Abstract
In view of their pain‐relieving effect, the non steroidal anti‐inflammatory drugs are more and more used as a pain‐reducing component in modern wound dressings. To analyse the effect on new blood vessel growth, implants from Biatain Ibu™, a polyurethane foam containing ibuprofen, were inserted into the dorsal skinfold chamber of BALB/c mice. Implants from ibuprofen‐free polyurethane foam Biatain™ served as controls (n = 10 per group). Blood vessel growth and the functional vessel density (FVD) as a parameter for microvascularisation of implant’s border zone were assessed by intravital fluorescence microscopy (IVFM). IVFM was performed on days 3, 7 and 12 after implantation. Direct comparison showed no significant differences in FVD (mm/mm2) for the border zone of the ibuprofen‐releasing implants versus controls on day 3 (185·49 ± 4·75 versus 197·17 ± 5·21) and day 7 (229·60 ± 8·53 versus 247·99 ± 5·39). However, the IVFM showed a significant increased FVD for ibuprofen‐releasing implants (301·30 ± 8·44 versus 279·24 ± 5·78) on day 12 (P < 0·05). Also, a significant increase of FVD was detected for the ibuprofen‐releasing implants throughout the implantation time of 12 days. This study shows that local release of small‐dose ibuprofen from a polyurethane dressing does not decrease new blood vessel growth during the implantation time of 12 days. In the end, the microvascularisation of implant’s border zones in both groups was found comparatively undisturbed.
Keywords: Angiogenesis, Cyclooxygenase, Ibuprofen, Intravital microscopy, Skinfold chamber
Introduction
Angiogenesis, the formation of new vasculature, plays a crucial role in the wound healing. A disturbed angiogenesis in damaged tissue may be a major cause of impaired wound healing and increased rate of wound infection 1, 2. The development of a new vascular network consists of several steps that are regulated by various pro‐ and anti‐angiogenic factors 3, 4. One of the best investigated regulators of angiogenesis is the vascular endothelial growth factor (VEGF). The VEGF is indispensable for physiological and reparative angiogenesis. Its expression is induced by numerous mediators, including hypoxia; inflammatory cytokines; other growth factors, such as basic fibroblast growth factor, epidermal growth factor, transforming growth factor and platelet‐derived growth factor‐BB, as well as mediators such as nitric oxide and reactive oxygen species 5, 6, 7. Beside these inducers, prostaglandins (PGs) have been reported to regulate VEGF expression. The production of PG can directly stimulate endothelial cell growth factor‐induced angiogenesis 8, 9, 10, 11. The PG synthesis is mediated by the enzyme cyclooxygenase (COX). COX is found in many tissues and organ systems. To date, three COX isoforms have been identified. The isoenzymes are regulated differently and exhibit distinct functions. COX‐2 enzyme is present on new angiogenic endothelial cells. Recent findings indicate that this enzyme acts as a potent inducer of angiogenesis. Its expression may be induced by trauma, growth factors, tumour promoters and cytokines. COX‐2 was found overexpressed in inflamed and malignant tissue 12, 13, 14.
COX is a major target of non steroidal anti‐inflammatory drugs (NSAIDs). NSAIDs are classical inhibitors of COX enzymes that are widely prescribed for the treatment of inflammation, pain and fever. Because of the contribution of COX to angiogenesis, the pharmacological inhibition of the enzyme is supposed to affect the development of new blood vessels and disturb wound healing 15, 16, 17. Therefore, we sought to analyse the effect of a non selective COX inhibitor on new blood vessel growth during a long‐term implantation period. Ibuprofen‐releasing polyurethane foam (Biatain Ibu™) was studied by means of intravital microscopy after implantation into the transparent dorsal skinfold chamber of BALB/c mice.
Materials and methods
Animal model
The investigations were performed in an established microcirculatory model using the transparent dorsal skinfold chamber. Direct visualisation of the angiogenesis and microcirculatory measurements within the striated skin muscle were performed by means of intravital fluorescence microscopy (IVFM). Total number of 20 female BALB/c mice was used. The animals were 12–14 weeks old and had a body weight of 18–22 g (Charles River, Sulzfeld, Germany). They were kept at 21°C in a normal 12/12 hour light/dark cycle and fed with a laboratory diet (Spezial diaeten, Soest, Germany) and water ad libitum. The experiments were conducted in accordance with the German law for the protection and the welfare of laboratory animals.
Implantation technique of the dorsal skinfold chamber
The implantation of the dorsal skinfold chambers was performed as described earlier 18, 19. Briefly, the animals were anaesthetised by subcutaneous injection of ketamine (100 mg/kg body weight, Ketavet; Parke‐Davis, Berlin, Germany) and xylazine 2% (10 mg/kg body weight, Rompun; Bayer, Leverkusen, Germany) injection. The mouse’s back was depilated and the extended double‐layered skinfold fixed to the titanium frame. After that, a circular area of 15 mm of the one skin layer was completely removed. The striated skin muscle of the opposite skin layer was then covered by second titanium frame incorporating a glass coverslip. All surgical procedures were performed under sterile conditions.
Implants
The wound dressings Biatain Ibu and Biatain™ (Coloplast, Hamburg, Germany) are based on polyurethane foam. The three‐dimensional structure of the polymer is responsible for the high absorption capability of wound exudate. Biatain dressings are used in the clinic and are indicated for treatment of leg ulcers, pressure ulcers and superficial and partial‐thickness burns 20, 21. Biatain Ibu is a sustained‐release ibuprofen foam dressing. The dressing consists of a soft, hydrophilic, non adhesive polyurethane foam containing ibuprofen (0·5 mg/cm2) as an integral part of the matrix. The foam is bonded to a semi‐permeable polyurethane film. Two discoid implant types (diameter 2 mm and thickness 300 μm) were prepared for the study. Ten implants per group were studied. The implants from the sterile wound dressings were single packed, randomised, labelled and stored dark prior to implantation. Forty‐eight hours following chamber preparation, the animals were again anaesthetised. The cover glass of the chamber was opened and a single implant positioned onto the striated skin muscle. The chamber preparation was closed with a sterile cover glass.
Experimental design
Following the implantation, animals were allowed a recovery period of 72 hours to exclude surgical effects on the microvasculature. The awake mice were then immobilised in a Plexiglas tube and placed on a computer‐controlled stage to perform the investigations with standard IVFM. Intravital microscopical investigations were performed on days 3, 7 and 12 after implantation. A 40‐fold water immersion objective (magnification: 335‐fold) was used for observations. For contrast enhancement, 0·2 ml of 5% fluorescein isothiocyanate‐labelled dextran (FITC‐dextran, molecular weight 500 kDa; Sigma Chemicals Co., St Louis, MO) was injected through a tail vein. Epi‐illumination was attained using a 12 V, 100 W halogen lamp in conjunction with a Zeiss filter set (BP 450490, FT 510 and LP 520; Carl Zeiss Microimaging GmbH, Göttingen, Germany) for measurements after injection of FITC‐dextran. Observations were performed in two different areas of the implant’s border zone. In each area, two different microvascular regions of interest were selected and recorded and their x–y coordinates stored on the computer to relocate the identical areas within the chamber. The images were recorded digitally (Pinnacle MovieBox; Avid Technology GmbH, c/o Pinnacle Systems, Munich, Germany). Analysis of the digital images was performed off‐line using the commercially available CapImage® computer program (Zeintl, Heidelberg, Germany) (22). The functional vessel density (FVD) (mm/mm2) as a parameter of microvascularisation was assessed as the length of perfused vessels per observation area. At the end of the protocol, the animals were euthanised by an overdose of intravenously given pentobarbital.
Data acquisition and statistical analysis
Data were calculated in a single‐blinded fashion. SigmaStat™ (Jandel Scientific, San Raphael, CA) was used for statistical analysis of the data. Comparison between groups was performed using a paired parametric t‐test (Student’s t‐test). P < 0·05 was regarded as statistically significant.
Results
During the implantation time, no signs of infection within the chamber preparations were detected. The IVFM showed an intact microcirculation for the surrounding skin muscle tissue throughout the implantation period. FVD of the surrounding skin muscle tissue did not change significantly over the observation period in both groups. Here, the FVD (mean) ranged from 116·52 to 129·65 mm/mm2 on day 3 and from 130·75 to 136·38 mm/mm2 on day 12. The FVD (mean ± SE) measured at the start of observation shows 197·17 ± 5·21 mm/mm2 for the controls and 185·49 ± 4·75 mm/mm2 for the ibuprofen‐treated group with no statistically significant difference. An increased concentration of perfused newly developed microvessels in the border zone of the implants was already evident by fluorescence microscopy on day 7 after implantation (Figure 1A, B). Although the FVD of the border zone of implants in the control group at day 7 (247·99 ± 5·39 mm/mm2) was found increased compared with that of the ibuprofen‐releasing implants (229·60 ± 8·53 mm/mm2), no statistically significant differences could be found as well as no irregularity concerning the morphological alteration of the microvessel network detected. No considerable disturbances as well as any qualitative differences between the groups in the development of new blood vessels around the border zones of implants were observed at this time point. The transformation from originally parallelly arranged capillaries to dilated blood vessels of stronger elongation and torsion could be observed within the border zone of both groups (Figure 2A, B). At day 12, the IVFM still showed a constant development of a well‐perfused microvessel network surrounding the implant’s border zones in both groups (Figure 3A, B). The FVD of ibuprofen group (301·30 ± 8·44 mm/mm2) at this observation time point was measured significantly (P < 0·05) higher compared with that of controls (279·24 ± 5·78 mm/mm2) (Figure 4).
Figure 1.
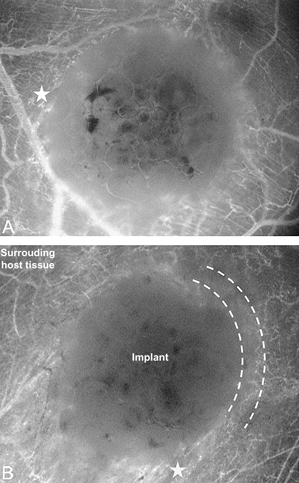
An increased vascular injection caused by perfused newly developed microvessels (asterisk) was evident by fluorescence microscopy within the border zone (marked by curves) of ibuprofen‐releasing implants (A) as well as of controls (B) already on day 7 after implantation. Magnification: 35‐fold.
Figure 2.

The intravital fluorescence microscopy images show the morphological alterations of the capillary network of ibuprofen‐releasing (A) and ibuprofen‐free (B) implants. The transformation from originally parallelly arranged capillaries to dilated microvessels (arrow) of stronger elongation and torsion could be observed in both groups at the same time (a postcapillary venule is marked by an arrowhead). The angiogenic modification of the microvasculature was detected within the border zones of implants at day 7 after implantation. Magnification: 355‐fold.
Figure 3.
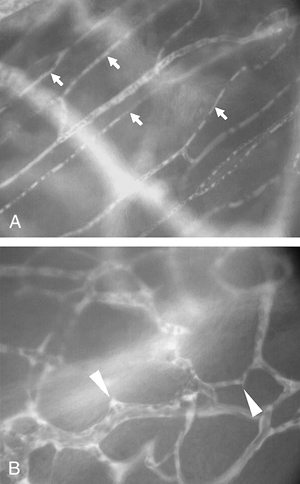
At the end of experiment, the ibuprofen‐releasing implants were surrounded by well‐organised and perfused new microvasculature (B) still detectable vessel sprouts (arrowheads). The architecture of the capillary network (arrows) within the surrounding host tissue areas remained morphologically unchanged (A). Magnification: 355‐fold.
Figure 4.
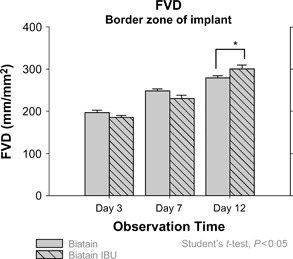
Vertical bar charts represent statistical values (mean ± SE) of the functional vessel density (FVD) measured within border zones at days 3, 7 and 12 after implantation. Comparison between groups (Biatain™ versus Biatain Ibu™) was performed using Student’s t‐test (*P < 0·05).
Discussion
Angiogenesis is known to be a critical factor in tissue regeneration. The inhibition of COX by the non selective inhibitor is able to reduce the growth of new blood vessel. It is assumed that the anti‐angiogenic effect of the NSAIDs is dependent on inhibition of COX activity and PG synthesis. A current study showed that both selective and non selective NSAIDs inhibit angiogenesis through direct effects on endothelial cells. It was shown that both COX‐1 and COX‐2 are important for the regulation of angiogenesis (15). Another study examined the effects of ibuprofen on tumour growth inhibition in mouse and human colorectal cancer tumour models. Angiogenesis was measured by in vitro endothelial cell tube formation and immunohistochemistry. It was shown that ibuprofen significantly inhibited cell proliferation in mouse and human colorectal cancer cell lines by modulating tumour angiogenesis. In vitro angiogenesis assays also indicated that ibuprofen decreased both cell proliferation and tube formation (23).
Ibuprofen is a relatively inexpensive, non selective COX inhibitor that is still widely used in clinical practice as pain‐relieving drug. The wound dressing Biatain Ibu is supposed to deliver low‐dose ibuprofen by a constant rate directly into the wound. By delivering ibuprofen into the wound, this dressing was shown to reduce the wound pain caused by tissue damage, providing a continuous pain‐relieving effect (24). However, the effect of ibuprofen‐releasing polyurethane foam on angiogenesis and microvascularisation in the wound tissue has not been studied as yet. In the present study, we sought to determine whether these two important aspects of wound healing would be affected by the ibuprofen treatment.
The direct comparison of both groups showed a reduced FVD in the border zone of the implants on days 3 and 7 for the group treated with ibuprofen‐releasing implants. However, no statistically significant difference could be detected. Also, no significant decrease of FVD was observed for the ibuprofen‐releasing implants throughout the implantation time of 12 days. The results of this study clearly show that the ibuprofen release by the dressing Biatain Ibu does not disturb the new blood vessel growth. The ibuprofen‐treated group did not show any relevant anti‐angiogenic effects by the drug in the border zone of implants. The microvessel density within the striated skin muscle of ibuprofen‐treated animals was not affected by the treatment and remained constantly increasing till to the end of experiment.
Conclusions
This study using our animal model shows by means of IVFM that ibuprofen‐releasing wound dressings Biatain Ibu do not disturb the angiogenesis as well as the microcirculation of a newly developed microvasculature. Data presented here do not support the assumption of suppressing the new blood vessel formation by releasing ibuprofen from the dressing to the skin muscle throughout the long‐term implantation period.
Further investigations are needed to confirm these findings compared with previous results.
Acknowledgements
We acknowledge the Coloplast, Hamburg, for providing the wound dressings for our study. The study was financially supported in part by Coloplast.
References
- 1. Hunt TK. Disorders of wound healing. World J Surg 1980;4:271–7. [DOI] [PubMed] [Google Scholar]
- 2. Hunt TK. The physiology of wound healing. Ann Emerg Med 1988;17:1265–73. [DOI] [PubMed] [Google Scholar]
- 3. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;6:389–95. [DOI] [PubMed] [Google Scholar]
- 4. Jain RK. Molecular regulation of vessel maturation. Nat Med 2003;9:685–93. [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto T, Claesson‐Welsh L. VEGF receptor signal transduction. Sci STKE 2001;2001:RE21. [DOI] [PubMed] [Google Scholar]
- 6. Dulak J, Jozkowicz A. Regulation of vascular endothelial growth factor synthesis by nitric oxide: facts and controversies. Antioxid Redox Signal 2003;5:123–32. [DOI] [PubMed] [Google Scholar]
- 7. Xie K, Wei D, Shi Q, Huang S. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev 2004;15:297–324. [DOI] [PubMed] [Google Scholar]
- 8. Cheng T, Cao W, Wen R, Steiberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci 1998;39:581–91. [PubMed] [Google Scholar]
- 9. Hoper MM, Voelkel NF, Bates TO, Allard JD, Horan M, Shepherd D, Tuder RM. Prostaglandins induce vascular endothelial growth factor in a human monocytic cell line and rat lungs via cAMP. Am J Respir Cell Mol Biol 1997;17:748–56. [DOI] [PubMed] [Google Scholar]
- 10. Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM. Cyclooxygenase 2‐ and prostaglandin E(2) receptor EP(2)‐dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res 2002;62:506–11. [PubMed] [Google Scholar]
- 11. Wang D, Dubois RN. Prostaglandins and cancer. Gut 2006;55:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miura S, Tatsuguchi A, Wada K, Takeyama H, Shinji Y, Hiratsuka T, Futagami S, Miyake K, Gudis K, Mizokami Y, Matsuoka T, Sakamoto C. Cyclooxygenase‐2‐regulated vascular endothelial growth factor release in gastric fibroblasts. Am J Physiol Gastrointest Liver Physiol 2004;287:G444–51. [DOI] [PubMed] [Google Scholar]
- 13. Tatsuguchi A, Matsui K, Shinji Y, Gudis K, Tsukui T, Kishida T, Fukuda Y, Sugisaki Y, Tokunaga A, Tajiri T, Sakamoto C. Cyclooxygenase‐2 expression correlates with angiogenesis and apoptosis in gastric cancer tissue. Hum Pathol 2004;35:488–95. [DOI] [PubMed] [Google Scholar]
- 14. Huang SP, Wu MS, Shun CT, Wang HP, Hsieh CY, Kuo ML, Lin JT. Cyclooxygenase‐2 increases hypoxia‐inducible factor‐1 and vascular endothelial growth factor to promote angiogenesis in gastric carcinoma. J Biomed Sci 2005;12:229–41. [DOI] [PubMed] [Google Scholar]
- 15. Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, Tarnawski AS. Inhibition of angiogenesis by nonsteroidal anti‐inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med 1999;5:1418–23. [DOI] [PubMed] [Google Scholar]
- 16. Bloch W, Huggel K, Sasaki T, Grose R, Bugnon P, Addicks K, Timpl R, Werner S. The angiogenesis inhibitor endostatin impairs blood vessel maturation during wound healing. FASEB J 2000;14:2373–6. [DOI] [PubMed] [Google Scholar]
- 17. Wu YL, Fu SL, Zhang YP, Qiao MM, Chen Y. Cyclooxygenase‐2 inhibitors suppress angiogenesis and growth of gastric cancer xenografts. Biomed Pharmacother 2005;59 Suppl 2:S289–92. [DOI] [PubMed] [Google Scholar]
- 18. Ring A, Langer S, Homann HH, Kuhnen C, Schmitz I, Steinau HU, Druecke D. Analysis of neovascularization of PEGT/PBT‐copolymer dermis substitutes in balb/c‐mice. Burns 2006;32:35–41. [DOI] [PubMed] [Google Scholar]
- 19. Ring A, Steinstraesser L, Muhr G, Steinau HU, Hauser J, Langer S. Improved neovascularization of PEGT/PBT copolymer matrices in response to surface modification by biomimetic coating. Eur Surg Res 2007;39:75–81. [DOI] [PubMed] [Google Scholar]
- 20. Pritchard V. Treatment of a patient with a deep leg ulcer using Biatain Adhesive. Br J Nurs 1999;8:1164–7. [DOI] [PubMed] [Google Scholar]
- 21. Lohmann M, Thomsen JK, Edmonds ME, Harding KG, Apelqvist J, Gottrup F. Safety and performance of a new non‐adhesive foam dressing for the treatment of diabetic foot ulcers. J Wound Care 2004;13:118–20. [DOI] [PubMed] [Google Scholar]
- 22. Klyscz T, Junger M, Jung F, Zeintl H. [Cap image – a new kind of computer‐assisted video image analysis system for dynamic capillary microscopy]. Biomed Tech (Berl) 1997;42:168–75. [DOI] [PubMed] [Google Scholar]
- 23. Yao M, Zhou W, Sangha S, Albert A, Chang AJ, Liu TC, Wolfe MM. Effects of nonselective cyclooxygenase inhibition with low‐dose ibuprofen on tumor growth, angiogenesis, metastasis, and survival in a mouse model of colorectal cancer. Clin Cancer Res 2005;11:1618–28. [DOI] [PubMed] [Google Scholar]
- 24. Jorgensen B, Friis GJ, Gottrup F. Pain and quality of life for patients with venous leg ulcers: proof of concept of the efficacy of Biatain‐Ibu, a new pain reducing wound dressing. Wound Repair Regen 2006;14:233–9. [DOI] [PubMed] [Google Scholar]


