Abstract
The aim of the study was to evaluate a novel foam dressing with continuous low‐level release of ibuprofen (Biatain‐Ibu foam dressing, Coloplast A/S, Humlebaek, Denmark) in persons with leg ulcers compared to local best practice. An open comparative and prospective block‐randomised study of 24 patients was conducted in a Canadian wound clinic. Twelve patients were randomised to ibuprofen–foam and 12 patients to local best practice. The study population consisted of patients with chronic, painful exudating leg ulcers. The patients rated their wound pain intensity at baseline and after the first dressing application. Pain intensity in the morning and evening was rated during a period of 1 week using a numeric box scale (NBS). A t‐test compared the main differences in pain intensity and a five‐point verbal rating scale measured the patients’ pain relief. At the last clinical visit, pain after dressing change was assessed using an NBS. In addition, wound size, percentage of healthy granulation tissue and the presence of peri‐ulcer erythema, were (all) evaluated at inclusion and the end of the study. The nurses and patients both evaluated the relative dressing performance and exudate management at the last study visit. This study demonstrates that the ibuprofen–foam dressing decreased wound pain in patients with leg ulcers compared to best practice. The ibuprofen–foam dressing was associated with: diminished chronic pain between dressing changes, reduced acute pain at dressing change, increased healthy granulation tissue, decreased peri‐wound erythema and excellent exudate handling capacity. It can be concluded from the results of the study that the combination of foam with a continuous low‐dose release of ibuprofen may offer a valuable new therapeutic approach to the reduction of wound pain.
Keywords: Arterial, Biatain–Ibu, Chronic wound pain, Dressing change pain, Persistent wound pain, Post marketing study, Pyroderma gangrenosum, Vasculitis, Venous
Introduction
Wound pain is the primary concern for most chronic wound patients and although it is widely reported, clinicians often offer inadequate treatment (1). Pain has been associated with reduced wound healing rates (2) and has a negative impact on patients’ quality of life including lack of sleep and increased anxiety 3, 4.
Previous studies have demonstrated that the pain experience for people with chronic wounds is characterised by a combination of unrelenting chronic pain with periods of unpredictable, intermittent pain as well as acute pain with dressing change that can disrupt and dominate all aspects of life 5, 6. Although pain is a significant problem in patients with chronic wounds, effective management strategies remain elusive. An ideal pain treatment product should be able to provide prompt and long‐lasting relief, be relatively non traumatic, safe and cost effective. Local and systemic side effects should be minimal.
Over the past several decades, moist interactive wound healing dressings have been the mainstay for chronic wounds treatment. One dressing class includes polyurethane foams; these simple foams have been improved by adding partial moisture retention (second generation) to surface moisture exchange (first generation) with products such as Biatain (Coloplast A/S). This second generation foam has now been combined with ibuprofen. It consists of a soft, hydrophilic, non adhesive polyurethane foam containing 0·5 mg/cm2 of ibuprofen homogeneously dispersed throughout the foam 5, 6. A 10 × 10 cm dressing contains 50 mg of ibuprofen that can be released directly into the wound over 1–7 days (depending on the exudate level).
The ibuprofen concentration in the dressing is low compared to a usual maximum daily oral dose of 1200 mg and in special cases up to 3200 mg (7). The continuous release of a low ibuprofen dose into the wound bed is appealing for its known anti‐inflammatory and pain‐relieving properties (5). Other clinical studies have indicated that ibuprofen–foam may promote wound healing by effectively managing exudate to minimise maceration and further reduce chronic pain 5, 6. The new dressing is indicated for painful exuding wounds and the direct release of ibuprofen into the wound bed may reduce tissue damage‐related pain (5).
This pilot study was conducted in a chronic wound clinic (real‐life setting). The ibuprofen foam dressing was compared to local best practice in the treatment of painful exuding chronic leg ulcers. In comparison to traditional clinical trials, this approach resembles real‐life situations because it allows the inclusion of patients encountered in everyday clinical practice.
Methods
This pilot study utilised an open, comparative and prospective block‐randomised design. Twenty‐four patients were recruited. The study sample consisted of patients with chronic, painful, exudating leg ulcers. All patients had the cause of the wound appropriately treated, including the use of compression bandaging for venous disease. The study was conducted in a Canadian wound care clinic between August and December 2005.
The inclusion criteria consisted of painful, chronic leg ulcers with moderate to high exudate. The minimum wound size was 0·5 × 0·5 cm and a maximum size was determined by the ability of the 10 × 10 cm. dressing to cover the wound. All patients experienced wound‐related pain (at least three out of 10 on Verbal Analogue Scale). They were adults over the age of 18 and were able to comprehend and rate their pain using a pain diary. All concomitant pain and prescribed medications were kept unchanged for 1 week prior to the commencement of the study. Exclusion criteria consisted of: known contraindication or allergy to ibuprofen or other NSAIDs, females of childbearing age that were breastfeeding or pregnant and deep ulcers extending to tendon, muscle or bone. To reduce the confounding effect from increased bacterial burden, patients with associated signs (non healing and one of the additional factors: deep red discolouration of the granulation tissue, surface slough or odour) were excluded.
All patients received both oral and written information about the purpose, potential risks, inconveniences and expected benefits from participation in the investigation. They were informed that their involvement was voluntary and that they could withdraw at any time, without affecting future care at the clinic. Written informed consent was obtained from all subjects by the study investigators. An independent official research ethics board has approved this study, according to the 1975 Declaration of Helsinki guidelines.
Patients were randomised to a 1‐week treatment period of either ibuprofen–foam or local best practice. The randomisation list was produced automatically by computer using software Medstat version 2.1. The study was comparative, with a control group that reflected real‐life clinical settings (usual patient care). Patients who were randomised to the local best‐practice group were treated according to the current treatment standards at the clinic, e.g. moist wound healing dressings and dressings containing active anti‐microbial and anti‐inflammatory components when necessary (Table 1). The wound management regime for individual patients in this group remained consistent during the 1‐week evaluation period.
Table 1.
Local best‐practice group wound treatments
| Wound contact dressing | Material covering the wound contact dressing | No. of patients | Compression bandage |
|---|---|---|---|
| Silver‐sulphadiazine cream | Gauze | 1 | No |
| Mepilex Foam™ | 1 | Yes | |
| Telfa™ | 2 | 1 = Yes, 1 = No | |
| Iodosorb™ | Telfa™ | 2 | Yes |
| 3M Foam™ | 1 | Yes | |
| Contreet®Foam | — | 1 | Yes |
| Promogran™ | Telfa™ | 1 | Yes |
| Aquacel Ag™ | ETE™ | 1 | Yes |
| Mesorb™ | 1 | Yes | |
| Intrasite gel™ | Biatain® | 1 | Yes |
A baseline assessment recorded the ulcer characteristics, including wound margin erythema and pain intensity. Patients were requested to keep their concomitant medication stable throughout the study. However, if changes in concomitant pain medication were unavoidable the changes were recorded in the diary as a ‘decreased dosage’, ‘no change’ or ‘increased dosage’.
Pain measurement
Patients were supplied with a diary for the purpose of recording chronic wound pain intensity and pain relief each morning at breakfast time and in the evening at bedtime. Repeated measures during the day provided a reliable evaluation of pain and eliminated the potential diurnal variability of pain. The pain intensity was measured using a 0–10 numeric box scale (NBS) with 0 ‘No Pain’ to 10 ‘Worst Possible Pain’. Subjects were asked to select a number on the scale to indicate the level of pain at the particular point in time when they were asked to evaluate it. Pain relief was measured utilising a verbal rating scale (VRS). Patients were asked to indicate if they experience any pain relief on a five point Likert scale with 0 representing no pain relief to 4 representing complete relief (Table 2).
Table 2.
Study design and visit schedule
| Visits | 1 | Between visits | 2 |
| Day | 1 | 2, 3, 4, 5, 6, 7 | 8 |
| Dressing change, pain assessment | Assessed | — | Assessed |
| Morning/Evening numerical box score | — | Twice daily pain readings by patients |
At the end of the study patients returned to the clinic for a final assessment where wound size was measured and characteristics of the wound margin and mature granulation were evaluated (four point Likert scale). At this point patients were asked to evaluate their acute wound pain intensity after dressing removal.
Statistical analysis
All statistical analyses were performed using SAS version 9 (SAS Institute Inc., Cary, NC). Significance level was set at P = 0·05. When computing the ulcer area, the shape was assumed to be an ellipse to provide a more accurate wound size (8). A two tailed t‐test was used to compare the mean group differences in pain intensity between the two groups at the end of the study. Differences in relative ulcer area were tested by Wilcoxon Rank Sum Test.
Pain intensity was regarded as a continuous variable and was measured on an 11‐point NBS. Pain intensity was analysed by computing the sum of pain intensity differences (SPID) from baseline for each patient. SPID values for the treatment groups were compared using a two‐sample t‐test assuming equal variances in the two treatment groups (9). The null hypothesis tested is that the mean values of SPID are the same. The pain intensity measured in the morning and evening were analysed separately as well as pooled into one data set containing both morning and evening values.
Chronic pain relief is a categorical variable with the descriptors: No relief, slight relief, moderate relief, a lot of relief and complete relief. It is measured each day (mornings and evenings) during 1 week on a five point VRS and analysed by computing a value of total pain relief (TOTPAR) for each subject. The full data set, both morning and evening observations were analysed with missing observations of pain relief substituted with the previous completed observation. These calculations assume that each descriptor has an equal numeric translation (9).
Results
A total of 24 patients were included in this study: 12 in the ibuprofen–foam group, 12 in the local best practice group. One patient from the local best practice group did not return for the second clinical visit. There were no adverse events reported during the study period. The baseline clinical characteristics are outlined in Table 3 and were comparable at baseline.
Table 3.
Patient demographics and clinical characteristics of wounds in study population
| Ibuprofen–foam | Local best practice | |||||
|---|---|---|---|---|---|---|
| N | X ± SD | Min–Max | N | X ± SD | Min–Max | |
| Mean age (years) | 12 | 58·8 ± 15·1 | 28–81 | 12 | 63·3 ± 17·8 | 39–84 |
| Mean duration of ulcer (years) | 12 | 2·6 ± 3·2 | 0·1–8·0 | 12 | 1·1 ± 2·8 | 0–10·0 |
| Median ulcer area (cm2) | 12 | 2·5 | 0·39–94·5 | 12 | 1·9 | 0·39–15·1 |
| Leg ulcer type | ||||||
| Venous leg ulcer | 7 | 58% | — | 10 | 83% | — |
| Venous and arterial ulcer | 2 | 17% | — | 1 | 8% | — |
| Pyoderma gangrenosum | 3 | 25% | — | 0 | 0% | — |
| Vasculitis | 0 | 0% | — | 1 | 8% | — |
At baseline the majority of study patients were taking oral pain‐relieving medication (92% of patients in the ibuprofen–foam group and 83% in the local best practice group). To minimise bias, pain intensity of the ulcers was measured before the patient was randomised to a treatment group. Mean pain intensity was 5·5 [standard deviation (SD) ± 2·2] in the ibuprofen–foam group and 6·3 (SD ± 1·7) in the local best practice group measured on a NBS (Figure 1). Sixty‐seven per cent (8) patients in the ibuprofen–foam group and 83% (10) in the local best practice group experienced baseline pain both in the wound and at the wound margin.
Figure 1.
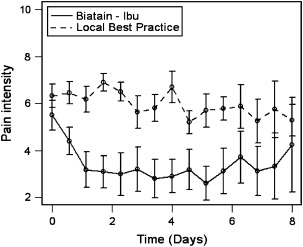
Pain intensity of ulcers in the morning and evening (pooled data) (P = 0·0217). The values represent 0 = No Pain to 10 = Worst Possible Pain.
Pain intensity
All of the patients in this study had painful ulcers at study inclusion. At the beginning of the study, there was no statistical difference in pain assessment scores between the two treatment groups. Over the 7‐day study, the additive morning pain intensity scores demonstrated a significant difference in favour of the ibuprofen–foam group (P = 0·0401, and the evening values had a similar significant reduction (P = 0·0202).
A correlation analysis between morning and evening pain intensity values showed significant correlation (Pearson’s, r = 0·85, P < 0·0001). When the chronic (persistent) wound pain intensity values from the morning and evening results were pooled, again there was a significant difference in favour of treatment with ibuprofen‐ foam (P = 0·0217, Figure 1).
Acute pain intensity was also assessed after the last visit dressing removal. Figure 2 illustrates the overall differences of acute wound pain intensity between the two treatment groups in favour of ibuprofen–foam (P = 0·0405).
Figure 2.
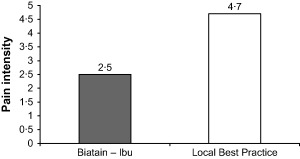
Pain intensity at dressing change (p = 0·04) at day 7 (6–8 days, second visit). The values represent 0 = No Pain to 10 = Worst Possible Pain.
Pain‐relieving medication
The overall changes in the participant’s pain‐relieving medication (increased dosage, no change, decreased dosage) during the study period are illustrated in Figure 3. Two patients (one from each group) changed their non pain‐related concomitant medication during the study period due to nausea and underlying rheumatoid disease.
Figure 3.
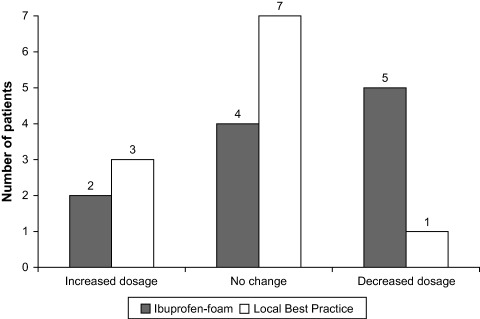
Patient reports of overall changes in pain relieving medication between inclusion and end of study (P = 0·1279). One patient in each group did not return with their patient diary.
Ulcer area
No ulcers healed during the 1‐week study period. Table 3 outlines the ulcer area at baseline and Table 4 the final ulcer areas. The reduction in ulcer area was significantly greater in the ibuprofen–foam group (P = 0·05).
Table 4.
Characteristics of leg ulcer type, exudate level, localisation of ulcer and condition of peri‐ulcer skin at the end of study
| Parameter | Local best practice | Biatain–Ibu | Statics P‐value |
|---|---|---|---|
| Relative wound size (100% baseline) | 125% | 83% | 0·05 |
| Increase in mature granulation tissue | 6% | 23% | 0·07 |
| Peri‐wound erythema (no. of patients) | 7 (Baseline 4) | 1 (Baseline 5) | 0·02 |
Appearance of ulcer bed and condition of peri‐ulcer skin
The condition of the wound bed was assessed at the beginning and at the end of the study. Wounds in the in the ibuprofen–foam group had more healthy red granulation tissue at the end of the study although the result is not significant (P‐value 0·07, Table 4).
There was a reduction of patients with peri‐wound erythema in the ibuprofen–foam group (baseline 5 versus 1 at the end of the study) as compared to increased peri‐wound erythema in the local best practice group (baseline 4 versus 7 at the end of the study) (P = 0·02, Table 4). No signs of clinical infection were observed in the ibuprofen–foam group, but two patients in the local best‐practice group developed a deep wound infection during the study period.
Dressing performance
The nurses reported no difference in the ease of dressing application or removal between the two study groups. The ibuprofen–foam managed exudate more effectively when measured on a four point scale (excellent, good, fair, poor) (Figure 4). The patients’ overall rating of the dressing was also superior in the ibuprofen–foam group, when compared to the local best‐practice group (P = 0·05, Table 5).
Figure 4.
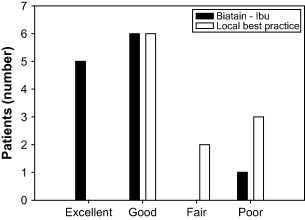
Nurses rating of exudate management with ibuprofen–foam as compared to local best practice.
Table 5.
Patients rating of dressing compared to their previous experience (P = 0·05 comparison between groups)
| Ibuprofen–foam | Local best practice | |||
|---|---|---|---|---|
| N | % | N | % | |
| Very good | 5 | 42 | 0 | 0 |
| Good | 5 | 42 | 3 | 27 |
| Moderate | 1 | 8 | 4 | 36 |
| Poor | 1 | 8 | 4 | 36 |
| Very poor | 0 | 0 | 0 | 0 |
Discussion
Chronic wounds are potentially painful. This study reinforces the importance of evaluating wound pain between dressing changes as well as acute pain elicited during dressing changes, debridement procedures and other events. Pain is often described as ‘what the patient says it is’(10). Through the use of validated measurement scales, the subjective nature of pain can be quantified and monitored to determine the alleviating and aggravating factors (11).
This study selected both pain relief and pain intensity as the primary outcomes. Management strategies are considered effective, not only because they can reduce pain intensity, but also bring relief from pain. Depending on the magnitude of pain, increasing evidence suggests that evaluation of pain relief may be as important an indicator as reduction in pain intensity. 11, 12. The NBS is a simple and reliable tool to measure pain 12, 13. In this small pilot study, both pain intensity and relief scales produced significantly reduced pain measurements in the older chronic wound patients studied.
The use of ibuprofen–foam significantly decreased chronic (persistent) wound pain in patients with leg ulcers compared to local best practice using modern treatment regimes with some local dressings known to minimise discomfort at dressing change. In the past, pain at dressing change has often been the focus of care without considering local management of chronic pain. The introduction of this new class of dressing combining moisture balance with ibuprofen release has given practitioners a new topical device to improve wound pain management.
Patients in the ibuprofen–foam group reported significantly lower wound pain intensity. The persistent pain often peaks in the morning for people with chronic wounds (6) and significant pain intensity reduction was noted with the ibuprofen–foam group. Patients considered the ibuprofen–foam provided better pain relief during the dressing wear‐time and at dressing change. Persons receiving the ibuprofen–foam were more likely to reduce their oral pain‐relieving medication, taken over the study period.
The exudate management capabilities of the ibuprofen–foam were superior compared to dressings in the local best practice group. This characteristic facilitated the improvement in the wound bed observed probably by promoting healthy granulation tissue and reducing erythema at the wound edge. The improvement in periwound erythema may also be a function of the anti‐inflammatory action of ibuprofen.
People with chronic wounds often suffer from round the clock fluctuating wound pain (6). It was therefore not surprising that most patients in this study preferred ibuprofen–foam with local continuous low‐dose ibuprofen release to improve baseline wound pain intensity.
Study limitations
Each new therapy needs proof of concept, but must also be evaluated for integration into everyday clinical practice. The scientific rigour that makes studies valuable as support for clinical decision‐making can isolate them from normal practice, because participants are carefully selected based on rigid and specific inclusion criteria. This study has attempted to overcome this limitation by comparing current best practice in a clinical setting with the ability of this new dressing in a real life setting for patients with painful exudative leg ulcers. Previous literature has identified the ideal patients in a randomised controlled study as proof of intervention efficacy and a study such as this on usual patients to be a measure of intervention efficiency (14).
Both researchers and participants were aware that the ibuprofen–foam dressing contained an active analgesic that may have biased the results. The measurement of an individual’s perception of pain is challenging and is influenced by many subjective variables. The elderly have low expectations of pain‐reducing interventions and often erroneously accept that pain is a normal part of their lives (15). Pain intensity ratings are known to have diurnal variations and may be influenced by factors such as medication schedule, anticipatory pain and mood that could vary and influence ratings the next day 6, 16, 17.
This study is a pilot with a small number of patients and precedes a multi national, multi centre randomised study to verify these conclusions. The improvement in leg ulcers may not be generalisable to other wound types and further investigations are warranted.
Conclusion
This open comparative and prospective block randomised study demonstrated that an advanced foam dressing with the capacity to minimise maceration, combined with low dose continuous release of ibuprofen can
-
•
decrease chronic wound pain in patients with leg ulcers
-
•
decrease acute wound pain intensity at dressing change
-
•
decrease peri‐wound erythema
-
•
provide superior exudate management
Conflicts of interest
The authors have declared no conflicts of interest.
Acknowledgement
This study was partially funded by an unrestricted grant from Coloplast A/S, Holtedam 1, 3050 Humlebæk, Denmark.
References
- 1. Briggs M, Ferris FD, Harding K, Hofman D, Hollinworth H, Krasner D, Lindholm C, Moffat CJ, Price P, Romanelli M, Sibbald RG, Stacey M, Téot L. Minimising pain at wound dressing‐related procedures: A consensus document. In: Calne S, editor. 2004. Ltd, 53 Hargrave Road, London N19 5SH, Medical Education Partnership. World Union of Wound Healing Societies. [Google Scholar]
- 2. Moffat CJ. Pain as a predictor of leg ulcer healing. How to decrease trauma and pain at dressing changes. Symposium on Advanced Wound Care& Medical Research Forum on Wound Repair; April 30May 3, 2001; Las Vegas, Nevada. [Google Scholar]
- 3. Hyde C, Ward B, Horsfall J, Winder G. Older women’s experience of living with chronic leg ulceration. Int J Nurs Pract 1999;5:189–98. [DOI] [PubMed] [Google Scholar]
- 4. Douglas V. Living with a chronic leg ulcer: an insight into patients’ experiences and feelings. J Wound Care 2001;10:355–60. [DOI] [PubMed] [Google Scholar]
- 5. Jørgensen B, Friis GJ, Gottrup F. Pain and quality of life for patients with venous leg ulcers: Proof of concept of the efficacy of Biatain‐Ibu, a new pain reducing wound dressing. Wound Repair Regen 2006;14:333–9. [DOI] [PubMed] [Google Scholar]
- 6. Flanagan M, Vogensen H, Haase L. Case series investigating the experience of pain in patients with chronic venous leg ulcers treated with a foam dressing releasing ibuprofen. World Wide Wounds 2006. [WWW document]. URL http://www.worldwidewounds.com/2006/april/Flanagan/Ibuprofen‐Foam‐Dressing.html [accessed on 2 March 2007]
- 7. Thomson MICROMEDEX DrugPoints® Ibuprofen . MICROMEDEX(R) Healthcare Series 2006 June128. [WWW document]. URL http://www.smi.dk [accessed on 1 December 2006]
- 8. Kantor J, Margolis DJ. Efficacy and prognostic value of simple wound measurements. Arch Dermatol 1998;134:1571–4. [DOI] [PubMed] [Google Scholar]
- 9. Max MB, Laska EM. Single‐dose analgesic comparisons. In: Max M, Portenoy R, Laska E, editors. Advances in pain research and therapy. New York: Raven Press Ltd, 1991: 55–95. [Google Scholar]
- 10. McCaffrey M. Nursing management of the patient with pain. Philadelphia: Lippincott, 1972. [Google Scholar]
- 11. Osterbrink J. Schmertzmanagement in der pflege: Der expertenstandard in der praxis [in German]. Pflege 2005;16:16–18. [Google Scholar]
- 12. Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain 2003;4:407–14. [DOI] [PubMed] [Google Scholar]
- 13. Anonymous. Assessment and Management of Acute Pain. Institute for Clinical Systems Improvement (ICSI) 2006 March; 2006 Mar. URL: http://www.guideline.gov/summary/summary.aspx?doc_id 9009 [accessed on 19 June 2006]
- 14. Price P. The challenge of outcome measures in chronic wounds. J Wound Care 1999;8:306–8. [DOI] [PubMed] [Google Scholar]
- 15. Schofield P. Pain management fo older people in care homes: A pilot study. Br J Nurs 2006;15:509–13. [DOI] [PubMed] [Google Scholar]
- 16. Hyde C, Ward B, Horsfall J, Winder G. Older women’s experience of living with chronic leg ulceration. Int J Nurs Pract 1999;5:189–98. [DOI] [PubMed] [Google Scholar]
- 17. Hyland ME, Ley A, Thomson B. Quality of life of leg ulcer patients: questionaire and preliminary findings. J Wound Care 1994;3:294–8. [DOI] [PubMed] [Google Scholar]


