Abstract
Infections of burn wounds are the source of significant problems in burn patients. Early excision of eschar tissue is an ideal solution to avoid sepsis. When early excision is not feasible, the application of topical antimicrobial formulations may be used to control burn wound sepsis. An understanding of the barrier properties of eschar tissue is essential for optimal design of topical antimicrobial formulations. To date, little research has been conducted on the permeability of eschar. Silver sulphadiazine (SSD) is the most frequently used topical agent in burn management. In this study, the permeation of sulphadiazine from aqueous saturated solutions of SSD through human full‐thickness burn eschar tissue was studied and compared with permeability through silicone and Carbosil as model membranes. The permeation of sulphadiazine through eschar tissue was significantly higher than that through silicone and Carbosil membranes (P < 0·05). Deconvolution of the data showed that the apparent sulphadiazine diffusion coefficient was much higher in eschar tissue and was comparable to transport through an aqueous protein gel. Further studies on a greater number of compounds are suggested to elucidate the utility of such membranes as predictive models of drug permeability through eschar tissue.
Keywords: Burn wounds, Carbosil, Eschar, Silicone, Silver sulphadiazine
Introduction
Major burns are associated with considerable mortality and morbidity (1). Early mortality relates to inhalational injury and circulatory shock (2), and later mortality is attributed to sepsis as well as the influence of the immunomodulation provided by the burn toxin in the coagulated tissue or eschar (3). In thermal injury, as temperature increases, protein disruption increases and protein denaturation progresses to coagulation. Cell necrosis is universal and complete, usually beginning at the skin surface where the heat energy is most intense. Eschar thus represents an open wound without the properties and benefits of normal skin. As this wound does not provide a mechanical and immunological barrier to infection, it constitutes a suitable medium for bacterial growth (4). In the absence of effective antimicrobial agents, pathogenic micro‐organisms proliferate in the tissue, penetrate and progress into the body. The resulting sepsis is a common complication of burns with consequent high mortality (5). Eschar tissue is metabolically inactive and contains heat‐derived products, toxins and pathogenic micro‐organisms, which may diffuse into the circulation, causing organ dysfunction and sepsis. Many of these problems are ameliorated by early excision of the eschar, a procedure that removes the agents responsible for immunosuppression and systemic sepsis (4).
While early burn excision is an ideal and practical solution, in some cases, early excision of large burns usually requires coverage with material other than autologous skin grafts because of their limited supply. Other possibilities are the use of stored allograft skin, xenografts or tissue‐engineered biosynthetic products that achieve temporary wound coverage (6). In many parts of the world, these relatively expensive products are not available and the options for early excision are limited (1). In a number of cases, patients have injuries to other organs, which prevent early operation under general anaesthesia (7). When early excision of the eschar is not possible, effective topical antimicrobial therapy may help to decrease the bacterial colonisation of the tissue, which would otherwise lead to sepsis. Topical drug delivery of antimicrobials improves burn wound treatment and decreases the rate of mortality and morbidity (8).
In vitro permeation studies using eschar tissue will provide information on the effectiveness of such drug formulations and will be useful in predicting drug levels in the wound tissue, plasma and other tissues adjacent to the wound. Knowledge of the eschar barrier properties should also facilitate the design and optimisation of effective topical formulations. For this purpose, mechanistic permeation studies using eschar tissue are necessary. There is no quantitative information in the literature on drug permeation through such tissue. This likely reflects the limited availability of such tissue as human eschar tissue for in vitro studies is usually obtained from cadavers or following plastic surgery. Particular care is necessary in handling and working with eschar tissue. The duration and method of tissue storage may also cause variability where the tissue is infected by serious pathogenic micro‐organisms (4).
Permeation studies with synthetic membranes instead of eschar may overcome the problems associated with studying eschar tissue. A range of artificial membranes has been evaluated as models for investigation of cutaneous permeation phenomena. Silicone is a non porous, hydrophobic, relatively inert and reproducible barrier, which has been used to evaluate factors such as drug concentration on permeation (9). Combinations of different polymers such as silicone and cellulose acetate have been used for modelling the hydrophilic and lipophilic domains of stratum corneum (10). A silicone–cellulose acetate multi‐laminate was reported to reproduce stratum corneum permeation for methyl nicotinate (11). Synthetic membrane systems are attractive as models for transdermal absorption for a number of reasons. In contrast to skin and eschar tissue, such polymeric membranes are readily available, homogeneous, chemically pure and easier to handle. Such membranes can provide predictive information about in vivo transdermal drug delivery when the stratum corneum represents the major resistance to drug transport and the drug is metabolically inert and not bound in viable skin (12). However, their usefulness as models for eschar tissue where the stratum corneum has been disrupted or is not present has not been investigated to date.
In this study, we compared the permeability of the sulphadiazine molecule when applied as an aqueous saturated solution of silver sulphadiazine (SSD) through eschar tissue from patients with full‐thickness burns and through silicone and Carbosil membranes. SSD is the first‐choice antimicrobial drug in the treatment of topical burn wounds (13). Silicone membranes have recently become available as skin substitutes (14), and Carbosil membranes have been used as air‐permeable non occlusive backing materials in covers for treatment of burns and wounds (12) . The aims of this study were first to characterise the permeability of sulphadiazine through these membranes to determine if they are useful models of eschar and second to determine the barrier properties of these synthetic membranes to an agent used to treat eschar tissue.
Materials and methods
SSD was purchased from Aldrich (St. Louis, MO, USA). Eschar tissue (1500 μm thick) from patients with full‐thickness burns was obtained from the Motahari Burn Centre (Tehran, Iran) with appropriate informed consent and approval. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Silicone sheeting (70 μm thick) was obtained from Dow Corning (Seneffe, Belgium), and Carbosil membrane (50 μm thick) was kindly donated by Pentapharm (Moscow).
A high‐performance liquid chromatographic (HPLC) method was developed for analysis of the sulphadiazine moiety. This method used a Hewlett Packard HPLC system (Houston, TX, USA) with C18 column (length: 15 cm, 4·6 mm id., 5 μm) and UV detector at 254 nm. The mobile phase was water:acetonitrile:phosphoric acid (900:99:1), and a flow rate of 1 ml/minute was used. A linear relationship (R 2 = 0·9996) between area under the curve and concentration was established, and the limit of quantification of this method was 12 ng/ml. Interday relative standard deviation (RSD) values were in the range of 1·0–1·9%, and intraday RSD values were in the range of 0·95–2·40%.
The saturated solubility of sulphadiazine in water was assessed by equilibrating an excess amount of SSD in aqueous solution using a stirrer at 37 ± 0·5°C for 24 hours. All samples were filtered through 0·45‐μm membrane filters, diluted and assayed by HPLC.
Large eschar tissue samples were obtained from males (mean age of 35 ± 10 years), 20–27 days post burn at the time of surgical debridement. In all cases, burns resulted from thermal damage, and only samples from abdominal and leg sites were used. Tissue samples were stored at −20°C until use. Samples were thawed initially and then washed with water. The samples were then cut into smaller pieces suitable for permeation studies as reported previously (15). Eschar samples used for this study had a measured thickness of 0·15 ± 0·02 cm.
Permeation studies were performed in Franz‐type diffusion cells (Figure 1) with a diffusion area of 2·5 cm2 for eschar samples and 1 cm2 for silicone and Carbosil. Eschar tissue, silicone and Carbosil membranes were mounted between the donor and the receptor chambers. Aqueous saturated solutions (1 ml) of SSD were applied to the membranes. The receptor chambers were filled with 25 ml of water adjusted with 0·4% phosphoric acid for eschar studies and 3 ml for silicone and Carbosil to ensure adequate solubility of the drug. The receptor chambers were maintained at 37 ± 0·5°C in a thermostatted water bath. At 1, 2, 3, 4, 5, 6 and 8 hours after application, 300 μl was withdrawn from the receptor chambers and replaced with the same volume of fresh receptor medium.
Figure 1.
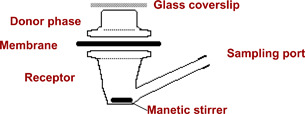
Schematic representation of a Franz diffusion cell.
Statistical analysis
The experimental data were fitted using a non linear curve‐fitting software package, Scientist® (Micromath Inc., St. Louis, MO, USA). Numerical inversion of the Laplace domain solution for steady‐state diffusion was used to obtain K, which is the membrane/donor partition coefficient, and D, which is the drug diffusion coefficient through the membrane. Drug permeation was subsequently calculated using Equation 1, (16).
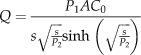 |
(1) |
P 1 and P 2 are defined as P 1 = Kl and P 2 = D/l 2, where D is the diffusion coefficient and K is the membrane/donor‐phase partition coefficient of a membrane with a diffusional area (A) and an effective length (l), following application of a concentration of drug (C 0) in the donor chamber, and s is the Laplace variable. Q is the mass permeated per unit area. C 0 is the solubility of the sulphadiazine; however, the donor solution will contain a number of species at equilibrium (17). If the dissolution of the SSD were rate limiting, there would be no differences in the rates of transport across the various membranes studied.
Fitting the permeation data with Equation 1 allows the permeability coefficient to be calculated from the product of P 1 and P 2 (k p = P 1 × P 2). The steady‐state flux, J, is given by Equation 2:
| (2) |
The lag time, T lag, was calculated from Equation 3:
| (3) |
For estimation of the effective diffusion coefficient and effective partition coefficient of sulphadiazine through the membrane, P 1 and P 2 were used together with the thickness of the membrane. For the eschar tissue, the thickness was measured as 0·15 ± 0·02 cm. The number of replicate experiments for eschar, silicone and Carbosil was at least five. The permeability coefficients and lag times were statistically evaluated by one‐tailed and two‐tailed analysis of variance with the level of statistical significance set to P < 0·05.
Results and discussion
The saturated solubility of sulphadiazine in water was 79·32 ± 2·08 μg/ml. The permeation profiles, based on detection of the sulphadiazine moiety, through eschar tissue, silicone and Carbosil membranes are shown in Figure 2. The permeation parameters are summarised in Table 1. These results indicate that sulphadiazine permeates to a greater extent across eschar tissue than across silicone and Carbosil. These differences are highly significant (P < 0·0005). The average diffusion coefficients across silicone and Carbosil are 2·12 × 10−6 ± 1·03 × 10−7 and 7·29 × 10−7 ± 1·98 × 10−8 cm2/h, respectively. The apparent diffusion coefficient of sulphadiazine across the eschar tissue was calculated as 1·1 × 10−3 cm2/h. It is interesting to note that the diffusion coefficient of dexamethasone has been measured as 3·3 × 10−4 cm2/h through human dermal tissue (18). This means that eschar tissue appears to have a 30‐fold smaller diffusional resistance than the dermis. Compared with the synthetic membranes, diffusion through eschar is 500 times faster than silicone and 1500 times faster than through Carbosil.
Figure 2.
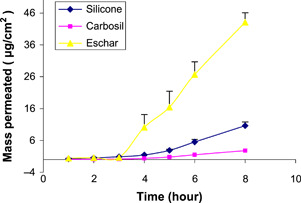
Permeation of sulphadiazine through eschar (▴), silicone (♦) and Carbosil (▪) (n = 5± SD).
Table 1.
Parameters for sulphadiazine permeation across all membranes and P 1 and P 2 calculated from the Equation 1 (n = 5± SD). Q 8 is mass permeated per unit area at 8 hours
| Membrane | Q 8 | P 1 | P 2 |
|---|---|---|---|
| Eschar | 43·5 ± 8·8 | 1·45 ± 0·077 | 0·051 ± 0·0044 |
| Silicone | 10·64 ± 1·18 | 1·08 ± 0·048 | 0·039 ± 0·005 |
| Carbosil | 2·97 ± 0·35 | 0·81 ± 0·029 | 0·031 ± 0·003 |
The difference between permeation rates is related to the structure and physicochemical properties of the membranes. The second synthetic membrane that was used in this study, Carbosil, is a polydimethylsiloxane–polycarbonate block copolymer that has previously been used as skin‐imitating permeation barriers (12). Feldstein et al. described the steady‐state permeation of a number of drugs through both stratum corneum and Carbosil membrane as a solubility–diffusion process. These authors also reported that Carbosil exhibits a considerably more polar diffusion medium than the stratum corneum. Stratum corneum is a lipophilic permeation barrier, and Carbosil exhibits rather an amphiphilicity or very moderate hydrophilicity. The unique morphology of the stratum corneum creates a highly tortuous diffusion path, and it has a low free volume available for diffusion in comparison with Carbosil.
In this study, the permeability coefficient and flux of sulphadiazine across eschar tissue are significantly higher than those across Carbosil and silicone. The permeability coefficient through silicone is also higher than Carbosil. Other hydrophobic membranes such as polyethylene have been shown to be impermeable to silver and sulphadiazine (17). The elastomeric nature of the silicone and the partitioning characteristics of both silicone and Carbosil provide a diffusional barrier that is significantly lower than that for polyethylene. The results are best explained in terms of the diffusion coefficients and partition behaviour. For eschar, the partition coefficient is approximately 10 (Table 2), which would suggest that the diffusing species is experiencing an environment that is slightly lipophilic. This would be commensurate with a tissue resembling an aqueous protein gel.
Table 2.
The effective membrane penetration parameters for sulphadiazine (n = 5± SD)
| Membrane | D eff (cm2/h) | k p (cm/h) | Flux (μg/cm2/h) | T lag (h) | K eff |
|---|---|---|---|---|---|
| Eschar | 0·0011 ± 0·0002 | 0·074 ± 0·01 | 5·86 ± 0·79 | 3·28 ± 0·28 | 9·66 ± 0·61 |
| Silicone | 2·12 E‐06 ± 1·03E‐07 | 0·044 ± 0·0045 | 3·34 ± 0·31 | 4·27 ± 0·16 | 154 ± 8·95 |
| Carbosil | 7·29 E‐07 ± 1·98 E‐08 | 0·023 ± 0·002 | 1·82 ± 0·18 | 5·27 ± 0·15 | 162 ± 11·37 |
Partitioning into the two synthetic membranes is much higher with a log (distribution coefficient) ∼2·2. The logD value for sulphadiazine (as opposed to SSD) between octanol and water at pH 6·4 is −0·13 (19). Although the analytical technique does not monitor the silver component, these results suggest that the diffusion of the species through the membrane is of the complex rather than the free sulphadiazine. The diffusion through eschar is three orders of magnitude greater than for silicone reflecting the ‘open’ nature of the tissue compared with silicone. In Carbosil, the diffusion coefficient is three times lower than that of silicone because of the more rigid nature of this polymer. The better partitioning behaviour, slower diffusion coefficients and different diffusional pathlengths compensate each other to provide overall steady‐state fluxes, which are not too dissimilar for the three membranes.
Conclusions
The barrier properties of eschar tissue to SSD are significantly less than those of the model membranes, silicone and Carbosil. The mechanism of drug transport within eschar tissue versus synthetic membranes requires more investigation with a wider range of permeants. Future studies will involve a comprehensive mechanistic evaluation of drugs with a wider range of molecular weight, lipophilicity or hydrophilicity to identify which drugs are suitable candidates for topical therapy of such tissue.
References
- 1. Garner JP, Heppell PSJ. Cerium nitrate in the management of burns. Burns 2005;31:539–47. [DOI] [PubMed] [Google Scholar]
- 2. White J, Thomas J, Maass DL, Horton JW. Cardiac effects of burn injury complicated by aspiration pneumonia‐induced sepsis. Am J Physiol Heart Circ Physiol 2003;285:H47–58. [DOI] [PubMed] [Google Scholar]
- 3. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 2006;19:403–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams WG. Pathophysiology of burn wounds. In: Herndon DN, editor. Total burn care. London: Saunders, 2002:514–22. [Google Scholar]
- 5. Fitzwater J, Purdue GF, Hunt JL, O’Keefe GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma 2003;54:959–66. [DOI] [PubMed] [Google Scholar]
- 6. Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg 2002;183:445–56. [DOI] [PubMed] [Google Scholar]
- 7. Ross DA, Phipps AJ, Clarke JA. The use of cerium nitrate‐silver sulphadiazine as a topical burns dressing. Br J Plast Surg 1993;46:582–4. [DOI] [PubMed] [Google Scholar]
- 8. Naoum JJ, Roehl KR, Wolf SE, Herndon DN. The use of homograft compared to topical antimicrobial therapy in the treatment of second‐degree burns of more than 40% total body surface area. Burns 2004;30:548–51. [DOI] [PubMed] [Google Scholar]
- 9. Iervolino M, Raghavan SL, Hadgraft J. Membrane penetration enhancement of ibuprofen using supersaturation. Int J Pharm 2000;198:229–38. [DOI] [PubMed] [Google Scholar]
- 10. Pellet MA, Watkinson AC, Hadgraft J, Brain KR. An ATR‐FTIR investigation of the interaction between vehicles and a synthetic membrane. Proc Int Symp Control Rel Bioact Mater 1994;21:439–40. [Google Scholar]
- 11. Nastruzzi C, Esposito E, Pastesini C, Gambari R, Menegatti E. Comparative study on the release kinetics of methyl‐nicotinate from topic formulations. Int J Pharm 1993;90:43–50. [Google Scholar]
- 12. Feldstein MM, Raigorodskii IM, Iordanskii AL, Hadgraft J. Modeling of percutaneous drug transport in vitro using skin‐imitating Carbosil membrane. J Control Release 1998;52:25–40. [DOI] [PubMed] [Google Scholar]
- 13. De Gracia CG. An open study comparing topical silver sulfadiazine and topical silver sulfadiazine‐cerium nitrate in the treatment of moderate and severe burns. Burns 2001;27:67–74. [DOI] [PubMed] [Google Scholar]
- 14. Atiyeh BS, Hayek SN, Gunn SW. New technologies for burn wound closure and healing–review of the literature. Burns 2005;31:944–56. [DOI] [PubMed] [Google Scholar]
- 15. Kasting GB, Filloon TG, Francis WR, Meredith MP. Improving the sensitivity of in vitro skin penetration experiments. Pharm Res 1994;11:1747–54. [DOI] [PubMed] [Google Scholar]
- 16. Okamoto H, Komatsu H, Hashida M, Sezaki H. Effects of [beta]‐cyclodextrin and di‐O‐methyl‐[beta]‐cyclodextrin on the percutaneous absorption of butylparaben, indomethacin and sulfanilic acid. Int J Pharm 1986;30:35–45. [Google Scholar]
- 17. Tsipouras N, Rix CJ, Brady PH. Passage of silver ions through membrane‐mimetic materials, and its relevance to treatment of burn wounds with silver sulfadiazine cream. Clin Chem 1997;43:290–301. [PubMed] [Google Scholar]
- 18. Walker M, Hulme TA, Rippon MG, Walmsley RS, Gunnigle S, Lewin M, Winsey S. In vitro model(s) for the percutaneous delivery of active tissue repair agents. J Pharm Sci 1997;86:1379–84. [DOI] [PubMed] [Google Scholar]
- 19. Yamazaki M, Kakeya N, Morishita T, Kamada A, Aoki M. Biological activity of drugs. XI. Relation of structure to the bacteriostatic activity of sulfonamides. (2). Chem Pharm Bull 1970;18:708–14. [DOI] [PubMed] [Google Scholar]


