Abstract
Deep tissue injury (DTI) is difficult to detect in the early phase. Creatine phosphokinase (CPK) as a muscle enzyme could represent a promising indicator of DTI. However, serum CPK levels reflect the systemic condition rather than the local wound environment. Wound exudates can be indicative of the local wound environment. This study aimed to investigate the usefulness of CPK levels in wound exudates as an indicator of DTI. Rats were divided into control, 6 hours 10‐kg and 6 hours 20‐kg loading groups. Serum samples were obtained before wounding, and at 8 and 12 hours, and 1, 2 and 3 days after wounding, while exudate samples were obtained on days 2 and 3. Serum CPK levels were markedly increased in the 10‐kg and 20‐kg groups at 8 and 12 hours after loading compared with the baseline value and control group, but decreased to the normal level on day 1. In both loading groups, exudate CPK levels were high on day 2 and decreased on day 3. Muscle necrosis was more severe in the 20‐kg group than in the 10‐kg group by histological examination. This is the first study to indicate the potential of CPK in wound exudates as an indicator of DTI.
Keywords: Creatine phosphokinase, Deep tissue injury, Exudate, Serum
Introduction
Pressure ulcers are a major health burden in many countries worldwide. The prevalence of pressure ulcers in the USA was reported to be 14·3–15·6% in acute care settings (1). Approximately 2·5 million pressure ulcers are treated each year in acute care facilities alone, with a staggering estimated cost of $11 billion (2). The cost of treating an individual pressure ulcer ranges from $500 to $40 000 depending on the severity of the wound (2). Although the prevalence of pressure ulcers in Japan is relatively low compared with the US and European countries, the proportion of severe pressure ulcers in Japan is high, with the proportions of stage III and stage IV pressure ulcers estimated as 22·5% and 12·1%, respectively (3). Previous studies have revealed that some of these severe ulcers progress from deep tissue injury (DTI) 4, 5.
DTI is defined as an ulcer that develops from a deep tissue (muscle) region and deteriorates towards the superficial skin, making it especially difficult to detect in clinical settings because ulceration is not present and the depth of tissue damage cannot be readily determined, resulting in progression to stage III and stage IV pressure ulcers (6). As DTI deteriorates into stage III and stage IV pressure ulcers 4, 5, 6, early detection of its occurrence is indispensable for early initiation of treatment to prevent such deterioration. However, the early stage of DTI remains difficult to detect in clinical settings because DTI cannot be evaluated solely by visual assessment (4). Other parameters reported in previous studies also remain insufficient for routine and simple application in clinical settings. A previous study used an in vitro model to monitor DTI occurrence and it is therefore difficult to apply the results to clinical settings (7). Other studies regarding indicators of DTI have focused on the use of magnetic resonance imaging (8) and ultrasound to assess the muscle damage (9). Although these devices are promising, none of them is specific for muscle damage (8). In addition, they are also difficult to perform routinely and require expertise to interpret the results.
As DTI begins in the muscle tissue, the enzymes found in muscle tissue could represent promising indicators for the detection of muscle damage. Creatine phosphokinase (CPK) is a skeletal muscle enzyme that is extremely sensitive to muscle damage (10). Under normal conditions, the CPK enzyme is mainly present in skeletal muscle and released into the blood at very low levels. When muscle damage occurs, the intracellular enzymes, including CPK, leak into the extracellular space, resulting in increased CPK levels in the blood 10, 11. Hagisawa et al. (10) applied loads of 2·3 kg/3·14 cm2 to the scapula and 3 kg/3·14 cm2 to the thoracic spine of porcines for 6 hours, and reported marked increases in the serum CPK levels at 2 hours after unloading. However, serum CPK levels reflect the systemic condition rather than the local wound microenvironment, and are therefore difficult to interpret when applied in clinical settings where patients may have muscle damage caused by issues other than DTI.
Wound exudates, which are produced during the inflammation process of tissue damage, represent a promising resource for wound assessment because they reflect the status of the wound microenvironment 12, 13, and also provide a simple and non invasive method for collecting samples from patients. Therefore, we focused on the detection of CPK in wound exudates as an indicator of DTI. However, it remained unclear whether CPK levels in exudate samples could act as indicators of DTI severity. Therefore, we conducted animal studies and measured the CPK levels in wound exudate samples to clarify whether these levels can act as indicators of DTI severity by creating muscle damage with different degrees of severity as confirmed by histological analysis as the gold standard.
Materials and methods
Animals
Wistar rats at 13–14 weeks of age, weighing 285–300 g, were used. The rats were purchased from Sankyo Labo Service Corporation (Tokyo, Japan), and maintained under controlled light conditions (12 h light:12 h dark) with free access to food and water. The experimental protocols were approved by the Animal Research Committee of The University of Tokyo, Tokyo, Japan.
Experimental protocols
A modified pressure ulcer model was used for DTI creation and exudate production (14). Briefly, after shaving the right flank region, two 2‐cm incisions (5 cm apart) that extended to the subperitoneum were created using a scalpel and a metal plate was inserted subperitoneally under anaesthesia with intraperitoneal injection of pentobarbital sodium (Somnopentyl; Kyoritsu Seiyaku Corporation, Tokyo, Japan; 30 mg/kg body weight). Pressure was applied by lowering a cylinder with a contact area of 3 cm2. After relieving the pressure, the metal plate was removed and the incisions were sutured (14). A transparent semipermeable dressing (Tegaderm HP; 3M Health Care Limited, Tokyo, Japan) was applied to the wound area and a polyurethane foam dressing (Hydrosite; Smith & Nephew Wound Management KK, Tokyo, Japan) was applied to the incision sites to prevent contamination of the wound by the exudates from the incision sites.
A total of eight rats were divided into three groups as follows: control group (two rats); 6 hours 10‐kg loading group (three rats); and 6 hours 20‐kg loading group (three rats). The rats in the control and experimental groups all underwent the same procedure for the incisions and plate insertion. As the model involved the creation of incisions and metal plate insertion, the control group was used to investigate the effects of the incisions and metal plate insertion on CPK release to differentiate these effects from those of the compressive loading.
Blood and exudate samples and measurements
Blood samples (0·2 ml) were obtained from the tail vein before wounding, and at 8 and 12 hours, and 1, 2 and 3 days after wounding. Exudate samples (30–80 μl) were obtained from the wound sites on days 2 and 3 by aspirating the pooled exudates under the transparent film using a syringe. The blood and exudate samples were centrifuged at 6000 rpm for 10 minutes and stored at −80°C until analysis. The CPK concentrations in the samples were determined enzymatically by a commercial laboratory (SRL Inc., Tokyo, Japan) using the method recommended by The Japan Society of Clinical Chemistry (15). The CPK activities were expressed as IU/l.
Histological examinations
The tissue samples were harvested on day 3, fixed with 4% paraformaldehyde, dehydrated through a graded series of alcohol and xylene, and embedded in paraffin. Next, the tissue samples were cut into 5‐μm‐thick sections and stained with haematoxylin and eosin (HE) to detect muscle damage. Histological observations of the muscle tissue were carried out under a light microscope.
Histological analysis was conducted using both qualitative and quantitative methods. The number of necrotic fibres in the deep muscle was measured quantitatively using the image processing package ImageJ developed by the National Institutes for Health (16). The images were obtained by dividing the HE‐stained wound area into five areas, and then randomly capturing images of two regions of interest in each area using a digital microscope under 100× magnification in both loading groups to reduce bias. Therefore, a total of 10 images were analysed for each tissue section. The number of damaged muscle fibres was quantified using unsupervised k‐clustering method through the k‐means algorithm in the ImageJ processing package 16, 17, 18. The k‐clustering method is widely used in medical areas to classify DNA patterns 19, 20 and quantify cancerous cells 21, 22, 23.
The images were divided into four clusters: necrotic fibres, healthy fibres, inflammatory cells and interstitial spaces. The clusters were divided into four groups to ensure that the necrotic muscle fibres and healthy muscle fibres were precisely grouped into one cluster and not included in the other clusters. After the necrotic and the total of muscle fibres pixels were calculated, the amount of muscle necrosis was determined and was expressed as percentage. Percentage of necrosis = (total pale‐stained areas/total areas of muscle) × 100.
Results
Changes in CPK levels in serum and exudate samples
The changes in the CPK levels in serum and exudate samples following compressive loading are shown in 1, 2. At 8 hours after wounding, the serum CPK levels were markedly elevated by 11·07‐fold and 4·2‐fold in the 10‐kg and 20‐kg loading groups, respectively, compared with the baseline value. Meanwhile, the control group showed only slight elevation by threefold compared with the baseline value. At 12 hours after wounding, the serum CPK level in the control group decreased to the normal level, whereas those in the 10‐kg and 20‐kg groups remained elevated by 6·7‐fold and 2‐fold, respectively, compared with the baseline value. On day 1, the serum CPK levels in the 10‐kg and 20‐kg groups decreased to the normal level (Table 1). The exudate CPK level was higher in the 20‐kg group than in the 10‐kg group on both days 2 and 3 (Table 2). In both loading groups, the exudate CPK levels were high on day 2 and low on day 3.
Table 1.
Serum CPK activities in rates following compressive loading*
| Group | Before Loading (U/L) | 8 Hours (U/L) | 12 Hours (U/L) | Day 1 (U/L) | Day 2 (U/L) | Day 3 (U/L) |
|---|---|---|---|---|---|---|
| Control | 50 (40–60) | 126 (84–68) | 33 (15–50) | 21 (12–30) | 51 (60–42) | 26 (22–30) |
| 10‐kg group | 26 (18–30) | 288 (216–521) | 174 (147–255) | 19 (10–23) | 30 (20–69) | 51 (69–84) |
| 20‐kg group | 38 (14–54) | 163 (120–590) | 72 (42–240) | 30 (15–60) | 15 (10–30) | 12 (10–13) |
Data are the median and range
Table 2.
CPK levels in exudate samples on days 2 and 3*
| Group | Day 2 (U/L) | Day 3 (U/L) |
|---|---|---|
| 10‐kg group | 1440 (876–2048) | 330 (108–460) |
| 20 kg group | 1860 (675–2330) | 894 (258–1530) † |
Data are the median and range
Exudate leakage occurred in one rat in the 20‐kg group, and these date were excluded
Macroscopic findings
The Macroscopic findings are shown in figure 1. After pressure unloading, a dark red circle with oedema at the circumference was seen in both 10‐kg and 20‐kg loading groups. In both groups, the colour tone of the wound surface area turned yellowish white on days 1, 2 and 3. On days 2 and 3, the exudate volumes were significantly increased. No deep/open ulcers were observed during the experimental period in either loading group. In addition, there were no differences in the macroscopic findings on days 1, 2 and 3 in both loading groups.
Figure 1.
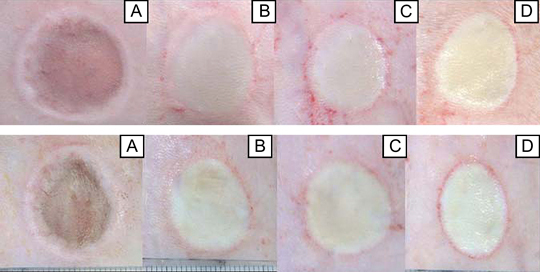
Macroscopic findings after wounding (A), and on day 1 (B), day 2 (C) and day 3 (D) after loading in the 10‐kg (upper) and 20‐kg (lower) groups using images from the same rat for each group. After pressure unloading, a dark red circle with oedema at the circumference was seen in the compressed areas in both 10‐kg and 20‐kg loading groups. In both groups, the tone colour of the wound surface area turns yellowish white on days 1, 2 and 3. There are no differences in the macroscopic appearances between the 10‐kg and 20‐kg loading groups.
Microscopic findings
Control slices, in which the myofibres showed normal multinucleated appearances with peripheral nuclei and small interstitial spaces, are shown in Figure 2A. These myofibres were large, homogeneous in size and polygonal in shape. The compressed area in the 10‐kg group (Figure 2B) showed extensive infiltration of polymorphonuclear cells (PMNs) and lymphocytes. These inflammatory cells were found in interstitial areas and internal muscle fibres. The numerous fibres were round shape, to pale‐stained, indicating the necrotic fibres. Most of the necrotic fibres were located in the deep muscle. However, normal muscle fibres could still be observed adjacent to the necrotic fibres. Large numbers of necrotic fibres were also observed in the 20‐kg group. The 20‐kg group showed more extensive infiltration of PMNs and lymphocytes and larger numbers of necrotic fibres than the 10‐kg group.
Figure 2.
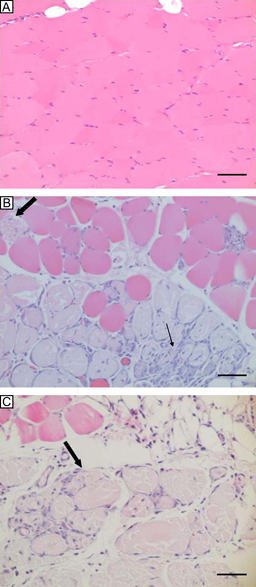
Transverse slices of HE‐stained abdominal muscle cross‐sections. (A) A control slice shows myofibres with normal appearances (scale bar, 50 μm). (B) Necrotic areas (big arrow) adjacent to normal muscle and massive infiltration of inflammatory cells (small arrow) are observed in the 10‐kg group (scale bar, 50 μm). (C) Larger necrotic areas (big arrow) are seen in the 20‐kg group (scale bar, 50 μm).
The numbers of necrotic fibres quantified by the k‐clustering method are shown in Table 3. The median percentages and ranges of necrotic fibres quantified by using the supervised k‐clustering method were 8.15 (7·40–8·90), 27·30 (27·30–34·33) and 44·25 (37·90–45·50) in the control group, 10‐kg loading group and 20‐kg loading group, respectively.
Table 3.
Numbers of necrotic muscle fibres quantified by image processing using the unsupervised k‐clustering method*
| Group | Necrotic fibres (percentage) |
|---|---|
| Control | 8.15 (7.40–8.90) |
| 10‐kg group | 27.30 (27.30–34.33) |
| 20‐kg group | 44.25 (37.90–45.50) |
Data are the median and range
Discussion
This study is the first attempt to measure the CPK levels in exudate samples from wound sites in comparison with the CPK levels in serum samples for detection of DTI following compressive loading. The results showed that the CPK levels in wound exudates more accurately reflected the status of the local wound environment and could be detected for a longer period than those in serum samples. These findings indicate that CPK in exudates may represent a useful indicator of DTI in clinical settings. Many studies have used serum CPK as a marker for muscle injury because serum CPK is sensitive to muscle damage 10, 24, 26, 27, 28. When muscle hypoxia, ischaemia, necrosis and other conditions that cause a change in fibre membrane permeability occur, the intracellular CPK leaks into the extracellular space, resulting in an elevated CPK level in blood 10, 11. However, serum CPK has a short half‐life as indicated by the results of the present study and previous studies 27, 29, 30. The CPK levels in the 10‐kg and 20‐kg groups showed marked elevation compared with the baseline value and control group at 8 hours after wounding, but returned to the normal level at day 1, consistent with a previous study showing that CPK returned to the normal level at 12 hours after exercise (27). In contrast to previous reports that serum CPK elevation corresponds to the degree of muscle injury 25, 26, 31, the present study showed that 20‐kg loading resulted in lower serum CPK elevation than in 10‐kg loading, contradicting the histological findings that the 20‐kg group exhibited more severe muscle damage than the 10‐kg group as indicated by the image processing analysis. This discrepancy may arise because of the large variability of serum CPK values, which has been reported by some researchers 29, 30. Previous research showed that serum CPK is sensitive to muscle damage. However, it is not sufficiently sensitive to measure the extent of muscle damage 29, 30.
It is interesting to note that the exudate CPK levels were high on days 2 and 3 when the serum CPK levels had returned to the normal level. Although the exudate CPK level cannot be compared with the level before loading, the exudate CPK levels on days 2 and 3 were higher than the serum CPK levels on the corresponding days. In addition, the exudate CPK level in the 20‐kg group tended to be higher than that in the 10‐kg group, particularly on day 3, corresponding to the histological findings that muscle necrosis was more severe in the 20‐kg group. These results indicate that the exudate CPK levels more accurately reflected the local status of the wound environment than the serum CPK levels. A limitation of the present study is that the exudate samples only increased significantly on days 2 and 3, and therefore measurements of CPK in exudate samples can only be conducted on days 2 and 3.
The macroscopic findings revealed no differences by visual assessment between the 10‐kg and 20‐kg loading groups, while the biochemical measurements of the exudate samples and histological findings showed that the 20‐kg group had more severe muscle damage than the 10‐kg group. This model of DTI represents clinical situations in which patients may show minimal skin damage by visual assessment, but muscle damage progressively develops into a more severe condition.
Interesting results were obtained regarding the different percentages of necrotic fibres in the 10‐kg and 20‐kg loading groups. In the present study, we used the unsupervised k‐clustering method to quantify the numbers of necrotic fibres in HE‐stained tissue sections. To the best of our knowledge, this study is the first trial to quantify HE‐stained necrotic tissue sections using the unsupervised k‐clustering method. In the present study, the clusters were divided into four groups to specify the types of cells based on their colour. The image analysis used in this study quantitatively differentiated among differences in the numbers of necrotic fibres after 10‐kg and 20‐kg loading, which might be difficult to measure qualitatively. Our image processing analysis could be useful for quantifying the numbers of damaged muscle fibres. However, this method has a limitation because the quantification is based on colour differences in HE‐stained tissue sections, and thus the results will also depend on the tissue processing procedures. In this study, the control group exhibited muscle fibres with normal appearances. However, in the k‐cluster method, small numbers of normal muscle fibres were quantified as necrotic because some tissue sections were not stained at the same intensity because of the presence of artefacts. When the method was applied to sections from the 10‐kg and 20‐kg groups without artefacts, the results confirmed that this method could distinguish among differences in the amounts of necrotic fibres between the 10‐kg and 20‐kg groups with small data variability among the rats. In addition, the amounts of necrotic fibres measured using this method in both groups corresponded with the qualitative findings indicating that muscle necrosis was more severe in the 20‐kg group than in the 10‐kg group. Considering this limitation, the presence of artefacts should be avoided when this method is conducted.
Although this was a pilot study with a small number of animals, the results provide important evidence regarding the potential of measuring CPK levels in exudate samples in clinical settings to detect the presence of DTI when a stage II pressure ulcer develops, thereby preventing wound progression into stages III and IV pressure ulcers. This study also provides a non invasive measurement that can be applied in clinical settings. Further studies with larger numbers of animals are required to confirm the usefulness of CPK measurements in clinical settings.
Conclusion
The present study is the first trial in the literature to detect DTI by measuring CPK levels in exudate samples. We found that the serum CPK level decreased to the normal level on day 1 after compressive loading. However, the exudate CPK levels remained high on day 2 and decrease on day 3, but still higher compared with the serum CPK in the same day. The results of the present study indicate that CPK in exudate samples could be a useful indicator of DTI.
References
- 1. Whittington KT, Briones R. National prevalence and incidence study: 6‐year sequential acute care data. Adv Skin Wound Care 2004;17:490–4. [DOI] [PubMed] [Google Scholar]
- 2. Woodbury MG, Houghton PE. Prevalence of pressure ulcers in Canadian health‐care settings. Ostomy Wound Manage 2004;50:22–38. [PubMed] [Google Scholar]
- 3. Japanese Society of Pressure Ulcers . Guideline for local treatment of pressure ulcers. Tokyo: Shorinsha, 2005. [Google Scholar]
- 4. Sato M, Sanada H, Konya C, Sugama J, Nakagami G. Prognosis of stage I pressure ulcers and related factors. Int Wound J 2006;3:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohura T, Ohura N, Oka H. Incidence and clinical symptoms of hourglass and sandwich‐shaped tissue necrosis in stage IV pressure ulcers. Wounds 2007;19:310–9. [PubMed] [Google Scholar]
- 6. Ankrom MA, Bennett RG, Sprigle S, Langemo D, Black JM, Berlowitz DR, Lyder CH, National Pressure Ulcer Advisory Panel . Pressure‐related deep tissue injury under intact skin and the current pressure ulcer staging systems. Adv Skin Wound Care 2005;18:35–42. [DOI] [PubMed] [Google Scholar]
- 7. Gawlitta D, Li W, Oomens CW, Baaijens FP, Bader DL, Bouten CV. The relative contributions of compression and hypoxia to development of muscle tissue damage: an in vitro study. Ann Biomed Eng 2007;35:273–84. [DOI] [PubMed] [Google Scholar]
- 8. Stekelenburg A, Oomens CW, Strijkers GJ, Nicolay K, Bader DL. Compression‐induced deep tissue injury examined with magnetic resonance imaging and histology. J Appl Physiol 2006;100:1946–54. [DOI] [PubMed] [Google Scholar]
- 9. Nagase T, Koshima I, Maekawa T, Kaneko J, Sugawara Y, Makuuchi M, Koyanagi H, Nakagami G, Sanada H. Ultrasonographic evaluation of an unusual peri‐anal induration: a possible case of deep tissue injury. J Wound Care 2007;16:365–7. [DOI] [PubMed] [Google Scholar]
- 10. Hagisawa S, Ferguson‐Pell MW, Palmieri VR, Cochran GV. Pressure sores: a biochemical test for early detection of tissue damage. Arch Phys Med Rehabil 1988;69:668–71. [PubMed] [Google Scholar]
- 11. Wagner JA, Critz JB. Effect of prednisolone on serum creatine phosphokinase response to exercise. Proc Soc Exp Biol Med 1968;128:716–20. [DOI] [PubMed] [Google Scholar]
- 12. World Union of Wound Healing Societies . Principles of best practice: wound exudates and the role of dressings. A consensus document. London: MEP Ltd, 2007. [Google Scholar]
- 13. Staiano‐Coico L, Higgins PJ, Schwartz SB, Zimm AJ, Goncalves J. Wound fluids: a reflection of the state of Healing. Ostomy Wound Manage 2000;46(1A Suppl):85S–93S. [PubMed] [Google Scholar]
- 14. Sugama J, Sanada H, Nakatani T, Nagakawa T, Inagaki M. Pressure‐induced ischemic wound healing with bacterial inoculation in the rat. Wounds 2005;17:157–68. [Google Scholar]
- 15. Hoshino T, Hosokawa N, Kumasaka K, Kawano K. The relationship of serum mitochondrial creatine kinase and rotavirus gastroenteritis in pediatric patients. Rinsho Byori 2001;49:597–602 (in Japanese). [PubMed] [Google Scholar]
- 16. Rasband W. Image processing and analysis in Java [homepage on the Internet]. Bethesda, MD: National Institutes of Health, 1997. http://rsb.info.nih.gov/ij/ [accessed 13 March 2008]. [Google Scholar]
- 17. Hung MC, Wu JP, Chang JH, Yang DL. An efficient k‐means clustering algorithm using simple partitioning. JISE 2005;21:1157–77. [Google Scholar]
- 18. Jain AK, Dubes RC. Algorithms for clustering data. Englewood Cliffs, NJ: Prentice Hall;1988. [Google Scholar]
- 19. Karaçali B, Vamvakidou AP, Tözeren A. Automated recognition of cell phenotypes in histology images based on membrane‐and nuclei‐targeting biomarkers. BMC Med Imaging 2007;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeung KY, Medvedovic M, Bumgarner RE. Clustering gene‐expression data with repeated measurements. Genome Biol 2003;4:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prastawa M, Bullitt E, Ho S, Gerig G. A brain tumor segmentation framework based on outlier detection. Med Image Anal 2004;8:275–83. [DOI] [PubMed] [Google Scholar]
- 22. Dalgin GS, Alexe G, Scanfekd D, Tamayo P, Mesirov JP, Ganesan S, DeLisi C, Bhanot G. Portraits of breast cancer progression. BMC Bioinformatics 2007;8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alexe G, Dalgin GS, Ganesan S, DeLisi C, Bhanot G. Analysis of breast cancer progression using principal component analysis and clustering. J Biosci 2007;32:1027–39. [DOI] [PubMed] [Google Scholar]
- 24. Kotil K, Tunckale T, Tatar Z, Koldas M, Kural A, Bilge T. Serum creatine phosphokinase activity and histological changes in the multifidus muscle: a prospective randomized controlled comparative study of discectomy with or without retraction. J Neurosurg Spine 2007;6:121–5. [DOI] [PubMed] [Google Scholar]
- 25. Arts MP, Nieborg A, Brand R, Peul WC. Serum creatine phosphokinase as an indicator of muscle injury after various spinal and nonspinal surgical procedures. J Neurosurg Spine 2007;7:282–6. [DOI] [PubMed] [Google Scholar]
- 26. Yousef MA, Vaida S, Somri M, Mogilner J, Lanir A, Tamir A, Yousef MA, Vaida S, Somri M, Mogilner, Lanir A, Tamir A, Shaoul R. Changes in creatine phosphokinase (CK) concentrations after minor and major surgeries in children. Br J Anaesth 2006;96:786–9. [DOI] [PubMed] [Google Scholar]
- 27. Sakamoto K, Nosaka K, Shimegi S, Ohmori H, Katsuta S. Creatine kinase release from regenerated muscles after eccentric contractions in rats. Eur J Appl Physiol Occup Physiol 1996;73:516–20. [DOI] [PubMed] [Google Scholar]
- 28. Aktas M, Auguste D, Lefebvre HP, Toutain PL, Braun JP. Creatine kinase in the dog: a review. Vet Res Commun 1993;17:353–69. [DOI] [PubMed] [Google Scholar]
- 29. Brancaccio P, Maffulli N, Limongelli FM. Creatine kinase monitoring in sport medicine. Br Med Bull 2007;81–82:209–30. [DOI] [PubMed] [Google Scholar]
- 30. Schneider CM, Dennehy CA, Rodearmel SJ, Hayward JR. Effects of physical activity on creatine phosphokinase and the isoenzyme creatine phosphokinase‐mb. Ann Emerg Med 1995;25:520–4. [DOI] [PubMed] [Google Scholar]
- 31. Carter WO, Bull C, Bortolon E, Yang L, Jesmok GJ, Gundel RH. A murine skeletal muscle ischemia‐reperfusion injury model: differential pathology in balb/c and dba/2n mice. J Appl Physiol 1998;85:1676–83. [DOI] [PubMed] [Google Scholar]


