Abstract
This prospective, non comparative study evaluated the safety and effectiveness of an adhesive gelling foam dressing in pressure ulcer management. Twenty‐three subjects with exuding pressure ulcers were recruited from seven centres in the USA and Canada. Study treatment included an adhesive gelling foam dressing, optional tape/roll bandaging and mandatory pressure‐reducing/relieving devices. Subjects were followed until ulcer healing, for up to 28 days, or on patient withdrawal from the study, whichever came first. Dressings were changed at least once every 7 days. Mean percentage change in ulcer area from baseline to final measurement was −13%. Investigators reported healing or subjective improvement of ulcer condition in 61% of patients. Mean dressing wear time was 4·2 days. Subjects found the dressing was comfortable, soothing and cushioning in situ at 80%, 64% and 70% of dressing changes, respectively. Subjects reported pain severity of none or mild for every dressing change. Fourteen subjects experienced adverse events, including seven subjects with study‐related maceration, erythema, wound enlargement, blister or infection. A regimen including an adhesive gelling foam dressing proved to be safe and effective for managing exudate, protecting the surrounding skin, minimising pain and supporting healing of pressure ulcers with exudate.
Keywords: Exudate, Gelling foam, Healing, Pressure ulcer, Wound dressing
Introduction
Pressure ulcers (also known as decubitus ulcers) are among the most prevalent form of chronic wounds, with a reported prevalence in acute‐care settings of 10–18% in the United States (1) and 10–11% in the United Kingdom, Germany and the Netherlands (2). Pressure ulcers increase the clinical and economic burden of care by adding excess bed‐days to procedures such as hip surgery or coronary artery bypass graft surgery (2). In hospitalised patients, pressure ulcers have been reported to contribute mean excess charges of $10 845 for treatment, with a mortality rate 7·2% higher than that for hospitalised patients without pressure ulcers matched on diagnosis‐related group, age, gender and white/non white race (3). For these reasons, reimbursement authorities and government agencies around the world are focused on decreasing the burden of managing these challenging wounds.
Current concepts of wound healing and the treatment of pressure ulcers (4) have evolved since Winter’s seminal paper 46 years ago describing the influence of a moist environment on wound healing (5). Use of moist cover dressings is strongly associated with decreased pressure ulcer area (6). In addition to providing an environment conducive to wound healing, pressure ulcers require management of pressure, friction and sheer forces, especially over heels, trochanters and the sacrum. Pressure ulcer dressings also should provide patient comfort (7) and cushion the wound while managing excess exudates (8), thereby protecting the wound and surrounding skin from prolonged exposure to wound or body fluids (9).
An adhesive gelling foam wound dressing (GFD‐A; Versiva® XC™ Gelling Foam Dressing—Adhesive; ConvaTec, a Division of E.R. Squibb & Sons, L.L.C., Princeton, NJ) was developed to address exudate management needs and to provide a comfortable, moist wound‐healing environment on moderately to heavily exuding wounds. When used in appropriate pressure ulcer protocols of care, GFD‐A provides both protection and cushioning of the wound surface, allowing wound healing to progress with minimal maceration of the surrounding skin. The GFD‐A dressing includes the following components: a polyurethane film/foam outer surface layer, a hydrocolloid adhesive layer and an island of Hydrofiber® technology (ConvaTec) (Figure 1). The island is composed entirely of highly absorbent, non woven, sodium carboxymethylcellulose to provide moist wound healing and manage exudate. The hydrocolloid layer holds the Hydrofiber® technology island in place and gels on contact with wound fluid to further support moist wound healing and provide a cushioning environment. The outer surface is designed to provide additional cushioning and comfort in situ.
Figure 1.
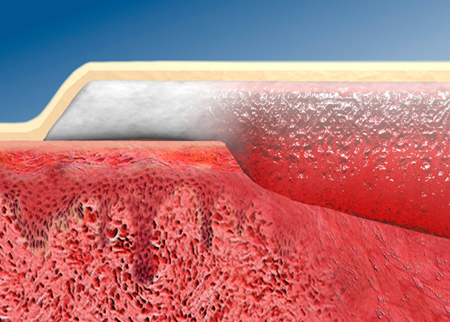
Cross section of adhesive gelling foam wound dressing (GFD‐A) at the edge of a wound. The Hydrofiber island (white) gels on contact with the wound bed to provide a moist wound healing environment (red) and manages exudate to minimise lateral spread of fluid to the periwound area. The hydrocolloid adhesive layer (tan) holds the dressing in place and gels on contact with wound fluid. The polyurethane film/foam outer surface layer (beige) also helps to cushion the wound. Picture reprinted with permission from E.R. Squibb & Sons, L.L.C.
This prospective multicentre study evaluated safety and performance of GFD‐A in managing subjects with an exuding pressure ulcer. The primary objective was assessment of safety, as measured by reported adverse events during study treatment. Secondary objectives were: (i) the evaluation of dressing performance, based on subject ratings of comfort and pain; and (ii) investigator ratings of ease of use, exudate management, leakage, adherence, trauma, ulcer condition, ulcer area and depth and appearance of the ulcer and surrounding skin.
Methods
Study design
This prospective, open‐label, multicentre, 4‐week study was conducted at six centres in the United States and one centre in Canada. Appropriate institutional review board (IRB) approvals were obtained in accordance with Good Clinical Practices and the principles contained within the Declaration of Helsinki. Patients were enrolled in physician‐supervised hospital or outpatient wound clinic settings, or practices of certified wound ostomy continence nurses (including one wound clinic and one consultant practice in various settings including home care, long‐term care and hospitals).
Materials
All subjects were managed with GFD‐A as the cover dressing. If wound filler was clinically necessary, AQUACEL® Hydrofiber Wound Dressing (ConvaTec) ribbon (2 cm × 45 cm) or dressing (9 cm × 9 cm) could be used. Clinicians were permitted to use any skin barrier cream or securing aids, such as conforming roll bandages. Use of a pressure‐relieving or pressure‐reducing device was mandatory, but the choice of device was at the clinician’s discretion.
Study eligibility
To enrol in the study, subjects were required to have a NPUAP stage II (partial thickness) pressure ulcer at least 2 cm2 in area or an NPUAP stage III or IV (full thickness) pressure ulcer of any area up to the maximum dimensions of the study dressing. Subjects with an NPUAP stage I pressure ulcer (i.e. a non broken, non exuding ulcer) were excluded regardless of ulcer area. Stage II pressure ulcers less than 2 cm2 in area were excluded. Stages II–IV pressure ulcers were excluded if their maximum dimensions were greater than 11·6 cm × 15·5 cm, which are the maximum dimensions of the absorbent island on GFD‐A.
Study treatment
The wound was debrided if clinically necessary using the conservative sharp method, cleansed, photographed, traced on acetate film for subsequent area determination using an image analysis system and measured. The appropriate‐sized GFD‐A dressing was applied with its island completely covering the wound and wound filler, if used. GFD‐A was available in square, oval and heel shapes in sizes that ranged from 9 cm × 9 cm (island size, 4·5 cm × 4·5 cm) to 18·5 cm × 20·5 cm (island size, 11·6 cm × 15·5 cm). All subjects were required to use an appropriate pressure relieving/reducing device. Investigators performed dressing changes at least once every 7 days. Final evaluations were performed at healing, after 28 days of study treatment or on patient withdrawal from the study, whichever came first.
Outcomes measured
The primary outcome was safety, which was evaluated as type and frequency of adverse events reported during study treatment. Secondary outcomes were exudate management, pain and comfort, clinical ulcer improvement and dressing performance.
At baseline, investigators recorded subject demographic characteristics and the duration, location and NPUAP stage of the study ulcer. The area and depth of the pressure ulcer were measured using acetate tracings and a cotton‐tipped applicator, respectively. The presence of clinical infection or undermining/tunnelling was recorded. Ulcer appearance was recorded as percentages of epithelium, granulation, slough, fibrin, eschar and ‘other’. Condition of the surrounding skin was recorded as percentages intact, erythematous, macerated, cellulitic and ‘other’.
At each dressing change, subjects reported wound pain associated with dressing removal using the Johns Hopkins Visual Analog Scale from 0 (no pain) to 10 (worst pain imaginable) (10). Subjects also responded ‘yes’ or ‘no’ to the following questions: has the dressing been soothing, has the dressing been cushioning, and has the dressing been comfortable? Investigators recorded the condition of the dressing (rolling, lifting, detachment or bunching), adherence of the dressing (dressing fell off, 25% adherent, 50% adherent, 75% adherent, edges lifted or fully adherent), whether there was any evidence of leakage, the level of exudate (none, minimal, moderate or heavy) and whether the dressing managed exudate satisfactorily. The area and depth of the ulcer were measured and the appearance of the surrounding skin was recorded.
At the final evaluation, ulcer condition and ulcer appearance were rated with the same scales as at baseline, and ulcer area and depth were recorded. Investigators rated ease of use as excellent, good, fair or poor for dressing application, dressing removal and dressing conformability. The same scale was used to rate dressing performance for leakage avoidance, wear time and condition of the surrounding skin. Investigators rated the overall change in ulcer condition and the overall change in the surrounding skin using a subjective scale of healed, marked improvement, mild improvement, no change, mild deterioration or marked deterioration.
Statistical and data management methods
All data were verified by double key entry and analyzed using the SAS® System for Microsoft Windows (SAS Institute Inc., Cary, NC). All statistical tests were evaluated at the α= 0·05 level of significance.
Safety and dressing performance data were to be analysed for all subjects who had a study dressing applied. Descriptive statistics (means, proportions) were calculated for demographic and baseline characteristics of subjects and ulcers. The nature and frequency of adverse events that occurred during the study were summarised overall by patient and also by relationship to study treatment. Summary statistics were calculated for ease of use and conformability at the initial application; dressing adherence, leakage, exudate, integrity, ease of use, comfort, trauma and pain at each dressing change; and ulcer area, ulcer depth, ulcer appearance, ulcer condition, condition of the surrounding skin and investigator global ratings of device performance at the final visit. Wear times were calculated from the differences between dressing change dates. Student’s t‐tests were used to test the significance of changes from baseline to final visit in ulcer appearance.
Results
Subject disposition
A total of 23 subjects were enrolled in the study and included in the analyses (Figure 2). Sixteen subjects (70%) completed the full 4‐week study. One subject (4%) healed during 28 days on the study protocol and six (26%) withdrew from the study before completion.
Figure 2.
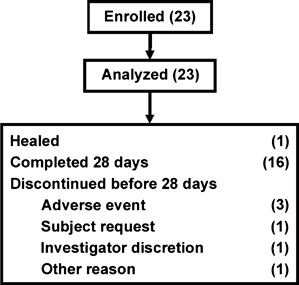
Subject disposition. All enrolled subjects were included in safety and effectiveness analyses. Most subjects completed the 28‐day study period.
Demographics and ulcer characteristics
Demographics and baseline characteristics of the enrolled patients are summarised in Table 1. Mean subject age was 57·6 years (SD 20·8; range 18–97). Subjects were predominantly male (n= 14, 61%). The most commonly listed pressure ulcer locations were specified as sacrum, heel or ‘other’, with the latter mostly at various locations on the foot, excepting the heel. The surrounding skin was predominantly intact at baseline, with some erythema, maceration or cellulitis reported.
Table 1.
Baseline demographic and ulcer characteristics
| Characteristics | Adhesive gelling foam dressing (n= 23) |
|---|---|
| Subject age (years) | |
| Mean ± SD (range) | 57·6 ± 20·8 (18–97) |
| Subject gender, n (%) | |
| Men | 14 (61) |
| Women | 9 (39) |
| Duration of ulcer (years) | |
| Mean ± SD (range) | 1·0 ± 1·8 (0–8) |
| Ulcer area (cm2) | |
| Mean ± SD (range) | 10·6 ± 16·4 (0·8–62·5) |
| Ulcer depth (mm) | |
| Mean ± SD (range) | 5·7 ± 8·8 (0·0–40·0) |
| Ulcer stage (NPUAP staging), n (%) | |
| Stage II | 9 (39) |
| Stage III | 10 (44) |
| Stage IV | 4 (17) |
| Ulcer location, n (%) | |
| Sacrum | 7 (30) |
| Heel | 5 (22) |
| Ischium | 1 (4) |
| Trochanter | 1 (4) |
| Other | 9 (39) |
| Undermining/tunnelling present, n (%) | |
| Yes | 5 (22) |
| No | 18 (78) |
| Ulcer clinically infected, n (%) | |
| Yes | 2 (9) |
| No | 21 (91) |
| Level of ulcer exudate, n (%) | |
| None | 0 (0) |
| Minimal | 9 (39) |
| Moderate | 12 (52) |
| Heavy | 2 (9) |
NPUAP, National Pressure Ulcer Advisory Panel.
On enrolment, the 23 study pressure ulcers had a mean area of 10·6 cm2 (SD 16·4; range 0·8–62·5) and a mean depth of 5·7 mm (SD 8·8; range 0·0–40·0). Nine (39%) of the ulcers were NPUAP stage II, 10 (44%) were stage III and 4 (17%) were stage IV. Nine (39%) had minimal exudate, 12 (52%) had moderate exudate and 2 (9%) had heavy exudate. Mean ulcer duration prior to enrolment was 1·0 year (SD 1·8; range 0–8). Twenty‐two per cent of ulcers were undermined or had tunnelling present and 9% were reported clinically infected at baseline.
Study treatment
A total of 128 dressing changes were recorded. The mean number of GFD‐A dressing changes after initial application was 5·8 (SD 3·4; range 2–16). Two subjects required more than nine dressing changes during the 4‐week study. Mean wear time per dressing was 4·2 days (SD 2·4; range 0–16). Similar mean wear times were reported among subjects with minimal (4·2 days), moderate (4·4 days) and heavy (3·6 days) exudate at baseline.
Hydrofiber dressings were used as wound filler for 15 subjects (65%) at the initial study dressing application and 64 (50%) of 128 total dressing changes.
Dressing safety
Fourteen subjects (61%) had adverse events. Seven subjects (30%) had adverse events considered related to the dressing, including three who discontinued from the study as a result of infection at the study location (n= 1), wound enlargement (n= 1) or erythema (n= 1). The four subjects who remained in the study with dressing‐related adverse events had maceration (n= 3) or blister (n= 1).
Three subjects had serious adverse events, none of which were considered related to the study product, as follows: two subjects had anaemia (one with elevated potassium and the other with a urinary tract infection) and the third subject had sepsis.
Skin condition
Clinicians rated protection of surrounding skin as good or excellent for 79% of subjects at the final evaluation. A barrier cream was applied to protect the surrounding skin at the initial study dressing application in eight subjects (35%). At the final evaluation, clinicians rated non traumatic dressing removal good or excellent for 91% of subjects. Skin condition healed or improved from baseline for 65% of subjects, remained stable for 22% and worsened for 13% (Table 2).
Table 2.
Change in condition of the ulcer and surrounding skin from baseline to the final evaluation
| Overall change | n (%) subjects | |
|---|---|---|
| Pressure ulcer (n= 23)* | Surrounding skin (n= 23)* | |
| Healed | 1 (4) | 2 (9) |
| Marked improvement | 7 (30) | 6 (26) |
| Mild improvement | 6 (26) | 7 (30) |
| No change | 6 (26) | 5 (22) |
| Mild deterioration | 1 (4) | 1 (4) |
| Marked deterioration | 2 (9) | 2 (9) |
The final evaluation was performed at the final visit for all patients, including the early discontinuation visit for patients who did not complete the study.
Dressing performance
At the final evaluation, clinicians judged dressing wear time as good or excellent for 61% of subjects and capacity to avoid leakage as good or excellent for 69% of subjects (Figure 3). Investigator ratings of dressing adherence at the final evaluation were good or excellent for 48% of subjects and fair or poor for 52%. Other investigator evaluations of dressing performance were rated mostly good or excellent at the final evaluation, including ease of application (92%), removal (96%) and conformability (69%; Figure 4). Thirteen subjects (57%) experienced leakage on a total of 50 (39%) of the 128 dressing changes. At the time of dressing change, most dressings (77%) were at least 75% adherent, whereas 20% of dressings became detached prior to the next visit.
Figure 3.
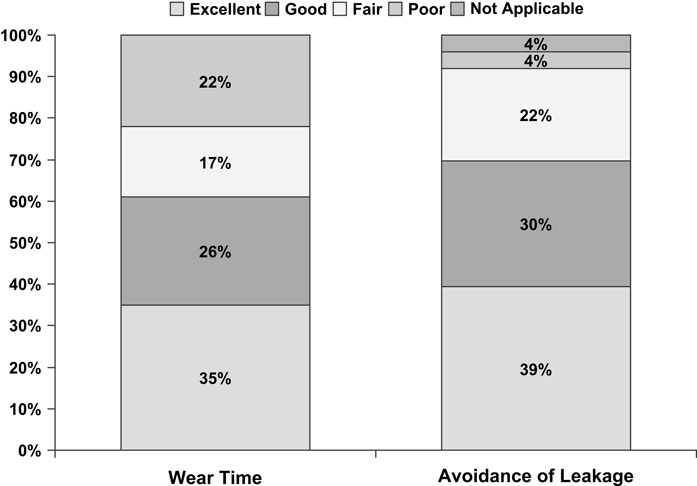
Exudate management. Investigators rated wear time and avoidance of leakage for each subject at the final evaluation (n= 23) (the final evaluation was performed at the final visit for all patients, including the early discontinuation visit for patients who did not complete the study). The majority of scores were either excellent or good for both outcomes.
Figure 4.
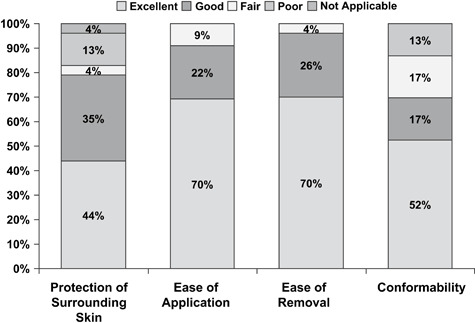
Dressing performance. Investigators rated protection of surrounding skin, ease of application, ease of removal and conformability for each subject at the final evaluation (n= 23) (the final evaluation was performed at the final visit for all patients, including the early discontinuation visit for patients who did not complete the study). Most scores were either excellent or good for each measure of dressing performance.
Change in wound condition
One subject (4%) healed during the 4‐week study. Clinicians reported the wound condition either healed or improved in 14 subjects (61%), was unchanged in 6 (26%) and deteriorated in 3 (13%; Table 2). Mean percentage change in ulcer surface area from baseline to final acetate tracings was −13% and mean change was −0·1 cm2. Mean percentage change in ulcer depth from baseline to final visit was +4% and mean change was −0·6 mm. Mean percentage composition of the ulcer bed increased in epithelium (P= ns) and fibrin (P= ns), and decreased in granulation tissue (P < 0·05) and slough (P= ns) (Table 3).
Table 3.
Ulcer bed condition at baseline and the final evaluation
| Type of tissue | Mean ± SD (%) of ulcer bed | P value† | |
|---|---|---|---|
| Baseline evaluation (n= 23)* | Final evaluation (n= 23)* | ||
| Epithelium | 63 ± 35 | 76 ± 30 | 0·140 |
| Granulation | 31 ± 31 | 15 ± 23 | 0·010 |
| Slough | 6 ± 12 | 2 ± 4 | 0·089 |
| Fibrin | 0 | 8 ± 25 | 0·145 |
| Eschar | 0 | 0 | — |
| Other | 0 | 0 | — |
The final evaluation was performed at the final visit for all patients, including the early discontinuation visit for patients who did not complete the study.
Student’s t‐tests for the significance of changes from baseline to final visit.
Pain and comfort
The median reported pain score was 0 of 10 (no pain) at every dressing change and the maximum reported pain score at any dressing change was 3 of 10 [i.e. mild pain (11)]. Subjects rated GFD‐A as comfortable for 102 (80%) of the 128 dressing changes, soothing for 82 (64%) and cushioning for 90 (70%), at 20–30% of dressing changes subjects being unable to respond to each question (Figure 5). Among those subjects who responded to the questions (i.e. excluding non responsive subjects), more than 90% rated GFD‐A as cushioning, soothing and comfortable.
Figure 5.
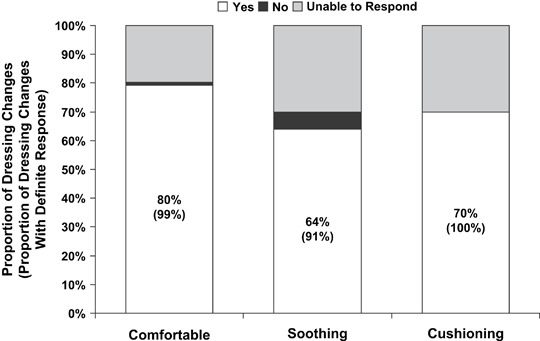
Dressing comfort in situ. At each clinic dressing change (n= 128), most of the subjects reported the adherent gelled foam dressing was comfortable, soothing or cushioning. Subjects were unable to respond to a question at 20–30% of study visits. More than 90% of recorded responses confirmed the dressing was comfortable, soothing or cushioning.
Discussion
In clinical practice, it is especially important to protect skin surrounding the pressure ulcer and manage exudate (9). GFD‐A was generally safe on the long‐duration pressure ulcers in this study. Despite skin fragility in ageing subjects like those participating in the study, GFD‐A was rated as protecting the surrounding skin in 79% of the subjects. Surrounding skin healed or improved in 65% of subjects and pressure ulcers healed or improved in 61% of subjects participating in this 4‐week study. The dressing performed well when used in a study regimen that included appropriate skin care, pressure relief in all subjects and Hydrofiber dressing as optional wound filler. GFD‐A managed pressure ulcer exudate well, with an average wear time of 4.2 days. Clinicians gave GFD‐A mainly good or excellent performance ratings for ease of application and removal, non traumatic removal and avoidance of leakage.
Pressure relief is required for healing in subjects with pressure ulcers, but often it is difficult to achieve fully. Uncorrected nutritional deficiencies and exposure to excess body or wound fluids may pose further challenges in pressure ulcer care. In the present study, the average ulcer duration was 1 year. Furthermore, most study ulcers were full thickness and most had moderate or severe exudate. Despite these difficulties, ulcer healing or improvement occurred in 61% of subjects during 4 weeks of treatment with a regimen that included GFD‐A.
Pressure ulcers often occur over bony prominences in areas that can be especially challenging to dress. Although the use of pressure‐relieving devices was mandatory, the ulcer dressing may have been subjected to the same pressures, friction and sheer that contributed to the original pressure ulcer tissue breakdown. Therefore, it is not unexpected that some dressings became detached during use in these circumstances. This experience is balanced by the gentle nature of the adhesive and the potential need for supplemental securing with adhesive tape in some patients. However, the average wear time and clinical safety of GFD‐A in this study are comparable to those for bordered hydrocolloid dressings.
Treatment of pressure ulcers should include consideration of patient‐centred concerns. However, pressure ulcers are more common among patients with reduced consciousness. In the present study, subjects did not respond to the questions about dressing comfort at 20–30% of dressing changes. Despite this limitation, more than 90% of subjects who were able to respond to the questions rated the dressing as comfortable, soothing or cushioning at dressing changes.
Several subjects in this study had at least one adverse event during GFD‐A therapy, including seven subjects who had adverse events that the investigator considered related to the dressing. The determination of relationship to treatment was based on the clinical judgment of the investigator and his/her knowledge of the specifics of each case. Three subjects were reported to have treatment‐related maceration, but the normal process of autolytic debridement of the wound under an occlusive dressing may present on the surrounding skin, a condition similar to maceration.
Because this was the first clinical study of GFD‐A, a small sample of subjects was enrolled and treated, as is common with an early clinical study of this type. Hydrofiber dressing was used as a wound filler in 65% of subjects and 50% of all dressing changes, which may have biased the study findings. However, a cover dressing such as GFD‐A is not intended for use alone in the management of deep wounds; the use of a wound filler such as Hydrofiber dressing is likely to be commonplace with the use of GFD‐A in the management of pressure ulcers in clinical practice.
Exudate management is especially important in all wound care, including pressure ulcers, because maceration of the surrounding skin can occur in the presence of excessive wound exudate. This can result in longer treatment time, excoriation and other complications (12). In this study, a regimen including GFD‐A was safe and effective in managing exudate, protecting surrounding skin and maintaining patient comfort in situ, while supporting pressure ulcer healing. The clinical implications of this research are that a gelling foam dressing may be a useful option in the management of exudate from stage II, III or IV pressure ulcers.
Acknowledgments
This study was supported and sponsored by a clinical grant from ConvaTec. We thank the following clinicians who served as principle investigators: Joy Schank, CWOCN, Himrod, New York, Bhavesh Shah, DPM, Poth, Texas, Gabriel J. Halperin, DPM, Los Angeles, California and R. Gary Sibbald, MD, Mississauga, Ontario, Canada. AQUACEL, Hydrofiber and Versiva are registered trademarks of ConvaTec.
References
- 1. Cuddigan J, Berlowitz DR, Ayelb EA, for the National Pressure Ulcer Advisory Panel. Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care 2001;14:208–15. [DOI] [PubMed] [Google Scholar]
- 2. Stausberg J, Kroger K, Maier I, Schneider H, Niebel W. Pressure ulcers in secondary care: incidence, prevalence, and relevance. Adv Skin Wound Care 2005;18:140–5. [DOI] [PubMed] [Google Scholar]
- 3. Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 2003;290:1868–74. [DOI] [PubMed] [Google Scholar]
- 4. Parish LC, Lowthian P, Witkowski JA. The decubitus ulcer: many questions but few definitive answers. Clin Dermatol 2007;25:101–8. [DOI] [PubMed] [Google Scholar]
- 5. Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962;193:293–4. [DOI] [PubMed] [Google Scholar]
- 6. Bergstrom N, Horn SD, Smout RJ, Bender SA, Ferguson ML, Taler G, Sauer AC, Sharkey SS, Voss AC. The National Pressure Ulcer Long‐Term Care Study: outcomes of pressure ulcer treatments in long‐term care. J Am Geriatr Soc 2005;53:1721–9. [DOI] [PubMed] [Google Scholar]
- 7. De Laat EH, Scholte op Reimer WJ, Van Achterberg T. Pressure ulcers: diagnostics and interventions aimed at wound‐related complaints: a review of the literature. J Clin Nurs 2005;14:464–72. [DOI] [PubMed] [Google Scholar]
- 8. White R. Managing exudate. Nurs Times 2001;97:59–60. [PubMed] [Google Scholar]
- 9. Agency for Health Care Policy and Research (AHCPR) . Treatment of pressure ulcers (clinical practice guideline, no. 15). Rockville: US Department of Health and Human Services, Public Health Service, AHCPR, 1994. [Google Scholar]
- 10. Grossman SA, Sheidler VR, McGuire DB, Geer C, Santor D, Piantadosi S. A comparison of the Hopkins Pain Rating Instrument with standard visual analogue and verbal descriptor scales in patients with cancer pain. J Pain Symptom Manage 1992;7:196–203. [DOI] [PubMed] [Google Scholar]
- 11. Palos GR, Mendoza TR, Mobley GM, Cantor SB, Cleeland CS. Asking the community about cutpoints used to describe mild, moderate, and severe pain. J Pain 2006;7:49–56. [DOI] [PubMed] [Google Scholar]
- 12. Cutting KF, White RJ. Maceration of the skin and wound bed. 1: Its nature and causes. J Wound Care 2002;11:275–8. [DOI] [PubMed] [Google Scholar]


