Abstract
Chronic wounds are a major health care problem worldwide. Wound healing is a holistic endeavour that requires an accurate identification of the specific entities interfering with wound healing in a particular patient. We present a case of fixed sporotrichosis as the cause of a chronic ulcer in the knee. Although a culture of Sporothrix schenckii could not be obtained, a positive response to the sporotrichin skin test, a skin biopsy showed a suppurative granuloma and an adequate response to oral administration of potassium iodide confirmed the diagnosis. The identification and correction of the underlying aetiology of any chronic wound is the first and most important step to restore wound healing.
Keywords: Chronic wound, Potassium iodide, Skin ulcer, Sporotrichin skin test, Sporotrichosis, Wound healing
Introduction
A chronic wound is defined as a break in the skin of long duration (>6 weeks) that does not heal at the expected rate or has frequent recurrence 1, 2. In today’s society, chronic wounds represent a major health care burden. Wound care is a holistic endeavour that requires an accurate identification of the specific entities interfering with wound healing in a particular patient (1). When approaching a non healing wound, a thorough medical history and physical examination are essential. Healthy patients heal in a timely manner, while patients with chronic non healing wounds almost always have factors that impair proper wound healing. Chronic wounds predominate in the lower extremities and venous ulcers, foot ulcer and arterial insufficiency are the most common causes of leg ulceration (2). However, chronic wounds may be the result of other not so common causes such as neoplasms or inflammatory (pyoderma gangrenosum, vasculitis) or chronic infections. Assessment of chronic wounds must be oriented towards identifying the underlying cause of the wound along with adequate wound bed preparation (debridement, infection control and exudate management). Sometimes, if the aetiology of the chronic wound is unclear or if it is not responding as expected despite appropriate wound bed preparation, a biopsy may be helpful.
Case report
A 53‐year‐old female patient presented to the Interdisciplinary Wound and Ostomy Care Center with a chronic ulcer on the right knee that had been present for over 3 years. The ulcer measured 5 × 3 cm, had regular verrucous borders and the wound bed had pink granulation tissue with scattered crusts (Figure 1). She stated that the lesion developed after suffering trauma on her right knee when falling down over some wooden branches and rocks approximately 3 years before. She had been seen by multiple physicians and had been prescribed many different systemic antibiotics and wound cleansing with diverse antiseptics without any improvement. The patient was otherwise healthy and had no history of chronic diseases. At the time she was seen, the patient was not taking any medications.
Figure 1.
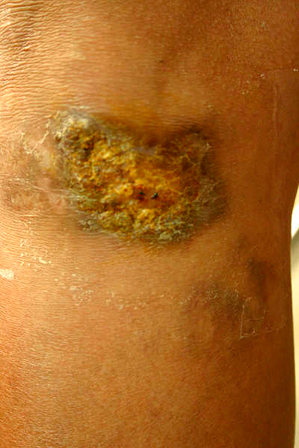
Ulcer appearance the first time the patient showed up at our Interdisciplinary Wound and Ostomy Care Center.
The patient was treated initially as a post‐traumatic wound. Surgical wound bed debridement was performed and topical povidone iodine was applied. After a couple of weeks, there had been no changes in wound size despite treatment. For this reason and because of the duration of the wound C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were determined along with a plain X‐ray to rule out chronic osteomyelitis. The radiograph had no suggestive changes and the CRP and ESR were slightly above normal limits, but not enough to suggest chronic osteomyelitis. She was started on oral clindamycin 300 mg t.i.d. and ciprofloxacin 500 mg b.i.d. A wound biopsy was performed to rule out a chronic foreign body reaction. The biopsy specimen showed a suppurative granulomatous dermatitis suggestive of a subcutaneous mycosis or mycobacterial infection. An intradermal sporotrichin test was positive and a tuberculin skin test was negative (Figure 2). A fungal culture was obtained and antibiotics were stopped.
Figure 2.
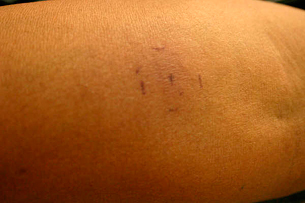
Forearm showing a positive sporotrichin skin test.
According to the histopathological findings and the positive sporotrichin skin test, the patient was started on a saturated solution of KI (SSKI) prepared with 300 ml of water with 20 g KI and given three tablespoons daily (2.7 g/day). Treatment duration was dictated by clinical response (Figure 3). A nano‐crystalline silver dressing was applied over the wound bed. After 4 weeks, the wound had completely healed and the patient had no signs or symptoms of iododerma, gastrointestinal upset or thyroid dysfunction (Figure 4). Treatment was stopped. The patient was followed up for 1 year without any signs of recurrence.
Figure 3.
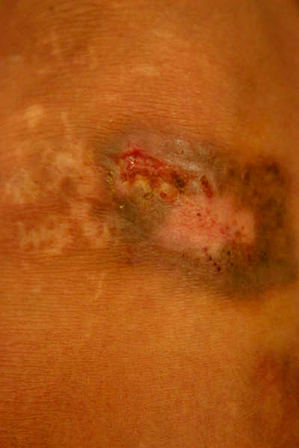
Wound appearance after 15 days of treatment with SSKI.
Figure 4.

Healed wound after 1 month of SSKI administration.
Discussion
Sporotrichosis is the most frequent subcutaneous mycosis in Mexico 3, 4. Three clinical forms are known: (i) lymphocutaneous (75%), (ii) fixed cutaneous (20%) and (iii) disseminated (5). Sporothrix schenckii, a dimorphic fungus, is the aetiological agent. The fungus has a worldwide distribution (6). Fixed sporotrichosis may be a clinical diagnostic challenge as it may mimic other dermatological diseases (7).
In our case, the fungal culture was negative and we were unable to isolate Sporothrix schenckii; but the positive sporotrichin skin test, the suppurative granuloma found histologically and the therapeutic response to SSKI support the diagnosis of fixed sporotrichosis.
The fixed form of sporotrichosis may appear as an ulcerated or verrucous lesion of variable duration. Clinically, it may be mistaken for other causes of a persistent, non healing ulcer (7). Sporotrichosis develops mainly through penetration of the fungus into the dermis after wounds or abrasions of the skin produced by infected materials such as thorns, hay, wood, splinters, barbed wire, gardening tools or flowers (5). Thus, the risk of sporotrichosis infection is determined mainly by the chance of a contact with Sporothrix schenckii. This risk, irrespective of age, race or sex, is particularly related to people’s occupations and activities (8). It is not clear yet why some people develop localised disease whereas others develop lymphocutaneous sporotrichosis. Some factors that have been related are the size and depth of inoculums, heat resistance of strains because fixed cutaneous lesions seem to be caused by strains that grow best at 35°C, whereas lymphocutaneous and extracutaneous disease are associated with strains that form colonies both at 35°C and 37°C, and host immune response as localised sporotrichosis has been considered a clinical form that occurs for patients with certain immunity against the fungus, whereas lymphangitic disease develops for patients who have not been in previous contact with the pathogen. However, these hypotheses still need to be validated 5, 8, 9, 10.
The basic histopathological pattern of the lesion is a combination of pyogenic and granulomatous reaction and includes epidermal hyperplasia, papillomatous acanthosis, hyperkeratosis, intraepidermal microabscesses and fungal elements such as rounded yeast‐like cells, cigar‐shaped cells and asteroid bodies 5, 11, 12.
Although infection with S. schenckii is rarely life threatening, all forms of sporotrichosis require some kind of treatment (13). Therapeutic options include SSKI, azoles (itraconazole or fluconazole), polyenes (amphotericin B), and allylamines (terbinafine) (14). According to results from current clinical trials, oral itraconazole is the drug of choice for the treatment of cutaneous sporotrichosis because of its safety, easy administration and high efficacy 9, 13, 14. However, in developing countries, where most endemic areas are located, its high cost is prohibitive and, therefore KI remains the mainstay of treatment 5, 9, 13.
Potassium iodide is usually administered in the form of a saturated solution (SSKI) at a dose of 47 mg/drop. SSKI is made by adding KI to hot purified water, using sodium thiosulphate as a preservative. To protect against gastrointestinal irritation, this solution can be added to water, fruit juice, or milk before drinking (15). The dose used to treat mycoses begins at 600 mg/day (approximately 12 drops of SSKI) orally three times daily and often increased to 6 g (approximately 127 drops of SSKI) daily if tolerated (16). After ingestion, KI is readily absorbed in the intestinal tract and distributes rapidly through the extracellular space. Iodide is concentrated in the thyroid gland, salivary glands, gastric mucosa, choroids plexus, mammary glands and the placenta. Ninety per cent of the orally administered dose is excreted in the urine. Sweat, breastmilk and faeces account for the remainder of the excretion (17). The precise mechanism by which KI exerts its therapeutic effect is unknown. It is unclear whether KI works against fungi by a fungicidal mechanism or by enhancing the body’s immunological and non immunological defence mechanisms. Sporothrix schenckii actually grows when plated in KI (18). Cell degeneration, however, has been shown by electron microscopy to occur in S. schenckii yeast dipped in varying concentrations of iodine (19). KI does not appear to increase monocyte or neutrophil killing of S. schenckii (20). Oral iodide was first used at the beginning of the 20th century and continues to be used in most of the world for fixed cutaneous disease because of its effectiveness and low cost (21).
Medicine generally and dermatology specifically must place great emphasis on quality wound care. The costs of wound care are escalating dramatically, and as our population ages, the incidence of chronic wounds will almost certainly increase. The staggering expenditures presently extant do not even quantify the loss of productivity, deleterious effects of wounds on quality of life or the indirect costs incurred by caregivers in time and effort spent caring for patients (2). Wound care is an interdisciplinary and holistic endeavour where medical dermatology has a major responsibility in the correct diagnosis and treatment of patients with skin ulcers.
This case report highlights the importance of properly identifying the cause of the wound and treating it accordingly. It also emphasises that in developing countries chronic infections are still a cause of chronic wounds.
References
- 1. Fowler E Chronic wounds: an overview. In: Krasner D, editor. Chronic wound care: a clinical source book for healthcare professionals. King of Prussia: Health Management Publications, Inc, 1990:12–8. [Google Scholar]
- 2. Fonder MA, Lazarus GS, Cowan DA, Aronson‐Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008;58:185–206. [DOI] [PubMed] [Google Scholar]
- 3. Méndez‐Tovar LJ, Anides‐Fonseca AE, Peña‐González G, Manzano‐Gayosso P, López‐Martinez R, Mernández‐Mernándoz F, Almerda‐Arvizu VM. Unknown fixed cutaneous sporotrichosis. Rev Iberoam Micol 2004;21:150–2. [PubMed] [Google Scholar]
- 4. Conti Díaz IA. Epidemiology of sporotrichosis in Latin America. Mycopathologia 1989;108:113–6. [DOI] [PubMed] [Google Scholar]
- 5. Da Rosa AC, Scroferneker ML, Vettorato R, Gervini RL, Vettorato G, Weber A. Epidemiology of sporotrichosis: a study of 304 cases in Brazil. J Am Acad Dermatol 2005;52:451–9. [DOI] [PubMed] [Google Scholar]
- 6. Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol 1994;33:38–41. [DOI] [PubMed] [Google Scholar]
- 7. Rathi SK, Ramam M, Rajendran C. Localized cutaneous sporotrichosis lasting for 10 years. Indian J Dermatol Venereol Leprol 2003;69:239–40. [PubMed] [Google Scholar]
- 8. Vismer HF, Hull PR. Prevalence, epidemiology and geographical distribution of Sporothrix schenckii infections in Gauteng, South Africa. Mycopathologia 1997;137:137–43. [DOI] [PubMed] [Google Scholar]
- 9. Bustamante B, Campos PE. Endemic sporotrichosis. Curr Opin Infect Dis 2001;14:145–9. [DOI] [PubMed] [Google Scholar]
- 10. Kwon‐Chung KJ. Comparison of isolates of Sporothrix schenckii obtained from fixed cutaneous lesions with isolates from others types of lesions. J Infect Dis 1979;139:424–31. [DOI] [PubMed] [Google Scholar]
- 11. Rippon JW. Sporotrichosis. In: Rippon JW, editor. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. 3rd edn. Philadelphia: WB Saunders, 1988:325–52. [Google Scholar]
- 12. Morris‐Jones R. Sporotrichosis. Clin Exp Dermatol 2002;27:427–31. [DOI] [PubMed] [Google Scholar]
- 13. Kauffman CA. Sporotrichosis: state‐of‐the‐art clinical article. Clin Infect Dis 1999;29:231–7. [DOI] [PubMed] [Google Scholar]
- 14. Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the management of patients with sporotrichosis. Clin Infect Dis 2000;30:684–7. [DOI] [PubMed] [Google Scholar]
- 15. Sterling JB, Heymann WR. Potassium iodide in dermatology: a 19th century drug for the 21st century‐uses, pharmacology, adverse effects, and contraindications. J Am Acad Dermatol 2000;43:691–7. [DOI] [PubMed] [Google Scholar]
- 16. Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol 1994;31(Suppl):S91–S102. [DOI] [PubMed] [Google Scholar]
- 17. Cavalieri R. Iodine metabolism and thyroid physiology: current concepts. Thyroid 1997;7:177–81. [DOI] [PubMed] [Google Scholar]
- 18. Tio T, De Vries G. Subcutaneous zygomycosis (phycomycosis). S Afr Med J 1977;52:77–8. [PubMed] [Google Scholar]
- 19. Hiruma M, Kagawa S. Ultrastructure of Sporothrix shenckii treated with iodine‐potassium iodide solution. Mycopathologia 1987;97:121–7. [DOI] [PubMed] [Google Scholar]
- 20. Rex J, Bennett J. Administration of potassium iodide to normal volunteers does not increase killing of Sporothrix schenckii by their neutrophils or monocytes. J Med Vet Mycol 1990;28:185–9. [DOI] [PubMed] [Google Scholar]
- 21. Mercurio MG, Elewski BE. Therapy for sporotrichosis. Semin Dermatol 1993;12:285–9. [PubMed] [Google Scholar]


