Abstract
Antimicrobial dressings such as those containing silver are now being used widely to control wound bioburden, and tests to demonstrate their efficacy predominantly involve in vitro models using free‐living or planktonic bacteria. In this present study a wide range of antibiotic‐sensitive and resistant bacteria were tested in their quasi‐sessile state using a standard agar assay and a second method used a poloxamer gel (true biofilm state – poloxamer encourages microorganisms to exhibit a more clinically relevant biofilm phenotype) technique. The antimicrobial activity of two silver dressings, a silver‐containing Hydrofiber® (SCH) dressing and a nanocrystalline silver‐containing dressing (NCS), were evaluated on a variety of microorganisms, using a zone‐of‐inhibition (ZOI) test. When grown on agar (presenting a quasi‐sessile state of each organism), the antibiotic‐susceptible microorganisms were generally more susceptible to the SCH dressing compared with the NCS. ZOIs associated with SCH dressing ranged between 5·7 and 17·5 mm; those for the NCS against the same group of organisms ranged between 1·9 and 8·6 mm. When grown on poloxamer gel, (presenting the biofilm state of each organism) the same group of microorganisms were less susceptible to both dressings. The SCH dressing was most effective against strains of Pseudomonas aeruginosa, Candida albicans and Staphylococcus aureus (ZOI range: 2·6–6 mm); the NCS was most effective against strains of Klebsiella pneumoniae, Enterococcus faecalis and Escherichia coli (i.e. ZOI range: 1–2·8 mm). Similarly to the antibiotic‐susceptible microorganisms, nine of ten antibiotic‐resistant bacterial strains when grown on agar were more susceptible to the SCH dressing compared with the NCS. Although the microorganisms tested were universally less susceptible to the silver dressings when in their biofilm state, in the majority of cases, the SCH dressing demonstrated greater biofilm‐inhibiting activity than the NCS.
Keywords: Biofilms, Poloxamer, Silver dressings, Wound care
Introduction
Wounds are susceptible to microbial contamination from both exogenous and endogenous sources, and it is likely that such organisms are involved in the formation of biofilms in wounds (1). Early contaminants of wound tissue are most likely to be skin flora (e.g. Staphylococcus epidermidis) that adhere to the wound (2), proliferate, synthesise extracellular polymeric substances and form a biofilm.
With the emergence and escalation of bacterial resistance, particularly to antibiotics (3) and associated increased health care costs, topical antiseptics are being used more extensively to control wound bioburden 1, 4, 5. In vitro techniques used to evaluate topical antimicrobial dressings are varied. One method is based on the zone of inhibition (ZOI), which measures bacterial clearance around a sample of dressing placed on an inoculated agar plate (6). However, it is now appreciated that culturing bacteria from viable tissue for use in the ZOI test alters a sessile pathogen into a lab‐adapted planktonic counterpart (7). Evidence presented by Christensen et al. (8) and Freeman et al. (9) have shown that non biofilm bacteria are able to grow as colony‐forming units on agar, suggesting that agar is not an ideal substratum for biofilm susceptibility testing of antimicrobials as it induces only a quasi‐sessile bacterial state. The quasi‐sessile state implies that the bacteria do express characteristic biofilm inducing genes and do not have the same phenotypic adaptations which lead to a true sessile and biofilm state and as such show an increased vulnerability to antimicrobials when compared to bacteria in a true biofilm state.
In this present study, a wide range of antibiotic‐sensitive and resistant bacteria and a yeast were tested using a standard agar assay (Kirby–Bauer‐type disc diffusion method (10), and a second method used a poloxamer technique to encourage the same strains of microorganisms to exhibit a more clinically relevant biofilm phenotype. Poloxamer gel cultures mimic many of the properties of biofilm‐grown bacteria and therefore provides a reproducible method for testing the antimicrobial efficacy of biocides against biofilm bacteria (11). Poloxamer hydrogels have been used to study biofilms of Streptococcus mutans in plaque (12), to study homoserine lactones and biocide efficacy in biofilms (13) and coaggregation in bacteria (14). Evidence of biofilm growth in the poloxamer model has also been confirmed using confocal laser microscopy (15). Data to date indicates therefore that poloxamer induces bacteria to grow in a biofilm, suggesting this to be an ideal surface to induce biofilm phenotypes.
In this study, we have utilised the biofilm‐inducing properties of poloxamer to provide a more relevant method to test the effectiveness of antimicrobial dressings on biofilm microorganisms.
Methods and materials
Media and reagents
Mueller–Hinton broth (MHB) and Mueller–Hinton agar (MHA; Laboratory M, Bury, UK) were used throughout. Poloxamer F127 was obtained from Univar (Essex, UK) and all other chemicals were purchased from BDH (Poole, UK), Bio Rad (Hemel Hempstead, UK) or Sigma (Poole, UK).
Test materials
The antimicrobial dressings used in this study were a silver‐containing Hydrofiber® (SCH) dressing (ConvaTec™, Princeton, NJ, USA) and a nanocrystalline silver‐containing dressing (NCS) (Smith and Nephew™, London, UK). The NCS dressing consists of a rayon/polyester non woven inner core laminated between two layers of silver‐coated high‐density polyethylene mesh. The layers are held together with ultrasound welds. The SCH dressing comprises of sodium carboxymethylcellulose Hydrofiber® and ionic silver. A Hydrofiber® dressing without silver was used as a control.
Microorganisms
A wide variety of aerobic bacteria (including antibiotic‐resistant strains) and Candida albicans known to be associated with wound colonisation and infection were included in the study. These included reference isolates: Staphylococcus aureus (National Collection of Industrial and Marine Bacteria [NCIMB] 9518), Pseudomonas aeruginosa (NCIMB 8626), Escherichia coli (NCIMB 8545), C. albicans (National Collection of Pathogenic Fungi [NCPF] 3179); clinical isolates: Enterococcus faecalis (clinical isolate 141), Klebsiella pneumoniae (clinical isolate 033); and antibiotic‐resistant bacteria: P. aeruginosa (NCIMB 8506), vancomycin‐resistant enterococcus (VRE) (National Collection of Type Cultures [NCTC] 12201), VRE (clinical isolate 1), VRE (clinical isolate 2), methicillin‐resistant S. aureus (MRSA) (NCTC 12232), MRSA (Cardiff clinical isolate 1), MRSA (clinical isolate 026), MRSA (clinical isolate 10371), MRSA (clinical isolate 10442) and Serratia marcescens (clinical isolate 1173146). An overnight culture of each microbial isolate was prepared in maximal recovery diluent (MRD; Laboratory M) at a concentration of approximately 1 × 105 colony‐forming units/mL. A 1‐ml volume of each organism suspension was inoculated onto the surface of MHA and poloxamer gel plates in duplicate, which were then gently agitated to allow full coverage of the inoculum on the agar or gel surface, and then allowed to dry.
Poloxamer hydrogels (biofilm phenotype induction)
Poloxamer F127 hydrogels are diblock copolymers of polyoxyethylene and polyoxypropylene that demonstrate thermoreversible gelation properties. At temperatures below 15°C, poloxamer is liquid and fully miscible with water but changes to a firm gel at temperatures in excess of 15°C. In this study, poloxamer hydrogels were prepared by mixing MHB with poloxamer F127 to make a final concentration of 30% poloxamer (w/v) (11). The solutions were chilled overnight to allow the poloxamer to go into solution. Prior to use, the poloxamer solution was autoclaved at 121°C for 15 minutes and then stored at 4°C. The solutions were mixed gently after incubation. Twenty‐five‐millilitre volumes of the poloxamer solutions were added to Petri dishes and then incubated at 35°C (±3°C) to allow the poloxamer to solidify.
Efficacy of silver dressings against poloxamer biofilms
Dressing preparation and evaluation
Circular samples (2‐cm diameter) of dressings were aseptically cut from a non SCH dressing (control), a SCH dressing and an NCS. With the use of sterile forceps, one circle of each dressing type was placed onto each inoculated MHA and poloxamer gel plate and pressed down gently to ensure close contact. A non SCH dressing and an SCH dressing were hydrated with 0·5 mL MRD to more closely mimic hydration in wound conditions. An NCS was hydrated with 0·5 mL sterile distilled water (as per the manufacturers’ instructions: the dressing requires hydration with sterile water before applying the dressing to the wound). Plates inoculated with bacteria were incubated at 35°C ± 3°C for 24 hours, and those inoculated with yeasts were incubated at 25°C ± 2°C for 48 hours.
After 24‐hour incubation, all plates were observed and the ZOIs around each sample on both the MHA and poloxamer gel plates were measured. All tests were carried out in duplicate.
Disodium ethylenediaminetetraacetic acid (EDTA, Fisher, Loughborough, UK) was used as a positive control 16, 17, 18 and saline as a negative control. For both the positive and negative controls, two sterile glass rings (diameter 15 mm) were placed aseptically onto each Petri dish containing poloxamer gel. A 400‐μL aliquot of 4% (w/v) disodium EDTA. pH 4·0, was added to one ring and 400 μL of 0·85% (w/v) saline (Oxoid) added to the other. The dishes were incubated at 35°C ± 3°C for 24 ±1 hour and then examined for signs of ZOIs of growth, which were measured using digital calipers (Mitutoyo, Singapore, Malaysia).
Statistical analysis
Data were analysed by the Mann–Whitney U‐test.
Results
Figures 1a–1d illustrate the antimicrobial efficacy data for the SCH dressing and the NCS dressing against a variety of aerobic bacteria (including antibiotic‐resistant strains).
Figure 1.
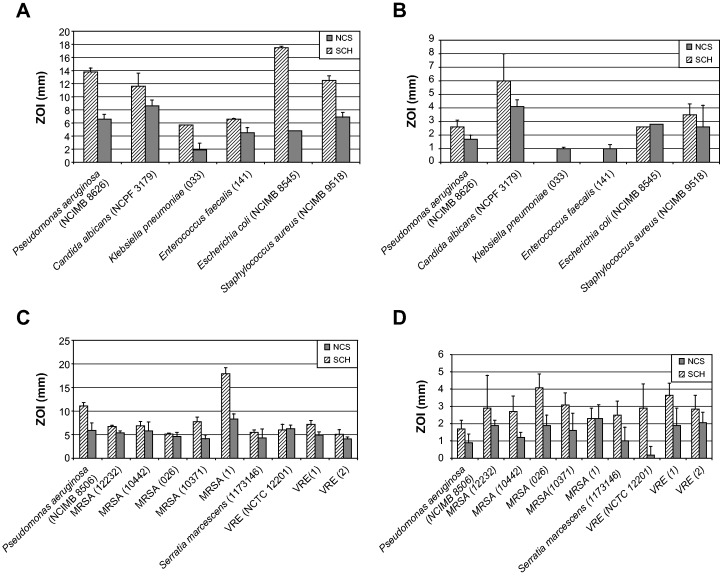
(a) Zone of inhibition (ZOI) induced by silver‐containing dressings against bacteria and yeast grown on Mueller–Hinton agar (MHA). Results are expressed as means ± standard error. (b) ZOI induced by silver‐containing dressings against bacteria and yeast grown on poloxamer gel. Results are expressed as means ± standard error. (c) ZOI induced by silver‐containing dressing against multiple resistant bacteria grown on MHA. Results are expressed as means ± standard error. (d) ZOI induced by silver‐containing dressings against multiple resistant bacteria on poloxamer gel. Results are expressed as means ± standard error. SCH, silver‐containing Hydrofiber® dressing; NCS, nanocrystalline silver‐containing dressing; MRSA, methicillin‐resistant Staphylococcus aureus; VRE, vancomycin‐resistant enterococcus.
On agar, the antibiotic‐susceptible microorganisms were generally more susceptible to the SCH dressing compared with the NCS (i.e. in 6/6 cases, Figure 1a). ZOIs associated with SCH dressing ranged between 5·7 and 17·5 mm; those for the NCS against the same group of organisms ranged between 1·9 and 8·6 mm. All data were shown to be statistically significant (P < 0·05). When presented in their biofilm form (poloxamer gel), the same group of microorganisms were less susceptible to both dressings (Figure 1b); the SCH dressing was most effective (P < 0·05) against strains of P. aeruginosa, C. albicans and S. aureus (ZOI range: 2·6–6 mm), and the NCS was most effective (P < 0·05) against strains of K. pneumoniae, E. faecalis and E. coli (ZOI range: 1–2·8 mm).
Similarly to the antibiotic‐susceptible microorganisms, nine of ten antibiotic‐resistant bacterial strains presented on agar were more susceptible (P < 0·05) to the SCH dressing compared with the NCS (Figure 1c) (ZOIs ranged between 5·1 and 17·9 mm for the SCH dressing and between 4·1 and 8·3 mm for the NCD). In their biofilm (poloxamer gel) form (Figure 1d), antibiotic‐resistant strains of bacteria showed reduced susceptibility to both dressings, but in nine of ten cases, the SCH dressing proved to be the more effective (P < 0·05) dressing, with equivalent activity being demonstrated against one clinical strain of MRSA (ZOI ranged between 1·7 and 4·1 mm for the SCH dressing and between 0·9 and 2·3 mm for the NCS).
The positive control produced a zone of clearing against P. aeruginosa and S. aureus biofilm. Both silver dressings produced a zone of clearing around both MRSA and P. aeruginosa when grown on agar (Figures 2a, 2c) and poloxamer gel (Figures 2b, 2d), although these were small, particularly for the NCS dressing.
Figure 2.
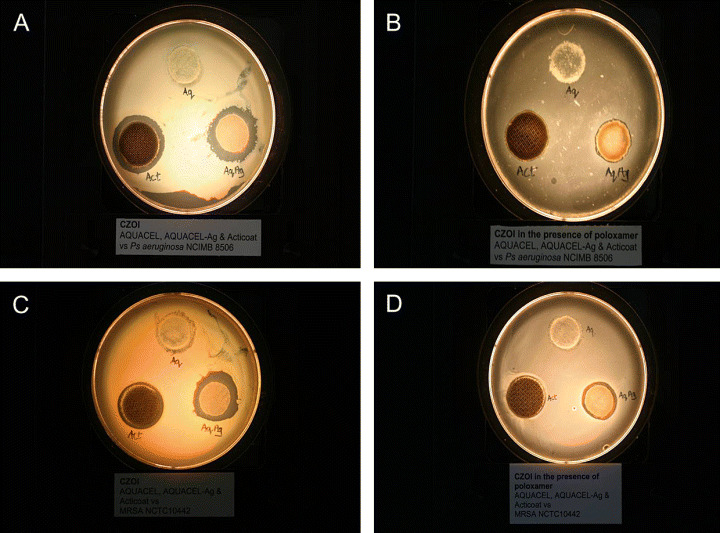
(a) Zone of inhibition (ZOI) against Pseudomonas aeruginosa (NCIMB 8506) on Mueller–Hinton agar (MHA). (b) ZOI of P. aeruginosa (NCIMB 8506) on poloxamer gel. (c) ZOI against methicillin‐resistant Staphylococcus aureus (NCTC 10442) on MHA. (d) ZOI of MRSA (NCTC 10442) on poloxamer gel Act (Acticoat™) is the nanocrystalline silver‐containing dressing, Aq (Aquacel®) is a non silver‐containing Hydrofiber® dressing, and AqAg (Aquacel® Ag) is a silver‐containing Hydrofiber® dressing).
Discussion
Using two silver‐containing dressings, the SCH dressing and the NCS, activity against biofilm microorganisms has been demonstrated, although differences in the level of activity was observed. In 15 of the 16 microorganisms tested on agar, the SCH dressing produced greater ZOIs compared with the NCS. Although the NCS dressing contains approximately ten times more silver (w/w of dressing) than the SCH dressing, this study showed that the concentration of silver did not correlate with the level of activity, and this has been reported elsewhere (19). Ionic silver (the active form of silver, Ag+) exerts antimicrobial activity at very low concentrations (20), and in vitro studies have shown that silver can be effective at concentrations well below the previously reported minimum inhibitory concentrations values (19).
In their biofilm form, antibiotic‐sensitive bacteria and C. albicans generally showed reduced susceptibility to both silver‐containing dressings. While the SCH dressing continued to demonstrate greater efficacy against five of the nine strains tested, the NCS dressing performed more favourably against strains of K. pneumoniae, E. cloacae, E. faecalis and E. coli. With respect to antibiotic‐resistant bacteria, the majority of which were MRSA and VRE strains, the SCH dressing performed more effectively than the NCS in nine out of ten cases (one strain of VRE being an exception) for the quasi‐biofilm forms, and in nine out of ten cases when the same bacteria were presented in their biofilm form. However, ZOIs were again generally smaller than for their quasi‐biofilm counterparts for both dressings.
As a test method to evaluate the efficacy of ionic silver on sessile (biofilm) bacteria, it is evident from this research that, ionic silver is less effective on poloxamer‐grown biofilm than on agar‐grown quasi‐sessile bacteria. Biofilms are well known to be recalcitrant to antimicrobials and have been documented in both infected and non‐infected chronic wounds (21), and therefore it is most likely that bacteria within biofilms needs to be controlled in order to facilitate wound progression.
It is recognised that in vitro data cannot be directly extrapolated to clinical outcomes. Nevertheless, in vitro models can be beneficial to enhance our understanding of how topical antimicrobial agents and wound dressings may facilitate wound management and every effort must be taken to ensure that in vitro models are both as stringent and realistic as possible, as was the case in this study.
Although the microorganisms tested in this in vitro model were universally less succeptable to the silver dressings when in their biofilm state, in the majority of cases, the SCH dressing demonstrated greater biofilm inhibiting activity than the NCS dressing.
Acknowledgement
Hydrofiber® is a registered trademark of E.R. Squibb and Sons, L.L.C. All other trademarks are the property of their respective owners.
References
- 1. Percival SL, Bowler PG. Biofilms and their potential role in wound healing. Wounds 2004;16:234–40. [Google Scholar]
- 2. Bowler PG. Progression toward healing: wound healing and the role of an advanced silver‐containing Hydrofiber® dressing. Ostomy Wound Manage 2003; 49 Suppl. 8A:2–5. 12856288 [Google Scholar]
- 3. Normark BH, Normark S. Evolution and spread of antibiotic resistance. J Intern Med 2002;252:91–106. [DOI] [PubMed] [Google Scholar]
- 4. Kingsley A. The wound infection continuum and its application to clinical practice. Ostomy Wound Manage 2003;49:1–7. [PubMed] [Google Scholar]
- 5. Cutting KF, White RJ. Criteria for identifying wound infection–revisited. Ostomy Wound Management 2005;49:1–7. [PubMed] [Google Scholar]
- 6. Jones SA, Bowler PG, Walker M. Antimicrobial activity of silver‐containing dressings is influenced by dressing conformability with a wound surface. Wounds 2005;17:263–70. [Google Scholar]
- 7. Costerton JW, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 2003;112:1466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christensen GD, Baddour LM, Madison BM, Parisi JT, Abraham SN, Hasty DL, Lowrance JH, Josephs JA, Simpson WA. Colonial morphology of staphylococci on Memphis agar: phase variation of slime production, resistance to beta‐lactam antibiotics, and virulence. J Infect Dis 1990;161:1153–69. [DOI] [PubMed] [Google Scholar]
- 9. Freeman DJ, Falkiner FR, Keane CT. New method for detection of slime production by coagulase‐negative staphylococci. J Clin Pathol 1989;42:872–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 1996;45:493–6. [PubMed] [Google Scholar]
- 11. Gilbert P, Jones MV, Allison DG, Heys S, Maira T, Wood P. The use of poloxamer hydrogels for the assessment of biofilm susceptibility towards biocide treatments. J Appl Microbiol 1998;85:985–90. [DOI] [PubMed] [Google Scholar]
- 12. Kim MM, Park HK, Kim SN, Kim HD, Kim YH, Rang MJ, Ahn HJ, Hwang JK. Effect of a new antibacterial agent, xanthorrhizol on the viability of plaque biofilm. Poster 3883, IADR/AADR/CADR 80th, San Diego, 6 9 March 2002.
- 13. MacLehose HG, Gilbert P, Allison DG. Biofilms, homoserine lactones and biocide susceptibility. J Antimicrob Chemother 2004;53:180–4. [DOI] [PubMed] [Google Scholar]
- 14. Rickard AH, Gilbert P, Handley PS. Influence of growth environment on coaggregation between freshwater biofilm bacteria. J Appl Microbiol 2004:96:1367–73. [DOI] [PubMed] [Google Scholar]
- 15. Sincock SA, Rajwa B, Robinson PJ. Characteristics and dynamics of bacterial populations with poloxamer hydrogel biofilm constructs. Abstract 6451, International Society for Analytical Cytology XX International Congress, 2025 May 2000, Le Corum, Montpellier, France. [Google Scholar]
- 16. Kite P, Eastwood K, Sugden S, Percival SL. Use of in vivo‐generated biofilms from hemodialysis catheters to test the efficacy of a novel antimicrobial catheter lock for biofilm eradication in vitro. J Clin Microbiol 2004;42:3073–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Percival SL, Kite P, Eastwood K, Murga R, Carr J, Arduino MJ, Donlan RM. Tetrasodium EDTA as a novel central venous catheter lock solution against biofilm. Infect Control Hosp Epidemiol 2005:26:515–9. [DOI] [PubMed] [Google Scholar]
- 18. Shanks RMQ, Sargent JL, Martinez RM, Graber ML, O’Toole GA. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant 2006;21:2247–55. [DOI] [PubMed] [Google Scholar]
- 19. Parsons D, Bowler PG, Myles V, Jones S. Silver antimicrobial dressings in wound management: a comparison of antibacterial physical, and chemical characteristics. Wounds 2005;17:222–32. [Google Scholar]
- 20. Russell AD, Hugo WB. Antimicrobial activity and action of silver. Prog Med Chem 1994;31:351–70. [DOI] [PubMed] [Google Scholar]
- 21. Ngo Q, Vickery K, Deua AK. PR21 Role of bacterial biofilms in chronic wounds. ANZ J Surg 2007;77:A66. [Google Scholar]


