmonary infections are of poor quality and limited by the lack of a clear definition of secondary infections versus co-infections. There is also an absence of a standardized definition with the heterogeneity of diagnostic criteria used to differentiate between true invasive pulmonary fungal infection from colonization.[33–35] (2) Moreover, secondary pulmonary infections observed are a bystander (minor secondary outcome) result or identified during subgroup analysis while assessing the many characteristics, risk factors, and outcomes of hospitalized COVID-19 patients [3, 24, 30–32, 56, 58]. (3) It is not uncommon for early and late secondary infections to be frequently clustered together in the currently available literature for COVID-19 patients that may lead to the under- or overestimation of the exact incidence of secondary pulmonary infections, depending on the duration of the study period, especially among the 78.6% retrospective studies included in our review [67]. (4) The wide range of incidence rates reported for secondary pulmonary infections might have been due to the differences in the patient population, severity of illness, diagnostic sampling, and frequency of surveillance cultures obtained across various observational studies from multiple different countries. The routine use of bronchoscopy with BAL in critically ill COVID-19 patients where many are intubated and requiring IMV may lead to the over-diagnosis of secondary pulmonary infections [14–16, 43]. (5) The restricted search methodology that is confined to English literature as we (authors) are not well-versed in other languages during this global pandemic likely contribute to the under-recognition of the true incidence of secondary pulmonary infections. (6) Furthermore, the high mortality rate associated with COVID-19 pneumonia may be an independent competing risk factor for the development of late secondary infection, leading to an unintended underestimation of the actual risk in non-deceased COVID-19 patients [67]. (7) Lastly, the widespread use of empirical antibiotics, analgesics, and corticosteroids likely mask underlying symptoms of infections, and lead to the delay and also underdiagnosis of secondary pulmonary infections. This could be due to the lack of routine surveillance cultures obtained because of fear towards COVID-19 transmission to health care professionals with prolonged patient contact [68]. These explain the variable incidence rate and inability to effectively perform a meta-analysis to determine better the incidence, risk factor, prognostic marker, and secondary pulmonary infection outcome in COVID-19 patients.
Conclusion
Our review on secondary pulmonary infections is limited by the lack of a clear definition of secondary infections versus co-infections, the inconsistency of the type microorganisms identified and time that surveillance cultures are obtained, the lack of information available on the associated antibiotic sensitivities of microorganisms, and duration of antibiotic treatment across various observational studies, small case reports and series, and variability in clinical characteristics reported in hospitalized COVID-19 patients. Additionally, with an observed strain being placed on the healthcare systems during the ongoing COVID-19 pandemic, there is a need for organized antimicrobial stewardship programs in the hospital to minimize the use of unnecessary empiric antibiotics and de-escalation of antibiotics when possible. As variation continues to exist on what constitutes a secondary infection (that we defined as infections occurring 48 h after admission) due to the lack of clear and consistent definition among many observational studies, we hope that a large, well-designed study can be performed in the future to accurately determine the incidence, microorganisms, risk factors, predictors, and outcomes of secondary pulmonary infections in hospitalized COVID-19 patients.
Author contribution
All authors had access to the data and were involved in writing the manuscript.
Funding
None.
Declarations
Conflict of interest
None.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. China JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 6.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 7.Lee N, Cameron P, Yung MY, Leung CB, Szeto CC (2003) A major outbreak of severe acute respiratory syndrome in Hong Kong. The New England Journal of Medicine 9. [DOI] [PubMed]
- 8.Seitz T, Hoepler W, Weseslindtner L, Aberle JH, Aberle SW, Puchhammer-Stoeckl E, et al. Successful management of the first reported case in Austria of COVID-19 with ARDS. Infection. 2020;48:647–651. doi: 10.1007/s15010-020-01458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiro JE, Sisovic S, Ockert B, Böcker W, Siebenbürger G. Secondary tension pneumothorax in a COVID-19 pneumonia patient: a case report. Infection. 2020;48:941–944. doi: 10.1007/s15010-020-01457-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng J-H, Liu Y-X, Yuan J, Wang F-X, Wu W-B, Li J-X, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torrego A, Pajares V, Fernández-Arias C, Vera P, Mancebo J. Bronchoscopy in COVID-19 Patients with Invasive Mechanical Ventilation: A Center Experience. Am J Respir Crit Care Med. 2020 May 15;rccm.202004–0945LE. [DOI] [PMC free article] [PubMed]
- 12.Chang SH, Jiang J, Kon ZN, Williams DM, Geraci TC, Smith DE, et al. Safety and Efficacy of Bronchoscopy in Critically Ill Patients With Coronavirus Disease 2019. Chest. 2020 Oct;S001236922034873X. [DOI] [PMC free article] [PubMed]
- 13.On behalf of the coVAPid study Group, Rouzé A, Martin-Loeches I, Povoa P, Makris D, Artigas A, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med [Internet]. 2021 Jan 3 [cited 2021 Jan 23]; http://link.springer.com/10.1007/s00134-020-06323-9 [DOI] [PMC free article] [PubMed]
- 14.Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with covid-19: a prospective study. Clin Infect Dis. 2020;28:ciaa1065. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutsaert L, Steinfort N, Van Hunsel T, Bomans P, Naesens R, Mertes H, et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10:71. doi: 10.1186/s13613-020-00686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Biesen S, Kwa D, Bosman RJ, Juffermans NP. Detection of invasive pulmonary aspergillosis in covid-19 with nondirected BAL. Am J Respir Crit Care Med. 2020;202:1171–1173. doi: 10.1164/rccm.202005-2018LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, Constantin J-M, et al. Occurrence of invasive pulmonary fungal infections in severe COVID-19 patients admitted to the ICU. Am J Respir Crit Care Med. 2020;203:307–317. doi: 10.1164/rccm.202009-3400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon AA, Berg DD, Brea EJ, Deutsch AJ, Kidia KK, Thurber EG, et al. A Case of COVID-19 and Pneumocystis jirovecii Co-infection. Am J Respir Crit Care Med. 2020;rccm.202003–0766LE. [DOI] [PMC free article] [PubMed]
- 19.Blanco JL, Ambrosioni J, Garcia F, Martínez E, Soriano A, Mallolas J, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7:e314–e316. doi: 10.1016/S2352-3018(20)30111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mang S, Kaddu-Mulindwa D, Metz C, Becker A, Seiler F, Smola S, et al. Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome coronavirus 2 coinfection in a patient with newly diagnosed HIV-1 infection. Clin Infect Dis. 2020;1:ciaa906. doi: 10.1093/cid/ciaa906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly S, Waters L, Cevik M, Collins S, Lewis J, Wu M-S, et al. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin Med. 2020;20:590–592. doi: 10.7861/clinmed.2020-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalised patients with COVID-19: a retrospective cohort study. Clinical Microbiology and Infection. 2020 Jul;S1198743X2030450X. [DOI] [PMC free article] [PubMed]
- 23.Fu Y, Yang Q, Xu M, Kong H, Chen H, Fu Y, et al. Secondary bacterial infections in critical Ill patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7:220. doi: 10.1093/ofid/ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrasa H, Rello J, Tejada S, Martín A, Balziskueta G, Vinuesa C, et al. SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020;39:553–561. doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63:766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice TW, Rubinson L, Uyeki TM, Vaughn FL, John BB, Miller RR, et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40:1487–98. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 29.Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis. 2018;31:471–480. doi: 10.1097/QCO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 30.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassetti M, Giacobbe DR, Grecchi C, Rebuffi C, Zuccaro V, Scudeller L, et al. Performance of existing definitions and tests for the diagnosis of invasive aspergillosis in critically ill, adult patients: a systematic review with qualitative evidence synthesis. J Infect. 2020;81:131–146. doi: 10.1016/j.jinf.2020.03.065. [DOI] [PubMed] [Google Scholar]
- 34.White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Machado M, Valerio M, Álvarez‐Uría A, Olmedo M, Veintimilla C, Padilla B, et al. Invasive pulmonary aspergillosis in the COVID‐19 era: An expected new entity. Mycoses. 2020 Nov 29;myc.13213. [DOI] [PMC free article] [PubMed]
- 36.Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh Y-H, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10:394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaillancourt M, Jorth P. The unrecognized threat of secondary bacterial infections with COVID-19. mBIO. 2020;11:e01806–20. doi: 10.1128/mBio.01806-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51:S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 39.Bereket W, Hemalatha K, Getenet B, Wondwossen T, Solomon A, Zeynudin A, et al. Update on bacterial nosocomial infections. Eur Rev Med Pharmacol Sci. 2012;16:1039–44. [PubMed] [Google Scholar]
- 40.Cabrera CE, Gómez RF, Zuñiga AE, Corral RH, López B, Chávez M. Epidemiology of nosocomial bacteria resistant to antimicrobials. Colombia Medica. 2011;1:117–125. doi: 10.25100/cm.v42i1.759. [DOI] [Google Scholar]
- 41.Silvestri L, Sarginson RE, Hughes J, Milanese M, Gregori D, Van Saene HKF. Most nosocomial pneumonias are not due to nosocomial bacteria in ventilated patients: evaluation of the accuracy of the 48 h time cut-off using carriage as the gold standard. Anaesth Intens Care. 2002;30:275–82. doi: 10.1177/0310057X0203000303. [DOI] [PubMed] [Google Scholar]
- 42.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wahidi MM, Lamb C, Murgu S, Musani A, Shojaee S, Sachdeva A, et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. J Bronchol Int Pulmonol. 2020;27:52–54. doi: 10.1097/LBR.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao CA, Bailey JI, Walter JM, Coleman JM, Malsin ES, Argento AC, et al. Bronchoscopy on intubated COVID-19 patients is associated with low infectious risk to operators. Annals ATS. 2021 doi: 10.1513/AnnalsATS.202009-1225RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. JoF. 2020;6:91. doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohamed A, Rogers TR, Talento AF. COVID-19 Associated invasive pulmonary aspergillosis: diagnostic and therapeutic challenges. JoF. 2020;6:115. doi: 10.3390/jof6030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson Iii GR, Cornely OA, Pappas PG, Patterson TF, Hoenigl M, Jenks JD, et al. Invasive aspergillosis as an under-recognized superinfection in COVID-19. Open Forum Infect Dis. 2020;7:242. doi: 10.1093/ofid/ofaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan. China Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. The Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong RSM. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du R-H, Liang L-R, Yang C-Q, Wang W, Cao T-Z, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karmen-Tuohy S, Carlucci PM, Zervou FN, Zacharioudakis IM, Rebick G, Klein E, et al. Outcomes among HIV-positive patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. 2020;85:6–10. doi: 10.1097/QAI.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 60.Chopra A, Chieng HC, Austin A, Tiwari A, Mehta S, Nautiyal A, et al. Corticosteroid administration is associated with improved outcome in patients with severe acute respiratory syndrome coronavirus 2-related acute respiratory distress syndrome. Crit Care Explor. 2020;2:e0143. doi: 10.1097/CCE.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreno G, Rodríguez A, Reyes LF, Gomez J, Sole-Violan J, Díaz E, et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med. 2018;44:1470–1482. doi: 10.1007/s00134-018-5332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. The Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yap FHY, Gomersall CD, Fung KSC, Ho P-L, Ho O-M, Lam PKN, et al. Increase in methicillin-resistant staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis. 2004;39:511–516. doi: 10.1086/422641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franks TJ, Chong PY, Chui P, Galvin JR, Lourens RM, Reid AH, et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tse GM-K. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J Clin Pathol. 2004;57:260–5. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bassetti M, Kollef MH, Timsit J-F. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. 2020;46:2071–2074. doi: 10.1007/s00134-020-06219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dupont D, Menotti J, Turc J, Miossec C, Wallet F, Richard J-C, et al. Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019 COVID-19. Med Mycol. 2019 doi: 10.1093/mmy/myaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gangneux J-P, Reizine F, Guegan H, Pinceaux K, Le Balch P, Prat E, et al. Is the COVID-19 pandemic a good time to include aspergillus molecular detection to categorize Aspergillosis in ICU patients? A monocentric experience. JoF. 2020;6:105. doi: 10.3390/jof6030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helleberg M, Steensen M, Arendrup MC. Invasive aspergillosis in patients with severe COVID-19 pneumonia. Clin Microbiol Infect. 2021;27:147–148. doi: 10.1016/j.cmi.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamoth F, Glampedakis E, Boillat-Blanco N, Oddo M, Pagani J-L. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect. 2020;26:1706–1708. doi: 10.1016/j.cmi.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roman-Montes Authors CM, Martinez-Gamboa A, Diaz-Lomelí P, Cervantes-Sanchez A, Rangel-Cordero A, Sifuentes-Osornio J, et al. Accuracy of galactomannan testing on tracheal aspirates in COVID-19-associated pulmonary aspergillosis. Mycoses. 2020 doi: 10.1111/myc.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segrelles-Calvo G, Araújo GRS, Llopis-Pastor E, Carrillo J, Hernández-Hernández M, Rey L, et al. Prevalence of opportunistic invasive aspergillosis in COVID-19 patients with severe pneumonia. Mycoses. 2020 doi: 10.1111/myc.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19 associated pulmonary Aspergillosis. Am J Respir Crit Care Med. 2020;202:132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdalla S, Almaslamani MA, Hashim SM, Ibrahim AS, Omrani AS. Fatal Coronavirus Disease 2019-associated pulmonary Aspergillosis; a report of two cases and review of the literature. IDCases. 2020;22:e00935. doi: 10.1016/j.idcr.2020.e00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Falces-Romero I, Ruiz-Bastián M, Díaz-Pollán B, Maseda E, García-Rodríguez J, SARS-CoV-2 Working Group Isolation of Aspergillus spp. in respiratory samples of patients with COVID-19 in a Spanish Tertiary Care Hospital. Mycoses. 2020 doi: 10.1111/myc.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koehler P, Cornely OA, Böttiger BW, Dusse F, Eichenauer DA, Fuchs F, et al. COVID‐19 associated pulmonary aspergillosis. :7. [DOI] [PMC free article] [PubMed]
- 79.Lahmer T, Rasch S, Spinner C, Geisler F, Schmid RM, Huber W. Invasive pulmonary aspergillosis in severe coronavirus disease 2019 pneumonia. Clin Microbiol Infect. 2020;26:1428–1429. doi: 10.1016/j.cmi.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lescure F-X, Bouadma L, Nguyen D, Parisey M, Wicky P-H, Behillil S, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharifipour E, Shams S, Esmkhani M, Khodadadi J, Fotouhi-Ardakani R, Koohpaei A, et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20:646. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antinori S, Rech R, Galimberti L, Castelli A, Angeli E, Fossali T, et al. Invasive pulmonary aspergillosis complicating SARS-CoV-2 pneumonia: a diagnostic challenge. Travel Med Infect Dis. 2020;38:101752. doi: 10.1016/j.tmaid.2020.101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blaize M, Mayaux J, Nabet C, Lampros A, Marcelin A-G, Thellier M, et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26:1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duployez C, Le Guern R, Tinez C, Lejeune A-L, Robriquet L, Six S, et al. Panton-valentine leukocidin-secreting staphylococcus aureus pneumonia complicating COVID-19. Emerging Infect Dis. 2020;26:1939–1941. doi: 10.3201/eid2608.201413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernandez NB, Caceres DH, Beer KD, Irrazabal C, Delgado G, Farias L, et al. Ventilator-associated pneumonia involving Aspergillus flavus in a patient with coronavirus disease (COVID-19) from Argentina. Med Mycol Case Rep. 2019 doi: 10.1016/j.mmcr.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghelfenstein-Ferreira T, Saade A, Alanio A, Bretagne S, Araujo de Castro R, Hamane S, et al. Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU—a case report. Medical Mycology Case Reports. 2020 Jul;S2211753920300439. [DOI] [PMC free article] [PubMed]
- 87.Meijer EFJ, Dofferhoff ASM, Hoiting O, Buil JB, Meis JF. Azole-resistant COVID-19-associated pulmonary aspergillosis in an immunocompetent host: a case report. JoF. 2020;6:79. doi: 10.3390/jof6020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nasri E. Fatal invasive pulmonary Aspergillosis in COVID-19 patient with acute myeloid leukemia in Iran. Mycopathologia. 2020;185:1077–1084. doi: 10.1007/s11046-020-00493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P. (2020) Invasive pulmonary aspergillosis complicating COVID-19 in the ICU—a case report. Med Mycol Case Rep. May;S2211753920300300. [DOI] [PMC free article] [PubMed]
- 90.Schein F, Munoz-Pons H, Mahinc C, Grange R, Cathébras P, Flori P. Fatal aspergillosis complicating severe SARS-CoV-2 infection: a case report. Journal de Mycologie Médicale. 2020;30:101039. doi: 10.1016/j.mycmed.2020.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharma A, Hofmeyr A, Bansal A, Thakkar D, Lam L, Harrington Z, et al. COVID-19 associated pulmonary aspergillosis (CAPA): an Australian case report. Medical Mycology Case Reports. 2020 Jun;S2211753920300397. [DOI] [PMC free article] [PubMed]