Abstract
Excessive levels of matrix metalloproteinases (MMPs) are present in chronic wounds preventing wound closure. Reducing detrimental components may be key in healing chronic wounds. Elta Protease‐containing wound dressings have been observed clinically to resolve inflammation and appear to aid healing in acute and chronic recalcitrant wounds. To investigate possible mechanisms of action, in vitro tests, zymography, collagenase assays and enzyme‐linked immunosorbent assays (ELISAs), were performed to evaluate the effect of the dressing proteases on detrimental and beneficial wound healing components such as MMPs, Tissue Inhibitor of Matrix Metalloproteinases (TIMPs), cytokines and growth factors. Standards of pro‐ and active MMP‐2, MMP‐9 and chronic wound fluid (CWF) were prepared. Degradation of target proteins was enhanced by increased Elta Protease concentration, temperature and incubation time. Incubation with serial dilutions of the Elta Proteases resulted in nearly complete degradation of all MMP standards. After a 30‐minute incubation, twofold diluted Elta Proteases degraded >90% of the gelatinases in CWF. ELISAs showed that Elta Proteases effectively degraded MMP‐9 and tumour necrosis factor (TNF‐α). In contrast, Platelet Derived Growth Factor (PDGF) and interleukin 1β were resistant to degradation by Elta Proteases. These results suggest that Elta Protease dressings appear to deactivate detrimental components in CWF, which may reduce wound bed contact with harmful proteins.
Keywords: Inflammation, MMP, Protease, TNF‐α, Wound
Introduction
Wound healing is a complicated and fascinating process. Unlike acute wounds that heal rapidly and proceed through the inflammatory, proliferation and remodelling phases of wound healing, chronic wounds often become senescent in the inflammatory or proliferation stages and cannot progress to closure 1, 2. In addition to implementing treatment regimens addressing the aetiology and symptoms, clinicians prepare the wound for healing by removing dead tissue, reducing the bacterial bioburden, decreasing oedema, managing exudate and enhancing angiogenesis (3). Although the wound bed may appear ready to heal, the microenvironment may be out of balance, thus impeding healing and frustrating both the patient and the clinician.
The microenvironment of the wound is a web of intertwining cells, proteins, enzymes, fluids and pathways that perform specific functions that normally are tightly regulated 4, 5. In wounds that chronically fail to heal, however, the microenvironment has become deregulated with key components being overexpressed, underexpressed, inactive or ineffective 6, 7, 8, 9, 10. Specific protein comparisons between acute and chronic wounds showed that chronic wounds generally have excessive levels of matrix metalloproteinases (MMPs), high levels of tumour necrosis factor α (TNF‐α), interleukin (IL)‐1 and IL‐6 and minimal levels of Tissue Inhibitor of Matrix Metalloproteinases (TIMPs), transforming growth factor β and Epidermal Growth Factor (EGF) 1, 11. To complicate matters, activated inflammatory cells stimulate MMP production and suppress TIMPs by secreting TNF‐α and IL‐1β that impairs the healing process via increased degradation of Extracellular Matrix (ECM) components, growth factors and receptors contributing to multiple negative feedback loops preventing wound closure.
Promoting, returning to and maintaining a normal wound microenvironment can be difficult. Past use of isolated molecules or compounds to modify the healing process has been met with limited success 12, 13, 14, 15. These limitations may be the result of one molecule trying to modify the entire wound environment in a narrowly selected function or because of the duplicity of multiple alternative pathways. Additionally, the hostile chronic wound environment probably degrades exogenously applied growth factors as easily as the intrinsic ones, resulting in little clinical or molecular impact 16, 17.
An alternative way to return to a more normal wound microenvironment is act directly on proteins such as MMPs and pro‐inflammatory cytokines, which help promote the hostile environment when in excess. Wound dressings containing a mixture of natural proteases (Elta Protease Technology; Swiss‐American Products, Inc., Dallas, TX) have been observed clinically to resolve inflammation and appear to aid healing in acute and chronic recalcitrant wounds. Natural proteases are enzymes present in human, animal and plant tissues. These enzymes act as catalysts to cleave specific internal peptide bonds in proteins. The proteases present in the Protease Technology product line are generally recognized as safe (GRAS) by the Food and Drug Administration (18).
The proprietary Elta Protease formulations were developed specifically to enhance wound healing. The protease blend should deactivate excessive amounts of MMPs and cytokines, thus disrupting the autocrine and paracrine regulatory loops and moving the wound environment from a dysregulated phase of non healing into a more normalized healing one.
The wound dressing protease formulation was tested to investigate the mechanisms and effects of action against detrimental and beneficial wound healing components of purified MMPs, TIMPs, cytokines, growth factors and chronic wound fluids (CWF). The hypothesis was Elta Proteases degrade detrimental wound healing components, but do not degrade beneficial wound healing components.
Methods
Test and control materials
Elta wound dressing protease formulation (Elta Proteases) was provided as a solution (Swiss‐American Products, Inc., Carrollton, TX). MMP standard (Calbiochem, San Diego, CA) was prepared from concentrated active‐ and pro‐MMP‐2 and MMP‐9 and sterile water. Serial dilutions (1×, 2×, 4×, 8× and 16×) of the sterile protease mix were prepared with sterile water. A uniform stock of CWF was prepared for the experiments by mixing samples obtained from multiple patients as previously described (1).
Zymography
Sample preparation
MMP standard was incubated (1:1) with each of the Elta Proteases 8× and 16× dilutions for 30 minutes. A 2× dilution of the MMP standard and 2× dilutions of the Elta Proteases dilutions were also prepared for comparison. Overnight and acute incubations of CWF with 1×, 2×, 4×, 8× and 16× Elta Proteases dilutions were prepared, along with 2× dilutions of the 8× Elta Proteases dilution and the CWF standard. Sample buffer (20 μl) was added at the end of the incubation of each sample. Ten minutes later, the samples were added to the zymogram gel.
Zymogram
Samples were added to a 10% zymogram gel (Invitrogen, Carlsbad, CA). The gel was run at a constant 125 V at 4°C. After 2 hours, the gel was incubated in renaturing buffer for 30 minutes. The buffer was then replaced with developing buffer. After 30 minutes at room temperature, the gel was placed on a rocker platform for overnight incubation at 37°C. The developing buffer was replaced with Coomassie stain (2 ml rapid Coomassie stain in 40 ml 7.5% methanol–5·0% acetic acid) and the gel was incubated at room temperature on an orbital shaker (70 rpm) for 60 minutes. The stain was replaced with destain (7·5% methanol–5·0% acetic acid) and incubated for 10 minutes on an orbital shaker. Destain was replaced with deionized water and the gel was photographed with a digital camera.
Azocoll assay
Test reactions
Four reaction mixtures were used to determine the effect of Elta Proteases on CWF collagenase activity. CWF was diluted 1:1 with phosphate‐buffered saline (PBS)/azide. Each of the four conditions was incubated at 37°C for 3 hours prior to starting the assay. Reaction 1, CWF diluted 1:1 with Elta Proteases; 2, CWF diluted 1:1 with PBS/azide; 3, Elta Proteases diluted 1:1 with PBS/azide and 4. PBS/azide.
Substrate preparation
The substrate was prepared by suspending insoluble collagen fibres labelled with a diazostain (azocollagen) (Sigma, St Louis, MO) in PBS/azide at a concentration of 5 mg/ml.
Azocoll assay
All samples were assayed in duplicate; mean values and standard deviation were calculated for each sample. Each tube had a total 600 μl substrate and each reagent was added in the following order: substrate, +/− protease inhibitor (Sigma), test Reaction 1, 2, 3 or 4 and finally PBS/azide. Sample tubes were incubated at 37°C with rotation and read spectrophotometrically (570 nm) at various times over 28 hours in a 96‐well plate. Absorbance readings are directly proportional to collagenase activity.
Enzyme‐linked immunosorbent assays
Sample preparation
Prior to running the enzyme‐linked immunosorbent assays (ELISA), the CWF standard was tested to determine the baseline levels of MMP‐9, TIMP‐1, TNF‐α, IL‐1β, and PDGF. CWF standards were spiked with purified concentrations of 640 pg/ml TNF‐α and 4000 pg/ml PDGF stock solutions to achieve an adequate baseline concentration. Aliquots were prepared by combining the target protein in a 1:1 ratio with Elta Proteases or PBS control. Aliquots were removed for a time‐zero reading. All reactions were incubated at 37°C and room temperature and additional aliquots removed at 1, 4, 8 and 24 hours. With the exception of the TIMP‐1 and IL‐1β samples, the aliquots were mixed 10:1 with a general‐purpose protease inhibitor (Sigma) and frozen at −80°C. TIMP‐1 samples were diluted 1:25 in the kit assay buffer prepared with and without the general purpose protease inhibitor (1:100) to determine the effect of Elta Proteases on TIMP‐1 in CWF and the TIMP‐1 ELISA standards. IL‐1β samples were not mixed with a protease inhibitor.
Enzyme‐linked immunosorbent assays
All ELISAs were performed per manufacturers specifications. All samples were run in duplicate wells and all ELISAs repeated at least twice on separate days.
Active MMP‐9 Active MMP‐9 concentrations were quantified using the MMP‐9 Biotrak Activity Assay System (Amersham, Piscataway, NJ) per manufacturer instruction. CWF had adequate MMP‐9 levels and was diluted 150× in ELISA standard diluent before running the ELISA.
TIMP‐1 TIMP‐1 concentration was quantified using the TIMP‐1, Human Biotrak™ ELISA (Amersham) per manufacturer instruction except that TIMP‐1 standards were prepared with and without a general purpose protease inhibitor (Sigma) diluted 1:100 in the kit assay buffer.
Tumour necrosis factor‐α TNF‐α concentration was quantified using the Tumour Necrosis Factor Alpha [(h) TNF‐α] Human Biotrak ELISA System (Amersham). CWF with TNF‐α added was run undiluted.
Interleukin‐1β IL‐1β concentrations were quantified using the Quantikine® human IL‐1β ELISA (DLB50; R&D Systems, Minneapolis, MN, USA) as per manufacturer instruction. No protease inhibitor was added before running the ELISA. The CWF had adequate levels of IL‐1β, so no exogenous protein was added. The reactions in CWF were diluted 100× in water.
PDGF‐AB PDGF‐AB concentrations were quantified using the Quantikine® human PDGF‐AB ELISA (DHD00B; R&D Systems) as per manufacturer instruction. CWF was diluted 2×.
Statistical methods
ELISA data were analysed using two separate repeated measures analysis of variance model with time, therapy, and time‐by‐therapy as fixed effects in the model with a compound symmetry correlation structure. The first model tested the therapy‐by‐time interaction effect in the model at 37°C, while the second model tested the therapy‐by‐time interaction at room temperature.
Results
Zymography
The ability of Elta Proteases to degrade purified active‐ and pro‐forms of MMP‐2 and MMP‐9 standards and gelatinases in pooled CWF was assayed using zymogram gels. Initial experiments showed that Elta Proteases degraded the gelatine contained within the zymogram gel. Serial dilutions of Elta Proteases (2×, 4×, 8× and 16×) were tested to detect the optimal dilutions to run on the zymogram gels (data not shown). The 8× and 16× dilutions had the least amount of background degradation, while allowing for reactions within the CWF to be observed.
Purified active‐ and pro‐forms of MMP‐2 and MMP‐9 standards were incubated with the 8× and 16× dilutions of Elta Proteases. All the MMP standards were completely degraded by both dilutions except the active MMP‐2 that was incubated for 30 minutes at room temperature (Figure 1).
Figure 1.
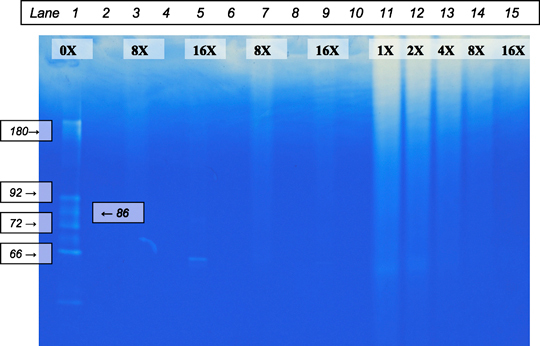
Zymography of purified matrix metalloproteinases (MMP)‐2 and MMP‐9 standards incubated with serial dilutions of Elta Proteases. Lane 1: MMP standard. Lanes 3 and 7: 8× Elta Proteases + MMP. Lanes 5 and 9: 16× Elta Proteases + MMP. Lanes 11–15: 1×, 2×, 4×, 8× and 16× dilutions of the Elta Proteases. Lanes 3 and 5 were incubated longer in sample buffer, which seemed to increase band intensity.
Gelatinases in pooled CWF were then incubated with the 8× dilution of Elta Proteases. Degradation of the CWF gelatinases by the 8× dilution was noticeable after only 30 minutes of incubation at 37°C. Degradation was even more pronounced after 24 hours of incubation, especially in samples containing less diluted Elta Proteases (Figure 2).
Figure 2.
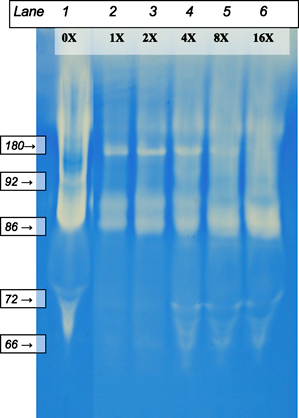
Zymography of chronic wound fluid (CWF) incubated with serial dilutions of Elta Proteases. Lane 1: 2× dilution of CWF standard. Lanes 2–6: CWF with 1×, 2×, 4×, 8× and 16× Elta Proteases dilutions, incubated overnight. Note the amount of degradation seen appears to be concentration dependent.
The zymograms clearly showed the ability of the Elta Proteases to degrade MMP standards and CWF gelatinases, even when diluted. Increased incubation temperature and time both enhanced the ability of Elta Proteases to degrade the MMPs and CWF gelatinases. An increase in Elta Protease concentration also improved the rate of degradation compared with diluted samples.
Azocoll assay
As the Elta Proteases easily degraded gelatinases, collagen degradation was then assessed using azocoll assays. An insoluble collagen fibre substrate labelled with azocollagen, was suspended PBS/azide. The protease mixture was able to degrade the collagen substrate with or without pre‐incubation and in the presence or absence of CWF. A protease inhibitor lacking EDTA was used to inhibit the Elta protease collagen degradation but not CWF collagenases, so the collagenase affect could be observed. The 3‐hour pre‐incubation period of the proteases and CWF increased net collagen degradation when compared with 6 and 28‐hour samples containing CWF but were not pre‐incubated with the Elta proteases. Interestingly, Elta samples that were not pre‐incubated had a slight effect on the net CWF collagen degradation even after 28 hours.
The azocoll assay suggests Elta proteases have collagenase activity and may slightly activate other CWF collagenases after a 6‐hour pre‐incubation. The increased degradation of collagen by the CWF collagenases may have been the result of an activation of collagenase pro‐forms. However, without pre‐incubation, collagen degradation decreased.
Enzyme‐linked immunosorbent assays
Matrix metalloproteinases ‐9
Active MMP‐9 concentrations in CWF were assessed using ELISA. Complete degradation of active MMP‐9 occurred within the first hour of incubation at 37°C (P < 0·001) with the Elta Proteases and within 8 hours at room temperature (P < 0·001) (Figure 3). These results confirmed the gelatine zymogram results previously discussed (1, 2). Controls of CWF alone had a slight degradation of active MMP‐9 over time regardless of incubation temperature.
Figure 3.
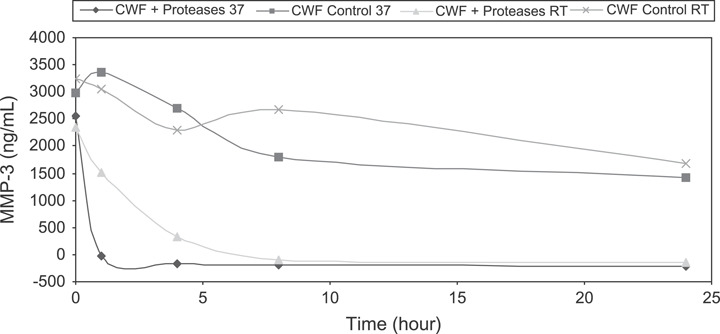
Enzyme‐linked immunosorbent assay results of matrix metalloproteinases (MMP)‐9 in chronic wound fluid showing MMP degradation by Elta Proteases over 24 hours.
TIMP‐1
TIMP‐1 concentrations in CWF were assessed using ELISA. Prior to initiating the ELISA, TIMP‐1 standard assay buffer with and without a general purpose protease inhibitor were tested and compared. Spectrophotometric absorbance readings were higher for the standards containing inhibitor than standards that were not exposed to the inhibitor suggesting TIMP‐1 was being degraded during the 2‐hour room temperature incubation period. Also observed was TIMP‐1 standards degraded slightly in the assay buffer over time. To assess the effect of the Elta Proteases on TIMP‐1 concentrations in CWF, samples were incubated and assayed by ELISA. At 24 hours, the decrease of TIMP‐1 levels was similar to the control, indicating TIMP‐1 was resistant to degradation of Elta Proteases (Figure 4).
Figure 4.
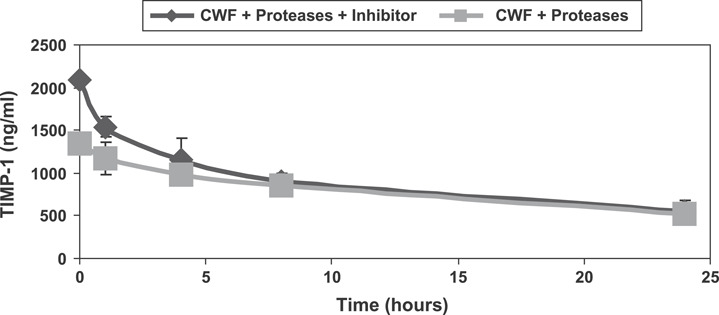
TIMP‐1 enzyme‐linked immunosorbent assay results showed a reduction similar to control at 24 hours.
Tumour necrosis factor‐α
TNF‐α concentrations in CWF were run undiluted and assayed using ELISA. Proteolysis occurred within the ELISA wells because the protease inhibitor was not added to the sample until after the incubation period. Complete TNF‐α degradation occurred within 8–10 hours in the presence of Elta Proteases at 37°C (P = 0·024) and were reduced greater than 90% at room temperature (P < 0·001) (Figure 5). Comparatively, TNF‐α levels in the controls were reduced 35% at 37°C and 2% at room temperature.
Figure 5.
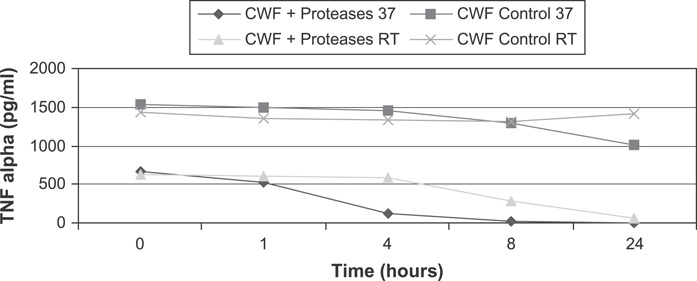
Enzyme‐linked immunosorbent assay results of tumour necrosis factor α (TNF‐α) in chronic wound fluid showing TNF‐α degradation by Elta Proteases over 24 hours.
Interleukin‐1β
IL‐1β concentrations in CWF were assessed using ELISA. Proteolysis occurred within the ELISA wells because the protease inhibitor was not added to the samples. At times up to 24 hours, the levels of IL‐1β exposed to Elta Proteases were similar compared with controls at both room temperature and 37°C. At both temperatures, the IL‐1β levels exposed to the Elta Proteases showed less degradation than the controls (Figure 6). A statistically significant therapy difference over time effect resulted at 37°C (P < 0·001) and at room temperature (P < 0·001). The interaction is visible at early time points, then the data sets split apart before eventually arriving at similar levels at 24 hours. These results suggest the Elta Proteases do not degrade the IL‐1β protein in CWF, but may also confer protection to the protein.
Figure 6.
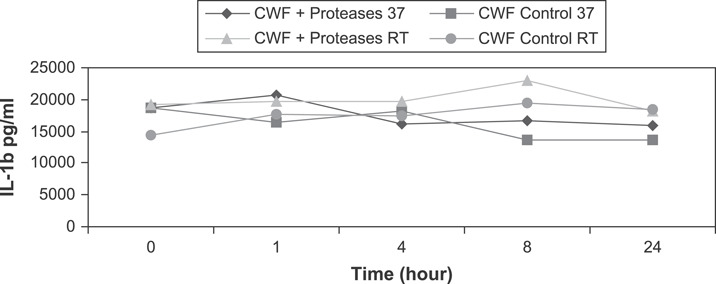
Enzyme‐linked immunosorbent assay results of IL‐1β in chronic wound fluid demonstrate interleukin‐1β resistance to degradation by Elta Proteases over 24 hours.
PDGF
PDGF‐AB concentrations in CWF were assessed using ELISA. CWF was spiked with exogenous PDGF‐AB to determine the effects of the Elta Proteases on the protein. At times up to 24 hours, the levels of PDGF exposed to Elta Proteases were similar compared with controls at 37°C. A statistically significant therapy difference over time effect resulted at room temperature (P < 0·001), but not at 37°C (P < 0·0931). Although the PDGF concentrations were above natural physiological levels, significant proteolysis of PDGF was not observed, suggesting resistance to degradation. At both temperatures, the PDGF levels exposed to the Elta Proteases showed less degradation than the controls (Figure 7). These results suggest the Elta Proteases do not degrade the PDGF protein in CWF, but may also confer protection to the protein.
Figure 7.
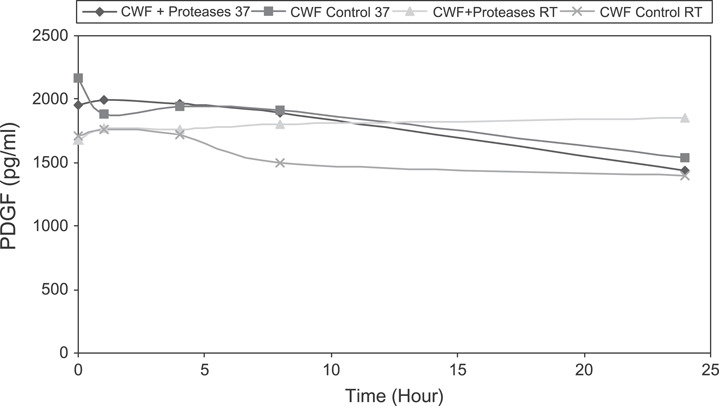
Enzyme‐linked immunosorbent assay results of PDGF in chronic wound fluid demonstrate PDGF‐AB resistance to degradation by Elta Proteases over 24 hours.
The ELISAs showed interesting and surprising results. The Elta Proteases were able to degrade active MMP‐9 and TNF‐α at room temperature, but more markedly at body temperature. Rapid and complete MMP degradation occurred within 1 hour and within 8–10 hours for TNF‐α in CWF from patient samples. Unlike MMP and TNF‐α, TIMP, IL‐1β and PDGF were not degraded by the Elta Proteases during the 24‐hour incubation period, even at 37°C. Even more interesting was the observation the controls had more proteolysis of target proteins IL‐1β and PDGF by CWF than in samples incubated with the CWF and the Elta Proteases.
Discussion
The geriatric population will continue to grow as medical advancements are made, ever increasing the number of chronic wounds present (19). In addition to contributing to failing health, chronic wounds are costly burdens (19). Morbidity and cost continue to increase as long as the wound remains open. Although rapid closure is desirable, conservative approaches are generally preferred for the elderly or debilitated patient because of health and cost issues.
There are many dressing choices available, but finding an inexpensive treatment to specifically improve or heal chronic wounds can be difficult. Using a conservative approach to modify the wound milieu can improve the clinical outcome. Elta Proteases act as immunomodulators when applied topically by altering the microenvironment. First, the Elta Proteases can degrade and thus deactivate certain proteins and enzymes, that in excess, impair wound healing. Second, removal of the excessive proteins and enzymes should modify cell–cell signalling and cross talk, thus further modulating the wound environment. Third, targeting multiple components from different regulatory pathways should ensure adequate impact to modulate the wound environment to promote healing.
In this study, Elta Proteases were observed to have extensive proteolytic activity against MMP‐2, MMP‐9 and TNF‐α both against purified forms and in pooled CWF. Elimination of excessive levels of MMP collagenases and gelatinases should improve ECM deposition, While elimination of the inflammatory response driver, TNF‐α, should substantially decrease the inflammation cycle. The inflammatory reaction would not be completely eliminated because intact alternate inflammatory pathways remain. As shown, Elta Proteases did not degrade pro‐inflammatory cytokine IL‐1β, which would allow for inflammation should the body need to mount a response.
Although Elta Proteases rapidly degraded multiple detrimental wound components, other tests were performed to determine the fate of beneficial wound components. As the results showed, Elta Proteases did not degrade TIMP, IL‐1β and PDGF. Of special interest, both PDGF and IL‐1β proteins appeared to be protected from CWF degradation when incubated with Elta Proteases. These results suggest that certain wound components beneficial to healing remain active in the presence of Elta Proteases.
Clinically, when Elta Proteases are applied, a reduction and sometimes, complete resolution of inflammation is observed (20). This clinical observation correlates well with the study results regarding TNF‐α. Healing of acute and chronic recalcitrant wounds also appears to be aided by Elta Proteases 20, 21. The clinical observations of healing also correspond well with the implications of the study results. Use of immunomodulators like Elta Proteases can have a substantial clinical impact on healing chronic wounds.
This in vitro study provided useful information on the actions of Elta Proteases. Future research incorporating dual clinical observations and in vitro results verifying the changes in the microenvironment while following the clinical outcomes would be particular interesting and strengthen the current study. Additional research on alternate Elta Protease formulations tailored to specific wound aetiologies and their microenvironments could increase the clinical impact.
Results from this study suggest Elta Proteases have proteolytic activity that effectively degrades different types of proteins including enzymes, cytokines and matrix proteins. The in vitro results also suggest not all proteins are degraded by Elta Proteases. Unlike other immunomodulators, Elta Proteases were not deactivated by CWF components. By affecting multiple targets and pathways, this immunomodulator may shift dysregulated physiological pathways and cross talk to become more balanced and normalized, thus leading to improved wound healing.
Acknowledgments
This study was sponsored by Swiss‐American Products, Inc. Dallas, TX. LKSP is an independent consultant and is currently retained by Swiss‐American Products, Inc. for their protease project.
References
- 1. Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wound fluid. Wound Repair Regen 1996;4:411–20. [DOI] [PubMed] [Google Scholar]
- 2. Falanga V. The chronic wound: failure to heal. In: Falanga V, editor. Cutaneous wound healing. Florence: Martin Dunitz Ltd, 2001:155–64. [Google Scholar]
- 3. Falanga V, Brem H. Wound bed preparation for optimal use of advanced therapeutic products. In: Falanga V, editor. Cutaneous wound healing. Florence: Martin Dunitz Ltd, 2001:457–68. [Google Scholar]
- 4. Mahendale F, Martin P. The cellular and molecular events of wound healing. In: Falanga V, editor. Cutaneous wound healing. Florence: Martin Dunitz Ltd, 2001:15–37. [Google Scholar]
- 5. Efron PA, Moldawer LL. Cytokines and wound healing: the role of cytokines and anticytokine therapy in the repair response. J Burn Care Rehab 2004;25:149–60. [DOI] [PubMed] [Google Scholar]
- 6. Mast BA. The skin. In: Cohen IK, Diegelmann RF, Lindblad WJ, editors. Wound healing: biochemical and clinical perspective. Philadelphia: WB Saunders Company, Harcourt Brace Jovanovich Inc., 1992:344–55. [Google Scholar]
- 7. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–25. [DOI] [PubMed] [Google Scholar]
- 8. Danon D, Kowatch MA, Roth GS. Promotion of repair in old mice by local injection of macrophages. Proc Natl Acad Sci U S A 1989;86:2018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leibovich SJ, Ross R. The role of the macrophage in wound repair; a study with hydrocortisone and antimacrophage serum. Am J Pathol 1975;78:71–91. [PMC free article] [PubMed] [Google Scholar]
- 10. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of Promogran, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 2002;10:16–25. [DOI] [PubMed] [Google Scholar]
- 11. Trengove NJ, Langton SR, Stacey MC. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen 1996;4:234–9. [DOI] [PubMed] [Google Scholar]
- 12. Fu X, Li X, Cheng B, Chen W, Sheng Z. Engineered growth factors and cutaneous wound healing: success and possible questions in the past 10 years. Wound Repair Regen 2005;13:122–30. [DOI] [PubMed] [Google Scholar]
- 13. Mustoe TA, Cutler NR, Allman RM. Phase II study to evaluate recombinant PDGF‐BB in the treatment of pressure sores. Arch Surg 1994;129:213–9. [DOI] [PubMed] [Google Scholar]
- 14. Steed DL, The Diabetic Ulcer Study Group. Clinical evaluation of recombinant human platelet derived growth factor for the treatment of lower extremity diabetic ulcers. J Vasc Surg 1995;21:71–81. [DOI] [PubMed] [Google Scholar]
- 15. Pierce GF, Tarpley JE, Tseng J. Detection of increased levels of PDGF‐AA rh activity healing human wounds treated with recombinant PDGF‐BB, and absence of PDGF in chronic nonhealing wounds. J Clin Invest 1995;96:1336–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yager DR, Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Cohen IK. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen 1997;5:23–32. [DOI] [PubMed] [Google Scholar]
- 17. Falanga V. Growth factors and chronic wounds: the need to understand the microenvironment. J Dermatol 1992;19:667–72. [DOI] [PubMed] [Google Scholar]
- 18. U.S. Food and Drug Administration . Summary of All GRAS Notifications. [WWW document]. URL http://www.cfsan.fda.gov/~rdb/opa‐gras.html (Accessed 3 January 2008).
- 19. Phillips L. Therapeutic options in management of pressure ulcers. In: Falanga V, editor. Cutaneous wound healing. Florence: Martin Dunitz Ltd, 2001:343–56. [Google Scholar]
- 20. Barnett L, Parnell LKS. Contact dermatitis treated with new topical products: a case study. Ostomy Wound Manage 2001;47:47–53. [PubMed] [Google Scholar]
- 21. Parnell LKS, Ciufi B, Gokoo CF. Preliminary use of a hydrogel containing enzymes in the treatment of stage II and stage III pressure ulcers. Ostomy Wound Manage 2005;51:50–60. [PubMed] [Google Scholar]


