Abstract
The role of tumour necrosis factor‐alpha (TNF‐α) in wound healing is not clear. Elevated levels of TNF‐α have been observed in fluids from chronic wounds and have been shown to decrease over time during the healing process. Therapeutic antibodies such as infliximab can inhibit TNF‐α activity. In this case series, we applied infliximab topically to eight patients with chronic ulcers of more than 4‐month durations. The ulcers had multifactorial aetiology, with chronic venous insufficiency being the most prominent factor. All the ulcers had failed to respond to any previous conventional treatment. Infliximab was applied repeatedly to ulcers either as a 10 mg/ml solution and covered with an adhesive sheet or as a gel formulation (0·45, 1, or 4·5 mg/g) under a hydrofiber dressing/adhesive sheet. Improvement was assessed by measuring the percentage of change in the ulcer surface area. Seven of the eight patients (12 of 14 ulcers) responded to treatment with infliximab. After 4 weeks of treatment, surface area was reduced by more than 50% in 6 of the 14 treated ulcers. Within 8 weeks, five ulcers completely healed, while another four were reduced by more than 75% in size. Chronic, therapy‐resistant leg ulcers responded well to repeated topical administration of a solution or a gel containing the TNF‐α antibody, infliximab. Randomised controlled studies should be conducted to further evaluate the effect of topical infliximab on chronic wound healing.
Keywords: Chronic ulcer, Infliximab, Topical treatment, Tumour necrosis factor‐alpha, Wound healing
Introduction
Wound healing is a dynamic and complex process involving interactions among soluble cytokines, blood constituents, extracellular matrix and cells (1). An acute wound generally heals within 2–4 weeks in a sequence of stages, including haemostasis/inflammation, proliferation and tissue remodelling. Biologically active substances, such as cytokines and growth factors, control each of these stages. A wound is considered to be chronic if it has not healed (epithelialised) within the expected physiological healing time (2). Dissemond et al. (3) defined a chronic wound as one that shows no tendency to heal within 3 months despite adequate treatment or as one that has not healed spontaneously within 12 months.
Studies comparing the cytokine patterns of acute and chronic wounds have shown that certain proinflammatory cytokines are elevated in chronic wounds. Tarnuzzer and Schultz (4) demonstrated that the levels of tumour necrosis factor‐alpha (TNF‐α) in fluid from chronic wounds were approximately 100‐fold higher than those in fluid from mastectomy incisions. Moreover, it has been shown that TNF‐α upregulates matrix metalloproteinase (MMP) production in fibroblasts, while it inhibits the tissue inhibitor of these proteolytic enzymes 5, 6, 7. These findings suggest that the presence of high levels of TNF‐α may inhibit wound healing. Infliximab is a chimeric immunoglobulin (Ig)G1 monoclonal antibody that binds with high affinity and specificity to the soluble and transmembrane TNF‐α. Infliximab has been shown to be effective in treating immune‐mediated inflammatory diseases such as rheumatoid arthritis 8, 9, 10, Crohn’s disease (11) and ankylosing spondylitis 12, 13. Infliximab is administered intravenously for all labelled indications. Neither topical application nor systemic use of infliximab to treat chronic wounds has been reported in the literature. Here, we report a case series of patients with chronic ulcers, who received topical infliximab therapy because conventional therapies had not been effective in wound healing.
Materials and methods
Eight patients with 14 chronic wounds (12 leg ulcers and 2 scrotal ulcers) were treated. Six leg ulcers (cases 4–7) were of venous origin. Chronic venous insufficiency (CVI) was a relevant aetiological factor in four other leg ulcers of multifactorial (case 1) or unclear (case 2) aetiology. The paraplegic patient (case 3) had two leg ulcers due to chronic traumatisation and a bleeding disorder. The scrotal ulcers appear to have been possibly artificially induced (case 8). All the wounds failed to respond to treatment of the underlying disease and to optimal, conventional, local wound care, including compression therapy. The wounds were present for more than 4 months (range: 4 months to 30 years), and the wound size ranged from 1 to 300 cm2. Written informed consent was procured from all the patients prior to treatment with infliximab. Infliximab was applied topically either as a solution (10 mg/ml) or as a gel formulation (0·45, 1 or 4·5 mg/g). Ulcers that were treated with the solution were subsequently covered with an adhesive sheet that remained on the wound for 24 hours. If the gel formulation was applied, a hydrofiber dressing or a transparent film was used to cover the ulcer for 24 hours.
The application of infliximab solution was repeated after 3–4 weeks. The gel formulation allowed application to be much easier to handle and was applied either once or twice a week. In all cases, conventional treatment was concomitantly continued.
Improvement was assessed by measuring the percent change (reduction) in ulcer area. Ulcer size was calculated by measuring the diameters of the wounds either in vivo or on photographs.
In case 1, 24 hours after the first application of infliximab solution, a punch biopsy was taken from the ulcer edge. In a few cases, punch biopsies were taken at 24 and 48 hours and 2 weeks after the application of infliximab. In all biopsies, direct immunofluorescence staining was performed on frozen tissue sections after incubation with antibody CNTO 1016 (a mouse IgG1, kappa monoclonal antibody provided by Centocor Inc., Horsham, PA), which specifically recognises the variable region of infliximab. CNTO 1016–infliximab complexes in the tissue were detected using a polyclonal rabbit anti‐mouse IgG‐fluorescein isothiocyanate (rabbit Fab′)2 from Dako (Copenhagen, Denmark) in a 1:200 dilution.
Case reports
Case 1
An 83‐year‐old woman was referred to our clinic with a 20‐cm2 V‐shaped ulcer, with an exposed tendon on the right lateral calf (Figure 1A). The ulcer had formed 16 months prior to admission. A split skin graft was attempted 13 months prior to admission but was not taken. Conventional treatment, for more than 10 months, with hydrocolloids, non adherent dressings and low compression was also unsuccessful. Another painful 6‐cm2 ulcer developed on the left lateral calf approximately 4 months prior to admission. Clinical signs of CVI such as oedema, dermatosclerosis and atrophie blanche were observed due to insufficient superficial veins. A biopsy from the edge of the second ulcer revealed only signs of CVI. However, several other possible aetiological factors were present. The patient had a history of diabetes, with polyneuropathy and macroangiopathy on the left leg. Angiography showed occlusion of the anterior and posterior tibial artery and relevant stenosis of the fibular artery of the left leg. On the right side, the tibiofibular trunk was completely occluded but with a sufficient collateralisation and an ankle brachial index score of more than 0·8. The patient also had polycythaemia vera and had been treated with hydroxyurea for 17 months.
Figure 1.
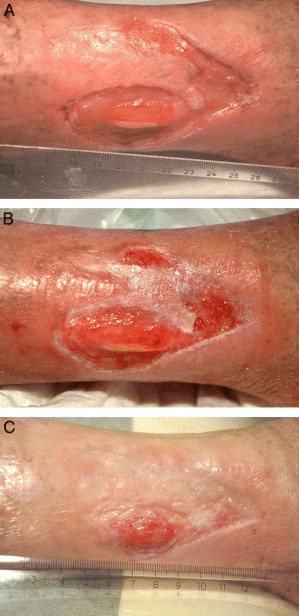
Case 1: (A) ulcer before treatment with infliximab solution, (B) ulcer 3 weeks after first application of infliximab solution, (C) ulcer 12 weeks after first infliximab application (3 weeks after second application of solution).
In order to treat the underlying disease, high compression therapy with Unna’s boots was initiated, and angioplasty was performed on the left leg. Hydroxyurea, which is known for its ulcerogenic potential, was discontinued. A swab sample from the ulcer showed the presence of Pseudomonas, Staphylococcus aureus and Streptococcus, and this was treated with topical antiseptics. All these therapeutic efforts provided no improvement.
Infliximab was initially applied on the right leg ulcer as a solution (10 mg/ml) under an adhesive film dressing, which remained in place on the wound for 24 hours. Local wound care and compression were continued after treatment with infliximab. Within 3 weeks, approximately one‐quarter of the wound reepithelialised (Figure 1B). A second application of infliximab solution was on the right leg ulcer at week 3. In addition, infliximab solution was applied on the left leg ulcer, which closed completely within 8 weeks. The more chronic ulcer on the right leg achieved a nearly 90% reduction in wound area within 12 weeks (Figure 1C). The patient received a third application of infliximab solution on the right leg at week 13. Despite alternative efforts including transplantations, a complete closure of this ulcer was not achieved, and after 1 year, the wound size remained stable at less than 2 cm2.
Case 2
A 72‐year‐old woman had large chronic ulcers of unclear origin on both legs for approximately 18 months. CVI was an aetiological factor; however, allergic contact dermatitis from substances used for local treatment appeared to be a significant contributing factor to an eczematous inflammatory process on both shins. Acute deep thrombosis in both legs was diagnosed shortly after referral to our clinic. Anticoagulation therapy was initiated, which led to bleeding in the granulation tissue and impaired healing. Both compression therapy and alginate dressings were ineffective, and new erosions occurred in the inflamed skin surrounding the ulcers. Histology results demonstrated signs of CVI, although no other underlying diseases were identified.
Infliximab was initially applied as a gel (4·5 mg/g) to the circumferential superficial ulcers and erosions of the left leg (initial size of approximately 200 cm2; Figure 2A). The gel was covered with a hydrofiber dressing for 24 hours, and conventional local treatment was continued thereafter. The surface area of the ulcer was reduced by approximately 50% within 1 week. Infliximab gel was applied again at week 2. By week 3, approximately 75% of the ulcer surface area had healed (Figure 2B). The superficial ulcer on the right leg (covering approximately 50 cm2) healed completely within 3 weeks after a single application of infliximab gel.
Figure 2.
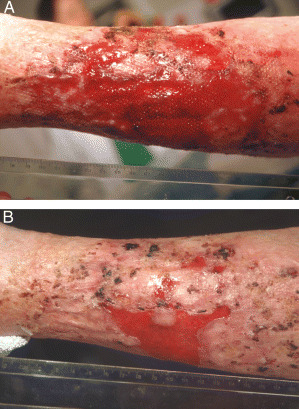
Case 2: (A) ulcerative lesions before treatment with infliximab gel, (B) ulcer 3 weeks after infliximab (two applications of the gel).
Seven weeks after the initial referral, the patient presented with new erosions covering the circumference of the left calf (approximately 300 cm2) and the dorsal aspect of the right calf (approximately 60 cm2). Infliximab gel (1 mg/g for 24 hours with a hydrofiber dressing) was applied twice a week. After 8 weeks of treatment, the right leg ulcer was completely closed. The left leg ulcers were 95% closed (of the initial erosive area of 300 cm2) after 12 weeks.
However, while the ulcerations showed a rapid wound closure after application of infliximab gel, new erosions developed around the treated lesions, with the initial aspect of large subepidermal pustules. New histology data demonstrated neutrophil dermatosis with formation of subepidermal pustules. We strongly suggest that this patient suffered from a disseminated superficial form of pyoderma gangrenosum. Topical treatment with infliximab proved to be beneficial for the ulcers but did not result in complete healing. A treatment with systemic steroids was initiated (prednisone 0·5 mg/kg daily). Five months post‐steroid treatment, all the ulcers were closed and no new pustules observed.
Case 3
A 50‐year‐old paraplegic man had small ulcers (2–3 cm2) on both lateral malleoli for approximately 4 years. Repeated trauma was responsible for the tissue injury, and a congenital factor VII deficiency led to instant bleeding. Dependency oedema impaired wound healing. Mild compression therapy was initiated, but the ulcers were refractory to treatment. Apligraf® (Organogenesis Inc., Canton, MA) transplantation was unsuccessful. A full‐thickness skin graft was not taken on both legs, and suction blister transplantations led only to partial, temporary success, with ulcers of 1 cm2 persisting for 12 weeks.
Infliximab was initially applied as a solution (10 mg/ml) under an adhesive sheet for 24 hours, and conventional local treatment was continued thereafter. After 3 weeks, the ulcer on the left lateral malleolus was reduced by more than 80%. Infliximab gel (4·5 mg/g) was applied at week 4, and the ulcer was completely closed by week 8. With the identical treatment, the ulcer on the right malleolus showed a similar tendency for healing. However, after repeated traumatisation of the right malleolus, the ulcer increased to 3 cm2. Three weeks of ambulatory, vacuum‐assisted, closure therapy did not result in improvement. After an additional 16 weeks of treatment with infliximab gel (1 mg/g twice a week), the ulcer was reduced to 0·8 cm2.
Case 4
An 87‐year‐old woman had a large venous ulcer for approximately 10 years, which encompassed the full circumference of the left shin. A split skin graft, which was transplanted 3 months prior to admission, was not taken. The wound worsened due to insufficient compression treatment and bacterial infection. In our institution, treatment with systemic antibiotics and high compression therapy was initiated, leading to a granulating wound bed. After failure of a split skin transplant, the patient refused additional surgical treatment.
Infliximab gel of different concentrations (0·45 and 4·5 mg/g for 24 hours under a hydrofiber dressing) was applied at weeks 0, 1 and 3 on the medial aspect of the calf (initial size of approximately 120 cm2). By week 12, 75% of the ulcer was epithelialised. During the same treatment period, Allox® (EpiSource/IsoTis SA, Lausanne, Switzerland) (a mixture of growth‐factor‐producing fibroblasts and keratinocytes in a fibrin spray) was applied on the other side of the ulcer. After another 6 months, the ulcer completely closed by using high compression therapy and hydrofiber dressings.
Case 5
An 81‐year‐old woman had venous ulcers on the medial and lateral malleolus of the right leg for approximately 1 year. Suction blister transplantation 7 months prior to admission had only led to a partial closure of the medial ulcer. Despite high compression therapy and systemic antibiotics, the ulcer did not improve.
Infliximab was applied as solution (10 mg/ml), with an adhesive sheet for 24 hours on both ulcers, and conventional local therapy was continued. At weeks 4 and 5, infliximab gel (0·45 and 4·5 mg/g, respectively) was applied on the wound and remained in place for 24 hours. The smaller ulcer (initial size of 7 cm2) on the medial side of the leg showed a reduction of more than 50% within 8 weeks. The larger ulcer (initial size of 17 cm2) on the lateral malleolus showed a slight reduction in size after 4 weeks. However, bacterial overload (Pseudomonas) after another 2 weeks impaired wound healing. The patient was hospitalised and treated with intravenous systemic antibiotics. Mesh grafts were transplanted on the remaining wounds, which led to complete closure within 12 weeks.
Case 6
An 86‐year‐old man presented with venous ulcer on the left medial malleolus existing for approximately 30 years. The patient refused surgical treatment. No local treatment improved healing, and Epidex® (Euroderm GmbH, Leipzig, Germany) transplantation was only partially successful. Infliximab solution (10 mg/ml) was initially applied on the ulcer (initial size of 13 cm2) with an adhesive sheet that remained in place for 24 hours. Infliximab gel was applied at week 4 (0·45 mg/g) and week 5 (4·5 mg/g). No change in ulcer aspect or ulcer area was observed after 8 weeks.
Case 7
A 77‐year‐old woman had been treated in our wound care outpatient unit for venous ulcers on both medial malleoli for approximately 4 years. Forty years prior to admission, the patient had recurrent phlebothrombosis in both legs. Since that time, the patient was receiving oral anticoagulation therapy with phenprocoumon. Ulcers occurred on the right medial malleolus shortly after the first thrombosis and then appeared on the left medial malleolus approximately 10 years later. Split skin transplantations were performed in the right leg 6 and 2 years prior to admission but were unsuccessful, and the ulcer never closed completely. In the year prior to admission, a 16‐cm2 ulcer developed and persisted in spite of various local treatments. Infliximab gel, 1 mg/g, with a hydrofiber dressing held in place for 24 hours and administered once a week, was initiated while established local therapy continued. After 8 weeks, the wound reduced in size and a strong edge effect was observed. In the meantime, a additional 14‐cm2 ulceration developed on the left malleolus in an area of inflamed atrophy blanche. The patient was admitted to the hospital, and infliximab gel (1 mg/g) was applied to both ulcers twice a week. After 8 weeks, the ulcer on the right leg decreased by more than 75%, while the ulcer on the left leg decreased by more than 85%.
Case 8
A 73‐year‐old man had pruritus of unknown origin for more than a year. Itching was most intense in the genital region, where continuous scratching had led to ulcers on both sides of the scrotum. Microbiological examinations, histology and serology were negative for an infectious or Behçets disease. Local wound care with mupirocin cream and hydrocolloid dressings did not improve healing. Infliximab gel was applied to the deeper ulcer (initial size of 5 cm2) on the left side of the scrotum twice a week with an adhesive film sheet for 24 hours. The ulcer completely healed within 4 weeks. The ulcer on the right side of the scrotum (initial size of 9 cm2) was initially treated with silver‐coated hydrofiber but did not heal. Because of the favourable outcome of the ulcer on the left side, infliximab gel was applied twice weekly, in the same manner, to the ulcer on the right side, which then healed within 2 weeks.
Results
Five of the 14 therapy‐resistant ulcers completely healed within 4–8 weeks (as shown in Table 1). Four of the remaining ulcers showed at least a 75% reduction in size within 8 weeks. Two other ulcers showed no reduction in wound size after 8 weeks, but only one failed to show any observable reaction to treatment. All the patients tolerated the treatment well, with no reported systemic or local side effects.
Table 1.
Results of the treatment
| Case number | Ulcer location | Infliximab application | Ulcer size (cm2) | Adverse reactions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 1 | Week 2 | Week 3 | Week 6 | Week 8 | Week 12 | ||||
| 1 | Right lateral calf | 3 ml of 10 mg/ml solution at weeks 0, 3 and 13 | 20 | 19 | 16 | 13·5 | 5 | 3·2 | 2 | None |
| Left lateral calf | 1 ml of 10 mg/ml solution at weeks 0 and 3 | 6 | 3 | 2·5 | 1·8 | 0·1 | 0 | 0 | None | |
| 2 | Semi‐circular ulcer on left calf | 4·5 mg/g gel at weeks 0 and 2 | 200 | 100 | 60 | 40 | Relapse | None | ||
| Right dorsal calf | 4·5 mg/g gel at week 0 | 50 | 20 | 2 | 0 | Relapse | None | |||
| Circular ulcer on left calf (second ulcer) | 1 mg/g gel twice a week | 300 | 280 | 220 | 160 | 50 | 20 | 10 | None | |
| Right dorsal calf (second ulcer) | 1 mg/g gel twice a week | 60 | 70 | 70 | 50 | 8 | 0 | 0 | None | |
| 3 | Left lateral malleolus | 0·5 ml of 10 mg/ml solution at week 0 and 4·5 mg/g gel at week 4 | 1 | 0·7 | 0·3 | 0·175 | NA | 0 | 0 | None |
| Right lateral malleolus | 0·5 ml of 10 mg/ml solution at week 0 and 4·5 mg/g gel at week 4 | 1 | 0·7 | 0·3 | 0·175 | NA | 0·2 | 0·8 | None | |
| 4 | Left shin full circumference | One side of ulcer treated with 0·45 mg/g gel at weeks 0 and 3 and 4·5 mg/g gel at week 1 | 120 | 120 | 100 | 80 | NA | 60 | 30 | None |
| 5 | Right lateral malleolus | 2 ml of 10 mg/ml solution at week 0, 0·45 mg/g gel at week 4 and 4·5 mg/g gel at week 5 | 17 | 17 | 15 | 15 | 16 | 16 | 18 | None |
| Right medial malleolus | 2 ml of 10 mg/ml solution at week 0, 0·45 mg/g gel at week 4 and 4·5 mg/g gel at week 5 | 7 | 5 | 4 | 4 | 4 | 3 | 2·6 | None | |
| 6 | Left medial malleolus | 2 ml of 10 mg/ml solution at week 0, 0·45 mg/g gel at week 4 and 4·5 mg/g gel at week 5 | 13 | 13 | 13 | 13 | 12 | 12 | 12 | None |
| 7 | Right medial malleolus | 1 mg/g gel once weekly until week 12 and twice weekly after week 12 | 16 | 16 | NA | 14 | 12 | 12 | 10 | None |
| Left medial malleolus | 1 mg/g gel twice weekly | 10 | 10 | 8 | 5 | 3 | 1·5 | 0·3 | None | |
| 8 | Left side of scrotum | 1 mg/g gel twice weekly | 5 | 2 | 1 | 0·5 | 0 | None | ||
| Right side of scrotum | 1 mg/g gel twice weekly | 9 | 5 | 0 | 0 | 0 | None | |||
NA, not applicable.
Localisation of infliximab antibody at the sites of wound closing, verified via direct immunofluorescence staining of skin biopsies (patients 1 and 4), resulted in a positive reaction to antibody complexes between the infliximab‐specific monoclonal antibody CNTO 1016 and the infliximab, in the upper layers of the treated wound bed, in the wall of dermal vessels (Figure 3A) and along the basal membrane of the epidermis (Figure 3B).
Figure 3.
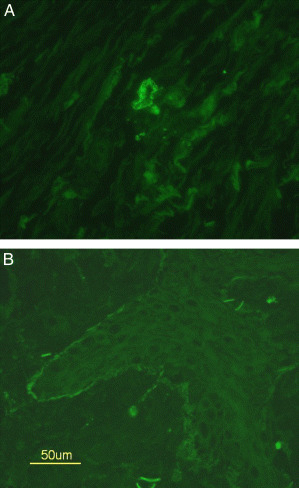
Case 1: (A) positive reaction to mouse anti‐infliximab antibody deposits around dermal vessel in direct immunofluorescence staining, indicating infliximab presence in the ulcer tissue, (B) positive reaction along basal membrane, indicating infliximab in the tissue.
Discussion
TNF‐α is produced by macrophages during the normal inflammatory process and in response to tissue injury. TNF‐α induces macrophages to produce interleukin (IL)‐1, which is mitogenic for fibroblasts (4). Both TNF‐α and IL‐1 upregulate the production of MMP 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14. The family of these zinc‐dependent proteases includes collagenases, gelatinases and stromelysins (14), which are responsible for degrading the extracellular matrix in the remodelling process, improving the tensile strength of the repaired tissue. While TNF‐α stimulates production of MMPs, it suppresses expression of the natural inhibitor of these proteases, the tissue inhibitor of metalloproteinases (TIMP) 5, 7.
The in vivo and in vitro data on the role of TNF‐α in wound healing have been controversial. Mooney et al. (15) reported that local application of TNF‐α increased disruption strength in incisional wounds and eventually accelerated wound healing in rats. In contrast, Rapala et al. (16) and Salomon et al. (17) reported that local application of TNF‐α solution impaired wound healing. Impairment of wound healing was also found after inoculation of TNF‐α‐producing hamster ovary cells in mice (18). Overproduction of TNF‐α has been associated with chronic wounds (4), and levels of TNF‐α, IL‐1 and IL‐6 decreased when the wound began to heal. Furthermore, MMPs have been shown to have higher activity in chronic wounds than in acute wounds (19). Conversely, TIMP, the natural inhibitors of MMPs, are diminished in chronic wounds. Since TNF‐α upregulates MMP production in fibroblasts and inhibits the expression of TIMP, it may be possible that elevated TNF‐α levels in chronic wounds lead to uncontrolled MMP activity.
TNF‐α is also involved in angiogenesis, which is indispensable in the formation of granulation tissue during the proliferative phase of wound healing. Mori et al. (20) examined the biological role of tumour necrosis factor (TNF) receptor p55 (TNF‐Rp55) in wounds of TNF‐Rp55‐deficient mice. These mice showed enhanced angiogenesis and collagen accumulation along with reduced infiltration of neutrophils and macrophages compared with wild‐type mice. Wound healing was accelerated in TNF‐Rp55−/− mice than in wild‐type mice. The lack of TNF‐Rp55 may enhance gene expression of fibrogenic factors and vascular endothelial growth factor receptors in skin wounds, thereby promoting angiogenesis and collagen accumulation.
TNF‐α is also known to be involved in apoptotic processes in inflammatory cutaneous diseases via activation of Nuclear Factor Kappa B (NFkB) (21). In normal wound healing, apoptosis is responsible for removal of inflammatory cells and evolution of granulation tissue into scar. Dysregulation in apoptosis could lead to abnormal wound healing (22).
To investigate whether the activity of TNF‐α is likely to contribute to delayed healing, Wallace and Stacey assessed immunoreactive versus bioactive TNF‐α levels in chronic venous leg ulcers (23). Even though the levels of immunoreactive TNF‐α were significantly higher in wound fluid from non healing ulcers than in wound fluid from healing ulcers, the levels of bioactive TNF‐α were not significantly different between the healing phases. The investigators concluded that TNF‐α‐mediated events do not seem to be key events contributing to impaired healing in chronic ulcers.
Despite the conflicting results concerning the role of TNF‐α in wound healing, the clinical data in this case series appear to suggest that the topical application of the TNF‐α antibody, infliximab, had a beneficial impact on wound healing. We provide the first clinical evidence that wound healing may be improved by inhibition of TNF‐α, an observation that has already been confirmed in animals by Regan et al., who observed the beneficial effects of rabbit anti‐TNF‐α serum in murine skin wounds (24). Although the mode of action is not yet clear, we hypothesise that inhibition of TNF‐α diminishes the expression of MMPs and may promote angiogenesis and/or affect apoptosis of inflammatory cells.
Furthermore, the results from our cases suggest that different modes of topical application of infliximab (e.g. solution or gel) appear to be equivalent in clinical effectiveness. We found evidence of infliximab presence in the tissue, by immunostaining, irrespective of the mode of application. Infliximab was found along the basal membrane of the epidermis and in the vessel walls from skin biopsies of ulcers in treated patients. This pattern of distribution requires further investigation.
For practical application purposes, the gel was easier to handle, particularly in an outpatient wound care facility. Some patients showed a dramatic positive response to infliximab treatment and an acceleration of wound closure after just one application. However, repeated application of infliximab in most patients resulted in the best clinical response. We observed good results when the gel, applied twice a week, remained on the wound for 24 hours and was covered by an adhesive sheet (Tegaderm®, 3M, St Paul, MN). However, the optimal dosing scheme needs to be determined.
Topical application of infliximab as solution or as gel was well tolerated by the patients; no systemic or local side effects were observed. As expected, the topical application of infliximab showed no systemic side effects, while it rapidly and effectively delivered the antibody into the wound. Interestingly, infliximab application did not affect the bacterial burden of the ulcers. A bacterial imbalance was diagnosed in 10 of the 14 ulcers and was treated with local antiseptics or systemic antibiotics before infliximab treatment was initiated. Even after repeated application of infliximab, we did not observe a higher rate of bacterial burden. One exception was noted in case 5 where infliximab was applied to an ulcer that was previously colonised with Pseudomonas. After treatment with infliximab, Pseudomonas was again identified.
In conclusion, preliminary data indicate that the topical administration of infliximab on therapy refractory wounds is safe and effective. Further investigation is necessary to demonstrate how the cytokine pattern in chronic wounds is altered by the inhibition of TNF‐α. In addition, randomised controlled studies are needed to determine the safety and efficacy of topical infliximab treatment of chronic wounds.
Acknowledgements
This case series is an observational, actual use, community experience description. Study medication was provided by Essex Chemie AG, Luzern, Switzerland. No funding was provided for this work. The authors would like to thank Dr Anastasia Papandrikopoulou of Centocor, for her contributions to this work; Scott Newcomer of Centocor, for editorial support and Essex Chemie for kindly providing the drug for ‘off‐label use’.
References
- 1. Singer A, Clark R. Cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 2. Gillitzer R. Modernes Wundmanagement. Hautarzt 2002;53:130–47. [DOI] [PubMed] [Google Scholar]
- 3. Dissemond J, Witthoff M, Brauns T, Haberer D, Goos M. pH values in chronic wounds, evaluation during modern wound treatement. Hautarzt 2003;54:959–65. [DOI] [PubMed] [Google Scholar]
- 4. Tarnuzzer R, Schultz G. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–5. [DOI] [PubMed] [Google Scholar]
- 5. Ito A, Sato T, Iga T, Mori Y. Tumor necrosis factor bifunctionally regulates matrix metalloproteinases and tissue inhibitor of metalloproteinases (TIMP) production by human fibroblasts. FEBS Lett 1990;269:93–5. [DOI] [PubMed] [Google Scholar]
- 6. Murphy G, Willenbrock F, Crabbe T, O’Shea M, Ward R, Atkinson S, O’Connell J, Docherty A. Regulation of matrix metalloproteinase activity. Ann N Y Acad Sci 1994;732:31–41. [DOI] [PubMed] [Google Scholar]
- 7. Han Y, Nien Y, Garner W. TNF‐alpha induced proteolytic activation of pro‐MMP‐9 by human skin is controlled by down‐regulating TIMP‐1 and mediated by tissue‐associated chymotrypsin‐like proteinase. J Biol Chem 2002;277:27319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maini RN, St Clair EW, Breedveld F, Furst DE, Kalden JR, Weisman M, Smolen JS, Emery P, Harriman GR, Feldmann M, Lipsky PE. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–9. [DOI] [PubMed] [Google Scholar]
- 9. Lipsky PE, Van Der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 2000;343:1594–602. [DOI] [PubMed] [Google Scholar]
- 10. Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, St Clair EW, Keenan GF, Van Der Heijde D, Marsters PA, Lipsky PE. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004;50:1051–65. [DOI] [PubMed] [Google Scholar]
- 11. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 12. Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, Gromnica‐Ihle E, Kellner H, Krause A, Schneider M, Sorensen H, Zeidler H, Thriene W, Sieper J. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187–93. [DOI] [PubMed] [Google Scholar]
- 13. Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, Golder W, Gromnica‐Ihle E, Kellner H, Schneider M, Sorensen H, Zeidler H, Reddig J, Sieper J. Long‐term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three‐month, randomized, placebo‐controlled trial. Arthritis Rheum 2003;48:2224–33. [DOI] [PubMed] [Google Scholar]
- 14. Herouvy Y, Trefzer D, Zimpfer U, Schöpf E, Vanscheidt W, Norgauer J. Matrix metalloproteinases and venous leg ulceration. Eur J Dermatol 2000;109:173–80. [PubMed] [Google Scholar]
- 15. Mooney D, O’Reilly M, Gamelli R. Tumor necrosis factor and wound healing. Ann Surg 1990;211:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rapala K, Laato M, Niinikoski J, Kujari H, Soder O, Mauviel A, Pujol JP. Tumor necrosis factor alpha inhibits wound healing in the rat. Eur Surg Res 1991;23:261–8. [DOI] [PubMed] [Google Scholar]
- 17. Salomon G, Kasid A, Cromack D, Director E, Talbot TL, Sank A, Norton JA. The local effects of cachectin/tumor necrosis factor on wound healing. Ann Surg 1991;214:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buck M, Houglum K, Chojkier M. Tumor necrosis factor‐alpha inhibits collagen alpha1 (I) gene expression and wound healing in a murine model of cachexia. Am J Pathol 1996;149:195–204. [PMC free article] [PubMed] [Google Scholar]
- 19. Trengove N, Stacey M, Macauley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 20. Mori R, Kondo T, Ohshima T, Ishida Y, Mukaida N. Accelerated wound healing in tumor necrosis factor receptor p55‐deficient mice with reduced leukocyte infiltration. FASEB J 2002;16:963–74. [DOI] [PubMed] [Google Scholar]
- 21. Victor F, Gottlieb A. TNF‐alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J Drugs Dermatol 2002;3:264–75. [PubMed] [Google Scholar]
- 22. Huang N, Zac‐Varghese S, Luke S. Apoptosis in skin wound healing. Wounds 2003;15:182–94. [Google Scholar]
- 23. Wallace H, Stacey M. Levels of tumor necrosis factor‐alpha and soluble TNF receptors in chronic venous leg ulcers – correlation to healing status. J Invest Dermatol 1998;110:292–6. [DOI] [PubMed] [Google Scholar]
- 24. Regan M, Kirk S, Hurson M, Sodeyama M, Wasserkrug H, Barbul A. Tumor necrosis factor‐alpha inhibits in vivo collagen synthesis. Surgery 1993;13:173–7. [PubMed] [Google Scholar]


