Abstract
Perianal fistula is a very common problem in general population. Ninety percent of perianal fistulae arise from infected anal glands, and they often give rise to perianal abscesses. Very rarely perianal fistulae and abscesses undergo malignant transformation and give rise to carcinomas, mainly adenocarcinomas. We are reporting a case of squamous cell carcinoma arising from long‐standing perianal fistula and how we managed it surgically.
Keywords: Perianal fistula, Squamous cell carcinoma
INTRODUCTION
Perianal fistula is a very common problem in general population. Ninety percent of perianal fistulae arise from infected anal glands, and they often give rise to perianal abscesses. Approximately 10% of perirectal fistulae are thought not to be because of infected anal glands but to be because of a consequence of more specific causes such as Crohn's disease, trauma, human immunodeficiency virus, sexually transmitted diseases, radiation therapy, or a foreign body. Very rarely perianal fistulae and abscesses undergo malignant transformation and give rise to carcinomas. Carcinomas can be squamous cell carcinomas or adenocarcinomas, of which adenocarcinomas are much more common. Squamous cell carcinomas rarely arise from perianal fistulae. In the literature only 10 similar cases have been reported 1, 2, 3, 4, 5, 6, 7, 8. Here, we are reporting a case of squamous cell carcinoma that arose from long‐standing perianal fistula and how we managed it surgically. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal.
CASE REPORT
A 56‐year‐old man presented in our clinic with multiple perianal fistulae of 32‐year duration. Starting from the age of 14, he used to develop some or other abscesses from theses fistulae. He had abscesses in the right perianal region many times in the past and undergone incision and drainage twice in the past, that is, at 30 and 18 years back. Now he presented with a recurrent abscess of 4 months' duration at the same site towards right gluteal area. He was referred to our centre after a biopsy, which showed squamous cell carcinoma. On examination, there was an ulcer which was 4 × 4 cm in size with everted margins, situated in the right perianal region towards gluteal area (Figure 1). There was also induration around the ulcer and a collection of pus at the medial end of the induration with discharge through the ulcer. There were multiple scars in the perineum probably occurred from perianal fistulae in the past. On proctoscopy, there were no visible lesions in the anal canal or any communication with the fistulae. Subsequent flexible sigmoidoscopy was also normal. Magnetic resonance imaging (MRI) scan showed growth with a communicating fistulous tract going up to anal canal (Figure 2). Metastatic work up including chest x‐ray and ultrasound abdomen ruled out metastatic disease elsewhere. We excised the lesion with wide margins all around (Figure 3). During the surgery exploration was performed to find out the fistulous tract, which was futile. Frozen section examination showed negative margins all around the tumour. The defect was reconstructed by inferiorly based gluteal rotation flap (Figure 4). Patient had a smooth recovery except for minor flap loss at the medial end, which was managed conservatively. Histopathology report showed well‐differentiated squamous cell carcinoma (Figure 5), and all margins were free of disease.
Figure 1.
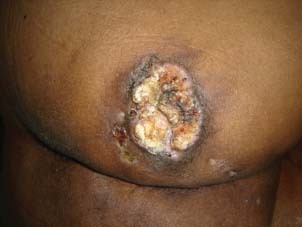
Squamous cell carcinoma arising from perianal region.
Figure 2.
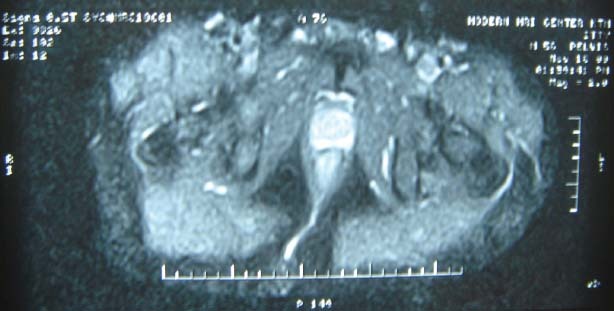
Magnetic resonance imaging (MRI) scan showing fistulous tract to anal canal.
Figure 3.
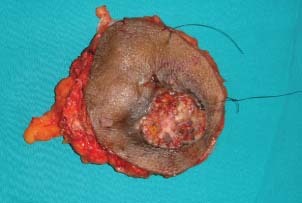
Wide excised specimen.
Figure 4.
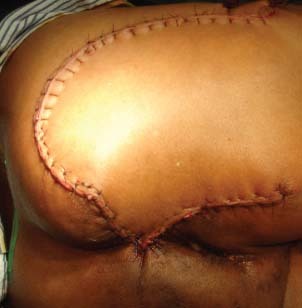
Defect reconstructed using gluteal rotation flap.
Figure 5.
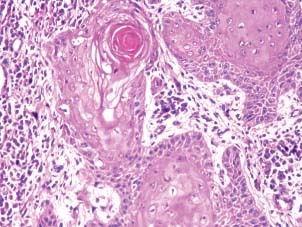
Photomicrograph showing well‐differentiated squamous cell carcinoma.
DISCUSSION
The incidence of perianal fistula in general population varies from 1·04–8·6 per 100 000 with male preponderance 9, 10. The etiology of squamous cell carcinoma of perianal region are smoking, sexual behaviour particularly homosexual anal intercourse, chronic inflammation (Crohn's, anal fistula, fissure, sepsis) and transmissible agents (human papilomma virus types 18, 16, condyloma accuminata). Perianal fistula is very common disease, especially in association with Crohn's disease. Malignant transformation of perianal fistula is a rare occurrence, if there is no history of Crohn's disease. In 1940, Ewing (11) quoted Krauske's statement, ‘there is no satisfactory evidence that cancer develops in tissues altered by haemorrhoids, fistulae or cicatrices,’ but today there is convincing evidence in the literature that chronic fistulas can give rise to malignancy. It was Rosser et al. who first described cancer developing in fistula in ano (8). Rosser (12) established three essential criteria to confirm that malignant transformation has occurred within a fistula. He stated firstly that the fistula must be present for 10 years to exclude the possibility that the malignancy predated the fistula; secondly, there should not be a tumour within the mucosa of the rectum or anal canal unless there is definite evidence that this is metastatic tumour; and thirdly that the opening of the fistula within the anal canal or rectum should not contain malignant tissue. Miller and Lypin (7) suggested that the best criterion for determining a causal relationship is the time factor. He arbitrarily set 10 years as the period over which a fistula must be present before neoplastic changes develop in it to prove an etiologic connection between the tumour and the tract. Relationship between chronic inflammation and carcinogenesis was originally suggested by Virchow, who first hypothesised in 1893 that the lymphoreticular infiltrate may be the origin of tumour at sites of chronic inflammation (13). Chronic inflammation caused by a variety of factors including bacterial, viral and parasitic infections, chemical irritants, and non digestible particles can predispose an individual to cancer, especially if the inflammation persists for a long time (14). Inflammatory cells and cytokines act as a tumour promoters by inducing proneoplastic mutations that affect cell survival, proliferation, invasion and angiogenesis (15). Free radicals produced during inflammatory response like reactive oxygen intermediates, superoxide and reactive nitrogen intermediates, nitric oxide and peroxynitrite lead to oxidative damage and nitration of DNA bases which increases the risk of DNA mutations (16). It is believed that repeated epithelial regeneration induced by chronic inflammation gives rise to a malignant process.
Anatomically midway up the anal canal lies the dentate line (pectinate line). This marks the true mucocutaneous junction between somatically innervated squamous epithelium distally and the viscerally innervated columnar epithelium proximally. Seminal work by Parks and Eisenhammer identified anal glands present at the level of the dentate line and showed that these glands are the etiology of most perirectal abscesses and fistulae 17, 18. Carcinoma arising from perianal fistulae and abscesses can be adenocarcinoma or squamous cell carcinoma because both squamous and columnar mucosas are present in this area.
In the retrospective cohort study including 45 186 patients by Nordenvall et al., standardised incidence ratio of squamous cell carcinoma of anal canal 1·3 times for anal fistulae and 5·3 times for anal abcesses (19). Among 53 patients who had squamous cell carcinoma, six occurred in patients suffering from benign rectal diseases like fistulae. In another study by Holly et al., the relative risk of cancer in fistulae and fissure are 2·4 in heterosexual men and women and 9·1 in homosexual men (20). Similarly another study showed relative risk of 31·7 (95% confidence interval, 3·6–114·5) for fistula and 17·7 (95% confidence interval, 2·0–63·9) for perianal or perirectal abscess (21) in 1st year of diagnosis. Here another error which might have occurred is that some of the cancers might have been misdiagnosed as fistulae in few patients, which is shown by the fact that the incidence of cancers in perianal fistulae drops to 9% (95% confidence interval 1–32·5%) in subsequent 4 years.
In case of perianal fistula of long duration, malignant transformation has to be ruled out. Symptoms suggesting malignant transformation are the onset of pain, induration or bleeding in an otherwise asymptomatic fistula, or the appearance of mucinous drainage. As malignancy progresses hard induration or mass develops around the fistulous tract. Rectal examination is usually surprisingly non contributory, and no lesion is visible on proctoscopic visualisation of the rectosigmoid, but on local examination ulcer with induration and indurated mass are signs of malignancy and should not be ignored. MRI and transrectal ultrasound (TRUS) are the investigations of choice to diagnose and stage underlying anal cancers. Some studies show that these two modalities are complimentary (22), some says that the TRUS is superior (23). Whenever surgery is performed for chronic perianal fistula, specimen should be subjected to histopathology, and patients with clinical suspicion of malignancy should be investigated adequately.
REFERENCES
- 1. Seya T, Tanaka N, Shinji S, Yokoi K, Oguro T, Oaki Y, Ishiwata T, Naito Z, Tajiri T. Squamous cell carcinoma arising from recurrent anal fistula. J Nippon Med Sch 2007;74:319–24. [DOI] [PubMed] [Google Scholar]
- 2. Ishizawa T, Koseki S, Mitsuhashi Y, Kondo S. Squamous cell carcinoma arising in chronic perianal pyoderma a case report and review of Japanese literature. J Dermatol 2000;27:734–9. [DOI] [PubMed] [Google Scholar]
- 3. Sundell B, Möller C. Squamous cell carcinoma occurring in chronic perianal pyoderma. Br J Plast Surg 1969;22:379–81. [DOI] [PubMed] [Google Scholar]
- 4. Abboud B, Ingea H, Tayar C, Abadjian G. Epidermoid carcinoma developing in a chronic anal fistula. Presse Med 2000;29:786–7. [PubMed] [Google Scholar]
- 5. McCune WS, Thistlettwaiter JR. Fistul cancer. Ann Surg 1959;149:815–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kline RJ, Spencer RJ, Harrison EG Jr. Carcinoma associated with fistula‐in‐ano. Arch Surg 1964;89: 989–94. [DOI] [PubMed] [Google Scholar]
- 7. Miller JM, Lypin RJ. Carcinoma in fistula‐in‐ano. Postgrad Med 1950;7:135. [DOI] [PubMed] [Google Scholar]
- 8. Rosser C. Etiology of anal cancer. Am J Surg 1931;11:328–33. [Google Scholar]
- 9. Sainio P. Fistula‐in‐ano in a defined population. Incidence and epidemiological aspects. Ann Chir Gynaecol 1984;73:219–24. [PubMed] [Google Scholar]
- 10. Zanotti C, Martinez‐Puente C, Pascual I, Pascual M, Herreros D, García‐Olmo D. An assessment of the incidence of fistula‐in‐ano in four countries of the European Union. Int J Colorectal Dis 2007;22:1459–62. [DOI] [PubMed] [Google Scholar]
- 11. Ewing J. Neoplastic diseases. Philadelphia: W. B. Saunders, 1940:727. [Google Scholar]
- 12. Rosser C. The relation of fistula in ano to cancer of the anal canal. Trans Am proct Soc 1934;35:65–70. [Google Scholar]
- 13. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- 14. Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16: 217–26,229. [PubMed] [Google Scholar]
- 15. Hong S, Lee HJ, Kim SJ, Hahm KB. Connection between inflammation and carcinogenesis in gastrointestinal tract: Focus on TGF‐β signaling. World J Gastroenterol 2010;16:2080–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003;3:276–85. [DOI] [PubMed] [Google Scholar]
- 17. Parks AG. Pathogenesis and treatment of fistula‐in‐ano. Br Med J 1961;1:463–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eisenhammer S. The internal anal sphincter and the anorectal abscess. Surg Gynecol Obstet 1956;103:501–6. [PubMed] [Google Scholar]
- 19. Nordenvall C, Nyren O, Ye W. Elevated anal squamous cell carcinoma risk associated with benign inflammatory anal lesions. Gut 2006;55: 703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holly EA, Whittemore AS, Aston DA, Ahn DK, Nickoloff BJ, Kristiansen JJ. Anal cancer incidence: genital warts, anal fissure or fistula, hemorrhoids, and smoking. J Natl Cancer Inst 1989;81:1726–31. [DOI] [PubMed] [Google Scholar]
- 21. Frisch M, Olsen JH, Bautz A, Melbye M. Benign anal lesions and the risk of anal cancer. N Engl J Med 1994;331:300–2. [DOI] [PubMed] [Google Scholar]
- 22. Halefoglu AM, Yildirim S, Avlanmis O, Sakiz D, Baykan A. Endorectal ultrasonography versus phased‐array magnetic resonance imaging forpreoperative staging of rectal cancer. World J Gastroenterol 2008;14:3504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siddiqui AA, Fayiga Y, Huerta S. The role of endoscopic ultrasound in the evaluation of rectal cancer. Int Semin Surg Oncol 2006;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]


