Abstract
Diabetic foot ulcers affect millions of people in the United States of America and impose tremendous medical, psychosocial and financial loss or burden. Negative pressure wound therapy (NPWT) is generally well tolerated and appears to stimulate a robust granulation tissue response compared with other wound healing modalities. This device may be a cost‐effective adjunctive wound healing therapy. This literature review will focus on the clinical outcome of diabetic foot ulcers treated with NPWT, its implication in the transition from acute care to home care, factors that might influence clinical outcomes in home care as well as quality‐of‐life aspects in these patients. Patient care for diabetic foot ulceration is complex and necessitates multiprofessional collaboration to provide comprehensive wound care. It is clear that when we strive for limb preservation in this most high‐risk population, it is important to have an available versatile, efficacious wound healing modality. There is a need for an easy transition from acute care to home care. Resources need to be combined in a collaborative and synergistic fashion to allow patient to perform many daily living activities while receiving the potential benefits of an advanced wound healing modality.
Keywords: Diabetes, Lower extremity ulcer, Negative pressure wound therapy, V.A.C.®, Wound healing
Introduction
The rapid rise in the incidence of diabetes, a serious lifelong condition, is of alarming concern to health care professionals. Recent data from the World Health Organization estimate that by the year 2025, more than 325 million people worldwide will be diagnosed with the disease. Diabetic foot ulceration is one of the most common complications associated with the disease and is notorious for its complexity and healing difficulties 1, 2, 3. The prevalence of foot ulcers ranges from 4% to 10% among persons diagnosed with diabetes mellitus (1). This translates to an annual population‐based incidence of 1·0–4·1%, and the lifetime incidence may be as high as 25% (1). Diabetic foot ulcers frequently become infected and are a major cause of hospital admissions 4, 5. They also account for most non traumatic lower limb amputations in this patient population (4). Diabetic foot ulcers impose tremendous medical and financial burden on our health care system with conservative cost estimates as high as US$45 000 per patient (6). These estimations, however, do not include the deleterious psychosocial effects on the patient’s quality of life because of impaired mobility and substantial loss of productivity (7).
Foot ulcerations are pivotal events in limb loss for two important reasons. They allow an avenue for infection (8) and can cause progressive tissue necrosis and poor wound healing in the presence of critical ischaemia. Approximately 56% of diabetic foot wounds become infected during their life cycle 9, 10, 11, and 20% of these patients with infected foot wounds require radical debridement of soft tissue and bone, resulting in some form of lower extremity amputation. Such wounds typically need some degree of preparation before delayed primary closure or healing by secondary intention because of their depth, size and presence of pre‐existing infection. The ensuing large wound deficit often requires prolonged healing time and extended hospital stays with a further risk for reinfection (Figure 1). Furthermore, this lengthened and sometimes interrupted healing process impairs patient mobility; causes substantial lost productivity; diminishes quality of life; imposes tremendous medical, psychosocial and financial impacts and presents a significant management challenge to health care professionals 12, 13, 14. Therefore, the advent of an effective wound healing modality that facilitates timely healing and shortens hospital stays is of immense value to the health profession.
Figure 1.
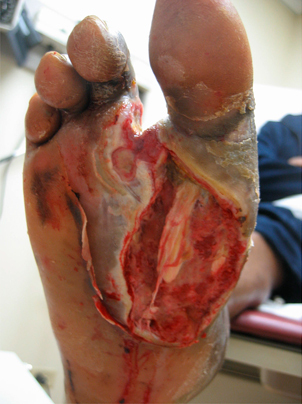
Diabetic foot wound following debridement of necrotic bone and soft tissue.
Negative pressure wound therapy
Recent advances in technology combined with better understanding of the complex cellular and biochemical mechanisms of wound healing have resulted in the development of a plethora of advanced wound healing modalities such as hyperbaric oxygen, topical growth factors, bioengineered skin and tissue equivalents and negative pressure wound therapy (NPWT) 15, 16, 17, 18, 19, 20. NPWT was first widely introduced in the United States of America more than a decade ago and has since evolved into a commonly used ‘complex wound’ advanced treatment option that may be used by all practitioner levels. NPWT is a non invasive wound closure system that uses controlled, localised subatmospheric pressure to help promote healing in chronic and acute wounds 21, 22, 23. NPWT delivered through the V.A.C.® device is US Food and Drug Administration (FDA) approved to promote wound healing in pressure ulcers; diabetic foot ulcers; other types of chronic, acute and traumatic wounds, in conjunction with meshed grafts and flaps and in partial‐thickness burns. Because of its ability to also manage wound exudate, NPWT is adjunctive for managing large defects and heavily draining wounds and can often be used both as a catalyst to secondary wound healing and as a bridge between debridement and definitive closure (24). NPWT is versatile as it can be applied at home, in an alternative care setting, and can simultaneously treat multiple wounds through connecting foam bridges or Y connectors. NPWT helps decrease the frequency of dressing changes and the time between debridement and definitive closure and lowers the costs of protracted hospital stays 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35. Many studies report that NPWT very effectively prepares wound beds for grafting or delayed primary closure and is also useful for patients who are poor surgical candidates 26, 36. Numerous authors advocate NPWT as a safe and effective adjunctive modality to promote a rapid granular bed and prevent further surgeries in this high‐risk population 26, 37, 38, 39. This literature review will focus on the clinical outcome of diabetic foot ulcers treated with NPWT, its implication in the transition from acute care to home care, factors that might influence clinical outcome in home care as well as quality‐of‐life aspects in these patients.
Clinical evidence
Armstrong et al. evaluated the efficacy of NPWT to heal 31 indolent diabetic foot wounds immediately after wide surgical debridement. A cessation of therapy protocol was used where NPWT was discontinued when the wound bed approached 100% coverage with granulation tissue and no exposed tendon, joint capsule or bone. They noted that 90·3% of the wounds treated with NPWT healed at the level of debridement without the need for further bony resection in a mean time of 8·1 ± 5·5 weeks (26). In a randomised trial, Eginton et al. compared the wound healing efficacy between NPWT and conventional moist dressings to treat large diabetic foot ulcers and noted that NPWT decreased the wound volume and depth significantly more than moist gauze dressings (59% versus 0% and 49% versus 8%, respectively) (37).
Recently published trials further showed the wound healing efficacy of NPWT. Blume et al. evaluated the safety and clinical efficacy of NPWT compared with advanced moist wound therapy (AMWT) in the treatment of diabetic foot ulcers in a multicentre, randomised controlled trial. Three hundred and forty‐two patients, 79% male, with a mean age of 58 years were randomised to receive either NPWT or AMWT and standard offloading therapy as needed (40). The authors noted that a greater proportion of foot ulcers achieved complete ulcer closure with NPWT (73 of 169, 43·2%) than with AMWT (48 of 166, 28·9%) within the 112‐day active treatment phase (P = 0·007). The Kaplan–Meier median estimate for 100% ulcer closure was 96 days (95% CI 75·0–114·0) for NPWT and not determinable for AMWT (P = 0·001). The authors noted no significant difference between the groups in treatment‐related complications at 6 months.
In a 16‐week, 18‐centre, randomised clinical trial involving 162 patients with wounds that were larger and more complex than those from previous randomised trials, Armstrong and Lavery noted that NPWT healed more wounds after partial foot amputation in patients with diabetes versus the standard of care [43 (56%) versus 33 (39%), P = 0·040]. Based on the time to complete closure, NPWT was noted to produce a faster wound healing rate (P = 0·005) (Figure 2) and faster granulation tissue formation rate versus standard of care (P = 0·002) (39).
Figure 2.
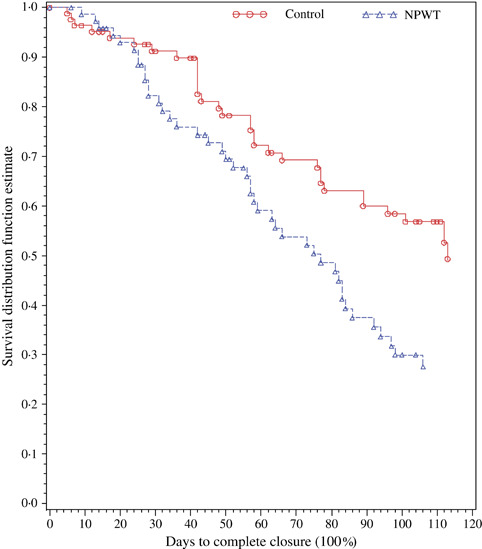
Kaplan–Meier estimates for time to complete wound closure. NPWT, negative pressure wound therapy.
Transition from acute care to home care
In addition to the wound healing efficacy of NPWT as shown by numerous trials in the literature, the fact that most of the patients who were randomised to NPWT were able to receive the therapy at home is of extreme importance. In the most recent study, the proportion of home care therapy days to total therapy days (including inpatient time) for NPWT was about 90% versus 95·3% for control. This is mirrored in Armstrong and Lavery’s study where no difference was noted between the NPWT group versus the control group for inpatient hospital stay (number of admissions or length of stay), with 89% of total duration of therapy classified as ‘outpatient’. Furthermore, patients who received NPWT experienced significantly (P = 0·035) fewer secondary operative revision amputations, which further decreased hospital stays. Two (3%) patients in the NPWT group and nine (11%) in the control group underwent a second admission for further amputation. Although the difference was not significant, it suggested a benefit in favour of NPWT (P = 0·060) with the relative risk suggesting that people receiving NPWT were only a quarter as likely as control patients to need a second amputation. This trend continued in patients who received a high‐level (above‐foot) amputation; five (6%) control patients received either a below‐knee (n = 3) or above‐knee (n = 2) amputation, but there were no high‐level amputations in the NPWT group (P = 0·060). This difference in reamputation may have resulted from the more rapid, higher proportion of healing and better wound coverage with granulation tissue seen in the NPWT group.
Resource utilisation for patients treated with NPWT also was evaluated in this same study population. Apelqvist et al. (41) reported that patients randomised to the NPWT group required fewer surgical procedures (including debridement) than the control group (43 versus 120, P < 0·001), fewer average number of dressing changes [41 (range 6–140) for NPWT versus 118·0 (range 12–226) for control AMWT (P < 0·0001)] and fewer outpatient treatment visits [4 (range 0–47) in the NPWT versus 11 (range 0–106) in the control (P < 0·05)]. This yielded a cost saving in excess of US$12 800 compared with standard therapy. This combined with the clinical data provides a compelling suggestion that NPWT is an efficacious, cost‐effective modality in healing wounds on an inpatient and outpatient basis.
The versatility of the NPWT device enables it to be applied at home or in an alternative care setting, making the transition from the hospital to the home setting extremely easy to facilitate. Furthermore, because dressing changes can be performed every 48 hours in the ambulatory setting, this helps decrease the frequency of dressing changes, decreases the time between debridement and definitive closure and lowers the costs of protracted hospital stays 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, making NPWT an ideal device for home care. The development of a portable NPWT unit (The Freedom V.A.C.® Therapy System; Kinetic Concepts Inc., San Antonio, TX) affords patients the freedom of mobility while sustaining continuous NPWT. This flexibility further reduces hospital inpatient utilisation costs allowing home patient recovery.
Criteria for treating patients in home care for best clinical outcomes with NPWT
NPWT in conjunction with adequate perfusion, debridement, a moist environment, pressure mitigation and management of contributing comorbid diseases may be effective at achieving complex wound closure. There are several essential questions to ask when assessing a diabetic foot wound to ensure maximal clinical outcome including location, size and depth of the wound, adequate perfusion and control of bioburden 42, 43.
The location of a wound and its aetiology go hand in hand. Generally, wounds on the medial aspect of the foot are caused by constant low pressure, whereas wounds on the plantar aspect of the foot are caused by repetitive moderate pressure. The size and depth of the wound also play a key role in determining duration of wound healing. Identification of ischaemia is of utmost importance when evaluating a wound. Ischaemic wounds were found to have a longer duration of healing compared with neuropathic wounds without deformities (44). In cases where there is a lack of adequate perfusion, a prompt vascular surgery consultation and possible intervention to improve perfusion are warranted. Bacterial colonisation and associated tissue damage of a wound is a recognised detrimental factor in the multifactorial process of wound healing (45). Adequate debridement along with appropriate antibiotic treatment may help decrease the bioburden and help enhance healing. When used in conjunction with adequate debridement and appropriate antibiotics, NPWT may be effective in enhancing wound closure in patients with treated osteomyelitis or soft tissue infections (33).
Despite technological advances in wound healing, it is important to emphasise the importance of pressure mitigation, especially in the treatment of lower extremity ulcerations. NPWT, although useful in the management of complex plantar foot wounds, has complicated plantar weight bearing because the tubing, which is contiguous with the foam dressing, often exits at a plantar site. By modifying the ‘bridging’ technique and running contiguous sponges from the plantar foot to the dorsal foot, an area that is more conducive to tube connection, NPWT can now be combined with a removable cast walker and secured with either a layer of cohesive bandage or a plaster to ensure compliance. This treatment, termed by Armstrong et al. the ‘V.A.C. Contact Cast (VCC)’, could allow a patient to perform many activities of daily living while still receiving the potential benefits of an advanced wound healing modality (46). In a gait laboratory study by Armstrong and coworkers, the VCC technique was compared with a removable cast walker alone. The documented difference is unlikely to be clinically significant in spite of the minimal pressure difference in favour of removable cast walker. It was therefore concluded that this very mild increase in pressure, when factored against the potential benefit garnered from topical negative pressure dressings, may weigh in favour of combined therapy (28). By allowing limited protected walking, this combined modality may also reduce inpatient length of stay in this high‐risk patient population (27).
Quality of life
Health‐related quality of life (HRQOL) has been noted to be worse among individuals with diabetes than among individuals without diabetes, and diabetic foot ulcers have a major negative effect on HRQOL 14, 47. Diabetic foot ulcers are associated with reduced mobility and deficits that adversely affect activities of daily living and HRQOL (14). Qualitative studies have confirmed clinical observations that diabetic foot ulcers have a huge negative psychological and social effect, including reduction in social activities, increased family tensions for patients and their caregivers (spouses or partners), limited employment and financial hardship (14). Quantitative studies utilising the SF‐36 Health Survey score confirm the findings of qualitative studies that diabetic foot ulcers exert a negative effect on physical functioning, psychological status and social situation and may be as severe as in similar patients with lower extremity amputation (47).
Summary
Foot ulcers are a common, serious and costly complication of diabetes, preceding 84% of diabetes‐related lower extremity amputations and increasing the risk of death by 2·4‐fold compared with people with diabetes but without ulcers (14). Randomised controlled trials that assessed clinical outcomes in diabetic wounds secondary to open amputation of the foot suggested that NPWT delivered through the V.A.C. Therapy System yielded a
-
•
higher proportion of healed wounds,
-
•
faster time to wound closure,
-
•
more rapid and robust granulation tissue response and
-
•
potential trend towards reduced second amputation risk versus controls.
When used appropriately, NPWT appears to be quite safe with no overt difference in proportion or distribution of adverse events compared with controls and can easily be transitioned from acute care to home care.
Conflicts of interest
The authors have declared no conflicts of interest.
References
- 1. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 2. Boulton AJ, Vileikyte L. The diabetic foot: the scope of the problem. J Fam Pract 2000;49(11 Suppl):S3–S8 [in process citation]. [PubMed] [Google Scholar]
- 3. Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non‐Hispanic whites from a diabetes disease management cohort. Diabetes Care 2003;26:1435–8. [DOI] [PubMed] [Google Scholar]
- 4. Dang CN, Boulton AJ. Changing perspectives in diabetic foot ulcer management. Int J Low Extrem Wounds 2003;2:4–12. [DOI] [PubMed] [Google Scholar]
- 5. Trepman E, Nihal A, Pinzur MS. Current topics review: Charcot neuroarthropathy of the foot and ankle. Foot Ankle Int 2005;26:46–63. [DOI] [PubMed] [Google Scholar]
- 6. Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower‐extremity ulcers among patients with diabetes. Diabetes Care 2004;27:2129–34. [DOI] [PubMed] [Google Scholar]
- 7. Ragnarson Tennvall G, Apelqvist J. Health‐economic consequences of diabetic foot lesions. Clin Infect Dis 2004;39 Suppl 2:S132–S139. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong DG, Lipsky BA. Advances in the treatment of diabetic foot infections. Diabetes Technol Ther 2004;6:167–77. [DOI] [PubMed] [Google Scholar]
- 9. Smith D, Weinberger M, Katz B. A controlled trial to increase office visits and reduce hospitalization in diabetic patients. J Gen Intern Med 1987;2:232–8. [DOI] [PubMed] [Google Scholar]
- 10. Gibbons G, Eliopoulos GM. Infection of the diabetic foot. In: Kozak GP, Hoar CS, Rowbotham JL, editors. Management of diabetic foot problems. Philadelphia: WB Saunders, 1984:97–102. [Google Scholar]
- 11. Block P. The diabetic foot ulcer: a complex problem with a simple treatment approach. Mil Med 1981;146:644–6. [PubMed] [Google Scholar]
- 12. Paquette D, Falanga V. Leg ulcers. Clin Geriatr Med 2002;18:77–88, vi. [DOI] [PubMed] [Google Scholar]
- 13. Ulbrecht JS, Cavanagh PR, Caputo GM. Foot problems in diabetes: an overview. Clin Infect Dis 2004;39 Suppl 2:S73–S82. [DOI] [PubMed] [Google Scholar]
- 14. Goodridge D, Trepman E, Embil JM. Health‐related quality of life in diabetic patients with foot ulcers: literature review. J Wound Ostomy Continence Nurs 2005;32:368–77. [DOI] [PubMed] [Google Scholar]
- 15. Introduction. Healing chronic wounds: technologic solutions for today and tomorrow. Adv Skin Wound Care 2000;13(2 Suppl):4–5. [PubMed] [Google Scholar]
- 16. Gough A, Clapperton M, Rolando N, Foster AV, Philpott‐Howard J, Edmonds ME. Randomised placebo‐controlled trial of granulocyte‐colony stimulating factor in diabetic foot infection. Lancet 1997;350:855–9. [DOI] [PubMed] [Google Scholar]
- 17. Steed DL. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg 1995;21:71–8. [DOI] [PubMed] [Google Scholar]
- 18. Donaghue VM, Chrzan JS, Rosenblum BI, Giurini JM, Habershaw GM, Veves A. Evaluation of a collagen‐alginate wound dressing in the management of diabetic foot ulcers. Adv Wound Care 1998;11:114–9. [PubMed] [Google Scholar]
- 19. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg 1996;183:61–4. [PubMed] [Google Scholar]
- 20. Hopf HW, Humphrey LM, Puzziferri N, West JM, Attinger CE, Hunt TK. Adjuncts to preparing wounds for closure: hyperbaric oxygen, growth factors, skin substitutes, negative pressure wound therapy (vacuum‐assisted closure). Foot Ankle Clin 2001;6:661–82. [DOI] [PubMed] [Google Scholar]
- 21. Banwell P, Withey S, Holten I. The use of negative pressure to promote healing. Br J Plast Surg 1998;51:79. [DOI] [PubMed] [Google Scholar]
- 22. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 23. Mullner T, Mrkonjic L, Kwasny O, Vecsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. Br J Plast Surg 1997;50:194–9. [DOI] [PubMed] [Google Scholar]
- 24. Agarwal JP, Ogilvie M, Wu LC, Lohman RF, Gottlieb LJ, Franczyk M, Song DH. Vacuum‐assisted closure for sternal wounds: a first‐line therapeutic management approach. Plast Reconstr Surg 2005;116:1035–40 [discussion 1041–3]. [DOI] [PubMed] [Google Scholar]
- 25. McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum‐assisted closure versus saline‐moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Manage 2000;46:28–32, 34. [PubMed] [Google Scholar]
- 26. Van Schie CH, Whalley A, Armstrong DG, Vileikyte L, Boulton AJ. The effect of silicone injections in the diabetic foot on peak plantar pressure and plantar tissue thickness: a 2‐year follow‐up. Arch Phys Med Rehabil 2002;83:919–23. [DOI] [PubMed] [Google Scholar]
- 27. Giovannini UM, Settembrini F, Colonna MR, Teot L, Giopre C, Amadeo G, Strano A, Stagno d’Alcontres F. Topical negative therapy and vacuum assisted closure. New strategies and devices in surgical reconstruction. Minerva Chir 2005;60:191–4. [PubMed] [Google Scholar]
- 28. Arca MJ, Somers KK, Derks TE, Goldin AB, Aiken JJ, Sato TT, Shilyansky J, Winthrop A, Oldham KT. Use of vacuum‐assisted closure system in the management of complex wounds in the neonate. Pediatr Surg Int 2005;21:532–5. [DOI] [PubMed] [Google Scholar]
- 29. Caniano DA, Ruth B, Teich S. Wound management with vacuum‐assisted closure: experience in 51 pediatric patients. J Pediatr Surg 2005;40:128–32 [discussion 132]. [DOI] [PubMed] [Google Scholar]
- 30. O’Connor J, Kells A, Henry S, Scalea T. Vacuum‐assisted closure for the treatment of complex chest wounds. Ann Thorac Surg 2005;79:1196–200. [DOI] [PubMed] [Google Scholar]
- 31. Bickels J, Kollender Y, Wittig JC, Cohen N, Meller I, Malawer MM. Vacuum‐assisted wound closure after resection of musculoskeletal tumors. Clin Orthop Relat Res 2005;441:346–50. [DOI] [PubMed] [Google Scholar]
- 32. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C‐reactive protein, interleukin‐6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 2005;112:976–83. [DOI] [PubMed] [Google Scholar]
- 33. Cowan KN, Teague L, Sue SC, Mahoney JL. Vacuum‐assisted wound closure of deep sternal infections in high‐risk patients after cardiac surgery. Ann Thorac Surg 2005;80:2205–12. [DOI] [PubMed] [Google Scholar]
- 34. Dosluoglu HH, Schimpf DK, Schultz R, Cherr GS. Preservation of infected and exposed vascular grafts using vacuum assisted closure without muscle flap coverage. J Vasc Surg 2005;42:989–92. [DOI] [PubMed] [Google Scholar]
- 35. Paul JC. Vacuum assisted closure therapy: a must in plastic surgery. Plast Surg Nurs 2005;25:61–5. [DOI] [PubMed] [Google Scholar]
- 36. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76 [discussion 577]. [PubMed] [Google Scholar]
- 37. Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative‐pressure wound dressings for diabetic foot wounds. Ann Vasc Surg 2003;17:645–9. [DOI] [PubMed] [Google Scholar]
- 38. Mendonca DA, Cosker T, Makwana NK. Vacuum‐assisted closure to aid wound healing in foot and ankle surgery. Foot Ankle Int 2005;26:761–6. [DOI] [PubMed] [Google Scholar]
- 39. Wu SC, Crews RT, Armstrong DG. The pivotal role of offloading in the management of neuropathic foot ulceration. Curr Diab Rep 2005;5:423–9. [DOI] [PubMed] [Google Scholar]
- 40. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy utilizing vacuum‐assisted closure to advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2007;31:631–6. [DOI] [PubMed] [Google Scholar]
- 41. Apelqvist J, Armstrong DG, Lavery LA, Boulton AJM. Resource utilization and economic costs of care based on a randomized trial of vacuum‐assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg 2008. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42. Wongworawat MD, Schnall SB, Holtom PD, Moon C, Schiller F. Negative pressure dressings as an alternative technique for the treatment of infected wounds. Clin Orthop Relat Res 2003;414:45–8. [DOI] [PubMed] [Google Scholar]
- 43. Bihariesingh VJ, Stolarczyk EM, Karim RB, Van Kooten EO. Plastic solutions for orthopaedic problems. Arch Orthop Trauma Surg 2004;124:73–6. [DOI] [PubMed] [Google Scholar]
- 44. Armstrong DG, Peters EJ. Classification of wounds of the diabetic foot. Curr Diab Rep 2001;1:233–8. [DOI] [PubMed] [Google Scholar]
- 45. Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg 2004;52:276–9 [discussion 279–80]. [DOI] [PubMed] [Google Scholar]
- 46. Armstrong DG, Kunze K, Martin BR, Kimbriel HR, Nixon BP, Boulton AJM. Plantar pressure changes using a novel negative pressure wound therapy technique. J Am Podiatr Med Assoc 2004;94:456–60. [DOI] [PubMed] [Google Scholar]
- 47. Willrich A, Pinzur M, McNeil M, Juknelis D, Lavery L. Health related quality of life, cognitive function, and depression in diabetic patients with foot ulcer or amputation. A preliminary study. Foot Ankle Int 2005;26:128–34. [DOI] [PubMed] [Google Scholar]


