Abstract
This retrospective study compared the clinical outcomes of negative pressure wound therapy with reticulated open cell foam (NPWT/ROCF) as delivered by Vacuum‐Assisted Therapy® (V.A.C.® Therapy, KCI Licensing Inc., San Antonio, TX) to non‐NPWT/ROCF conventional therapy (CT) in split‐thickness skin graft (STSG) survival in all patients to determine whether NPWT/ROCF affects the outcome of the graft survival, in terms of overall graft take, duration of graft take, repeated grafts and complications.
The authors conducted a 10‐year retrospective review of 142 patients admitted to a level I trauma centre and treated with an STSG in foot and ankle reconstructive surgeries. Demographic data, wound etiology, dressing type used, time to graft take, NPWT/ROCF duration, complications and outpatient treatments were analysed. There were significantly fewer repeated STSGs required in the NPWT/ROCF group compared to CT [n = 3 (3·5%) versus n = 9 (16%); P = 0·006]. In assessing safety, there were fewer complications in graft failure (seroma, hematoma and infection) in the NPWT/ROCF group as compared to the CT group at 8·9 months (range: 1–12 months). NPWT/ROCF is an excellent alternative for securing an STSG and is associated with improved graft survival as measured by a reduction in the number of repeated STSGs and graft failure complications.
Keywords: Negative pressure wound therapy, Split‐thickness skin graft, Vacuum‐assisted closure
INTRODUCTION
Restoration of an intact skin barrier is of utmost importance following wounding to prevent infection, minimise wound contraction to maintain function, minimise cosmetic disfigurement and to avoid volume depletion. Traditionally, split‐thickness skin grafts (STSGs) are used to cover large areas of skin loss, granulating tissue beds, tissue loss across joints in areas where contraction will cause deformity and where epithelialisation alone will produce an unstable wound cover 1, 2. STSGs currently represent the most rapid, effective method of reconstructing large skin defects.
Graft survival is predicated on several physiological events; the initial ‘take’ (or incorporation) occurs by diffusion of nutrition from the recipient site, termed ‘plasmatic imbibition’. Revascularisation generally occurs between days 3 and 5 by reconnection of blood vessels in the graft to recipient site vessels or by ingrowth of vessels from the recipient site into the graft (3). STSGs must be placed on well‐vascularised beds with low bacterial counts to prevent infection. Skin grafts generally will not ‘take’ on poorly vascularised beds such as bare tendons, cortical bone without periosteum, heavily irradiated areas or infected wounds. However, once cultivated, virtually any tissue type with a vascular granulating stroma may be an acceptable bed for grafting (1). Successful STSGs require immobilisation of the graft to prevent shearing, infection and seroma or haematoma formation beneath the graft. To achieve all of the above requirements, we need uniform pressure over the entire grafted area through a non adherent, semi‐occlusive, absorbent dressing material.
The conventional therapy (CT) dressing of choice is a cotton bolster or sterile compressive or stainless steel gauze dressing that is used for at least five days. In the last decade, another therapy choice for a STSG is the use of negative pressure wound therapy using reticulated open cell foam (NPWT/ROCF) 4, 5. This reticulated open cell foam dressing conforms to the wound geometry by addition of negative pressure and promotes skin graft adherence while removing exudates and oedema from surrounding tissues.
The senior author has completed many reconstructive surgeries with the use of NPWT/ROCF in order to secure STSGs in foot and ankle surgeries in a variety of wound types. He has observed a decrease of repeated grafts as well as maximum graft take duration with the use of NPWT/ROCF. This study evaluates the clinical outcomes of STSGs treated with NPWT/ROCF or CT in foot and ankle reconstructive surgeries.
PATIENTS AND METHODS
A retrospective review over a 10‐year period was completed. After securing Western Institutional Review Board approval, medical charts of 142 patients who underwent foot and ankle surgery requiring STSGs were reviewed. These patients were admitted between January 1999 and November 2008 to the senior author's attending hospital. We excluded patients who were non compliant with follow‐up treatment after STSG placement.
The study included patients with pressure wounds (n = 27), posttraumatic wounds (n = 3), diabetic wounds (n = 54), planter fibroma (n = 6), osteomyelitis (n = 21) and miscellaneous chronic ulcers (n = 31). Anatomical placement of the skin grafts included dorsum of the foot (n = 19), planter aspect of the foot (n = 82), medial forefoot (n = 12), lateral forefoot (n = 10) and calcaneal region (n = 19). Patients medical records were examined for patient age, sex, diabetes (type‐1 or type‐2) (n = 117), active smoker (n = 38), peripheral vascular disease (PVD) (n = 41), vascular intervention before STSG (n = 11), hypertension (n = 106), hyperlipidemia (n = 58), cause of wound, wound bed preparation, type of dressing after STSG, time to dressing change (NPWT/ROCF or other therapy), weight bearing started after STSG in days, maximum percentage graft take, total length of hospital stay in days, post‐STSG length of stay (LOS), reoccurrence of wound at the place of STSG, repeated STSGs at same site and complications.
In both groups, NPWT/ROCF and CT, the wound was prepared for grafting using standard debridement techniques after initiation of appropriate anaesthesia, which can include either topical/local with or without sedation or general anaesthesia. Skin grafts (also called autografts) were harvested mostly from the lower extremity/thigh region to minimise the discomfort during reepithelialisation. Donor skin was harvested with an electric dermatome (thickness of 0·030–0·041 cm) and was meshed only to cover a larger area. The wound bed was optimally prepared with series of curets and rongeurs or hydrosurgery techniques and irrigated with sterile normal saline with the pulse irrigation system to ensure removal of all contaminants. Grafts were fenestrated and stapled in place with surgical staples or absorbable sutures circumferentially. A dressing system was then chosen to provide uniform pressure over the entire grafted area through a non adherent, semi‐occlusive, absorbent dressing material.
The V.A.C.® Therapy System includes a reticulated open cell foam (V.A.C.® GranuFoam™, KCI Licensing Inc., San Antonio, TX) dressing, a pressure‐sensing pad (SensaT.R.A.C.™ Pad, KCI Licensing Inc., San Antonio, TX), evacuation tubing, a collection canister and a software‐controlled therapy unit responsible for generating the negative pressure. Continuous therapy is recommended to provide a constant bolster, with a target pressure range between 75 and 125 mmHg. Higher pressure may help to hold the graft more firmly in place. The unit regulates negative pressure to ensure safe delivery of the prescribed therapy setting as well as consistent distribution of pressure across the wound surface.
We applied a non adherent dressing layer as an interface between the skin graft and the ROCF to prevent peeling off of the graft when removing the ROCF. NPWT/ROCF was applied under sterile conditions and checked to ensure that a complete dressing seal was achieved. The dressing was covered with a transparent adhesive drape, and continuous negative pressure of 125 mmHg was used (1, 2, 3). In CT, various types of dressing from the wound protocol used in the senior author's hospital were used. Depending on the wound and wound contamination, the following dressings were used: sterile compressive dressing, cotton bolster dressing and stainless steel gauze dressing (4, 5, 6). The dressing was placed for a mean of 5·3 days for NPWT/ROCF and 5·1 days for CT. All patients received off‐loading therapy, preventatively and therapeutically, as indicated. The graft was examined and evaluated within 5 days postoperatively. Haematoma and seroma formation were addressed on an individual basis.
Figure 1.
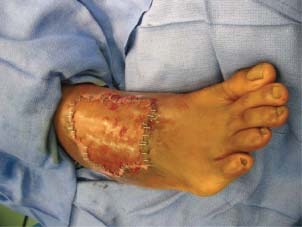
Post‐STSG before NPWT/ROCF dressing.
Figure 2.
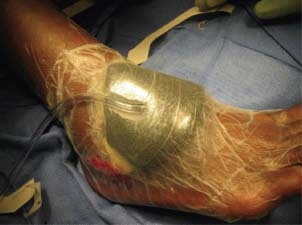
NPWT/ROCF after STSG.
Figure 3.
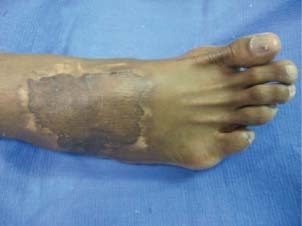
Completely healed wound after STSG and NPWT/ROCF.
Figure 4.
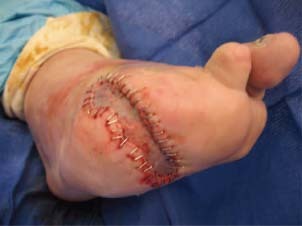
Post‐STSG before CT.
Figure 5.
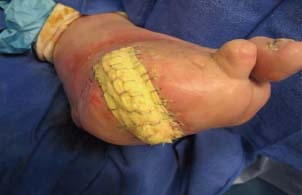
Conventional dressing after STSG.
Figure 6.
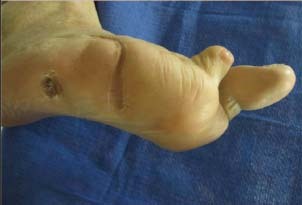
Completely healed wound after CT.
Differences in patient age, wound site, indication for STSG, PVD, vascular intervention before surgery, pre‐ or postoperative infection, partial or complete weight bearing after surgery, percentage of graft take at the first follow‐up, maximum graft take in days, graft failure and complications were assessed in all patients. We compared the group of patients treated with NPWT/ROCF to the group treated with CT using a one‐way analysis of variance or a two tailed t‐test with equal and unequal variance (Table 1). Significance was accepted at P < 0·05.
Table 1.
Population comparison of NPWT/ROCF versus CT patient groups
| Characteristics | NPWT/ROCF | Conventional therapy | P value* |
|---|---|---|---|
| n (total = 142) | 87/142 | 55/142 | |
| Age (years ± SD) | 54·6 ± 15·2 (range: 21–97) | 58·4 ± 11·9 (range: 27–85) | 0.11 |
| Sex (male/female) | 64/23 (73·6/26·4) | n = 35/20 (63·6/36·4) | 0.21 |
| Ethnic origin | 0.49 | ||
| African‐American | 23 (26·4) | 11 (20) | 0.38 |
| Caucasian | 40 (46) | 27 (49·1) | 0.72 |
| Hispanic | 19 (21·8) | 13 (23·6) | 0.68 |
| Native American | 4 (4·6) | 4 (7·3) | 0.5 |
| Other | 1 (1·2) | 0 (0) | 0.43 |
| Type of diabetes | 0.095 | ||
| Type 1 | 6 (6·9) | 3 (5·5) | 0.73 |
| Type 2 | 68 (78·2) | 40 (72·7) | 0.46 |
| Active smoker | 26 (29·9) | 12 (21·9) | 0.29 |
| Currently use alcohol | 13 (15) | 11 (20) | 0.29 |
| Hyperlipidaemia | 33 (38) | 25 (45·5) | 0.37 |
| Total length of stay (days ± SD) | 8·7 ± 6·4 (range: 5–56) | 9·74 ± 6·3 (range: 2–27) | 0.32 |
| Post‐STSG length of stay (days ± SD) | 5·6 ± 1·3 (range: 4–12) | 6·4 ± 2·4 (range: 4–16) | 0.01 |
| Duration of dressing (days ± SD) | 5·3 ± 0·6 (range: 4–7) | 5·1 ± 0·93 (range: 4–9) | 0.09 |
| Grafted area (cm2) (mean ± SD) | 45·4 ± 9·69 (range: 31·5–69·3) | 47·4 ± 10·3 (range: 31·5–74·7) | 0.26 |
| Average duration of first follow‐up (days ± SD) | 16·8 ± 7·7 (range: 5–40) | 15·1 ± 5·9 (range: 5–29) | 0.15 |
| % of graft take at first follow‐up (% ± SD) | 95·2 ± 8·7 | 86·2 ± 23 | 0.0001 |
| Total follow‐up (months ± SD) | 8·91 ± 3·2 (range: 1–24) | 8·3 ± 2·3 (range: 6–12) | 0.29 |
| Duration of maximum graft take (days ± SD) | 23·4 ± 11·9 (range: 6–67) | 29·3 ± 14·8 (range: 8–90) | 0.009 |
| Maximum graft take (% ± SD) | 95·7 ± 8·7 | 83·1 ± 32·9 | 0.0008 |
| Partial weight bearing started from surgery (days ± SD) | 32·9 ± 23·6 (range: 7–150) | 40·3 ± 25·1 (range: 2–95) | 0.07 |
| Graft failure | n = 3 (3·45%) | n = 9 (16·36%) | 0.006 |
| Complications in graft failure | |||
| Seroma | n = 1 (33·3%) | n = 4 (44·4%) | 0.8 |
| Haematoma | n = 0 (0%) | n = 2 (22·2%) | 0.4 |
| Infection | n = 2 (66·7%) | n = 3 (33·3%) | 0.4 |
RESULTS
One hundred forty‐two patients underwent STSG placement. The patients were divided into two groups and managed either with NPWT/ROCF [group 1, n = 87 (61·27%)] or CT [group 2, n = 55 (38·73%)]. The average age was 54·6 years for the NPWT/ROCF group and 58·4 for the CT group. All wounds were recalcitrant to prior multiple treatment modalities. Overall causes of wounds included diabetic foot ulcers (n = 79), pressure ulcers (n = 27), vascular insufficiency (n = 5), trauma (n = 3), fibroma (n = 5) and miscellaneous (n = 23). The average grafted area was 45·4 cm2 for the NPWT/ROCF group and 47·4 cm2 for the CT group. The average length of time for NPWT/ROCF was 5·3 days and 5·1 days for CT. There was no significant difference in age, total hospital LOS, mean wound size and site or type of wound grafted observed between the groups (by Fisher's Exact Test). A comparison of populations is shown in Table 1. Mean graft take at the first follow‐up was 95 ± 9% for NPWT/ROCF compared to 86 ± 23% for CT and maximum graft take was 96 ± 9% for NPWT/ROCF compared to 83 ± 33% for CT. Graft acceptance was 97% (84/87) for NPWT/ROCF compared to 84% (46/55) for CT (P = 0·009).
There were significantly fewer repeated STSGs required in the NPWT/ROCF group compared to the CT group [n = 3 (3·5%) versus n = 9 (16%) (P = 0·0067)]. When comparing patients who required repeated skin grafts with those who did not, we found no significant difference in age, graft size, location of wound, comorbidities or day of postoperative wound evaluation (Table 2). There were fewer complications in graft failure (seroma, haematoma and infection) in the NPWT/ROCF group as compared to the CT group.
Table 2.
Characteristics of patients with graft failure
| Age (years) | Sex | Etiology | Location | Grafted area (cm2) | Type of dressing | PVD | DM | HTN | Hb (g%) | S.Cr (mg/dl) | Blood glucose (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 | M | Diabetic ulcer | Lateral forefoot | 31.5 | NPWT/ROCF | No | Yes | Yes | 7.8 | 2.1 | 155 |
| 83 | M | Pressure ulcer | Planter forefoot | 61.6 | NPWT/ROCF | Yes | Yes | Yes | 9.6 | 0.9 | 210 |
| 49 | M | Pressure ulcer | Planter | 44.1 | NPWT/ROCF | No | Yes | Yes | 13.7 | 1.1 | 160 |
| 62 | F | Pressure ulcer | Medial forefoot | 35 | CT | Yes | Yes | No | 11.1 | 1.0 | 126 |
| 40 | F | Diabetic ulcer | Planter forefoot | 62 | CT | No | Yes | Yes | 13.6 | 1.0 | 215 |
| 67 | M | Diabetic ulcer | Calcaneal | 47.6 | CT | No | Yes | Yes | 13.3 | 1.2 | 190 |
| 72 | F | Diabetic ulcer | Planter forefoot | 50.4 | CT | Yes | Yes | Yes | 11.3 | 1.6 | 175 |
| 50 | M | Pressure ulcer | Planter forefoot | 54.6 | CT | No | Yes | Yes | 14.1 | 1.0 | 155 |
| 67 | M | Diabetes | Calcaneal | 33.6 | CT | No | Yes | Yes | 13.3 | 1.2 | 135 |
| 82 | M | Diabetic ulcer | Planter forefoot | 64.4 | CT | Yes | Yes | Yes | 13.2 | 0.9 | 140 |
| 62 | M | Diabetic ulcer | Planter forefoot | 50.1 | CT | Yes | Yes | Yes | 10.6 | 2.6 | 190 |
| 67 | M | Diabetic ulcer | Calcaneal | 32 | CT | No | Yes | Yes | 13.6 | 1.2 | 240 |
| 0·66 | 0·28 | 0·18 | 0·64 | 0.81 | 0·76 | 0·58 | 0.08 | 0.86 | 0·98 |
*Characteristics NPWT/ROCF conventional therapy P value.
DISCUSSION
This retrospective evaluation compared the clinical outcomes between NPWT/ROCF and the CT‐treated STSG patients. Our results showed no significant differences in age, total hospital LOS and mean wound size, site or type of wound grafted between the two groups. However, the percentages of overall graft take at first follow‐up and maximum graft take were higher in the NPWT/ROCF group when compared to the conventional therapy group. Graft take was significantly higher in the NPWT/ROCF group. Additionally, there were significantly fewer repeated grafts required in the NPWT/ROCF group compared to the conventional therapy group.
Despite numerous advances, chronic and other difficult‐to‐manage wounds continue to be a treatment challenge. The period of time between grafting and revascularisation of the graft is referred to as the phase of plasmatic imbibition. After graft placement, an initial adherence to the wound bed via a thin fibrin network temporarily anchors the graft until definitive circulation and connective tissue connections are established (6). This adherence begins immediately and is probably maximised by 8 hours postgrafting. This prevents graft desiccation, maintains graft vessel patency and provides nourishment for the graft. This process is entirely responsible for graft survival for 2–3 days until circulation is restored. Revascularisation of the graft begins at 2–3 days postgrafting by a mechanism that is not completely understood. Regardless of the true mechanisms, full circulation to the graft is restored by 6–7 days postgrafting. Without initial adherence due to poor technique, haematoma or shear, plasmatic imbibition and revascularisation will not take place and the graft will slough. If the wound bed is properly prepared and the graft is bolstered and the wound spared from shear, STSG survival should be near uniform (7). Our data showed an increase in graft take with NPWT/ROCF, which is supported by previous studies showing NPWT as an effective adjunct therapy for the management of skin grafts and as a bolster for graft take 5, 8, 9, 10, 11.
The ideal STSG dressing has three main components: elimination of fluid collection, immobilisation of the graft and stabilisation of the graft on an irregular surface. NPWT is a relatively new method in Western medical practice for treating acute and chronic wounds. The mechanisms of action of NPWT/ROCF include reducing oedema from extracellular tissues, decreasing the bacterial load on the wound and promoting tissue perfusion and healthy granulation tissue formation 12, 13, 14, 15. Recent cellular research speculates that microstrain, which is the mechanical stretching of cells under negative pressure, causes them to rapidly divide and proliferate (16) and results in positive growth factor production needed during wound healing (17).
A historical review of the literature shows that clinicians have experimented with the application of suction to wounds for over half a century. Russian clinicians developed its use during the 1980s with favourable reports in the medical literature 18, 19. There are 16 randomised controlled trials (RCTs) evaluating the clinical efficacy of NPWT/ROCF; however, at least nine of these RCTS are mostly concerned with chronic and diabetic wounds or their effect applied over STSGs 10, 20, 21, 22, 23, 24, 25, 26, 27. The majority of these trials report favourably that NPWT/ROCF can lead to a decrease in time to wound closure, promote perfusion and granulation tissue formation and that the therapy is safe. Recently, Scherer et al. (5) retrospectively compared bolster versus NPWT/ROCF in trauma patients and found a statistically significant decrease in repeated grafting during the same hospitalisation when NPWT was used in securing the STSG (5), which was similar to our findings. In this study, there was no statistically significant difference in mean graft size, compared to a 300 percent difference in the Scherer et al. study.
Reasons for skin graft failure can be multifactorial. The most common reason for skin graft failure is haematoma and seroma formation beneath the graft. Movement of the graft or shear forces may also lead to graft failure through disruption of the fragile attachment of the graft to the wound bed. This often occurs when the graft is placed over a flexor or extensor surface or over a mobile tendon sheath. Another common source of failure is a poor recipient site. The wound may have poor vascularity, or the surface contamination may have been too great to allow graft survival. Bacteria and the inflammatory response to bacteria stimulate release of enzymes and other harmful substances at the wound interface that disrupt the fibrin adherence of the graft. Technical error may also yield graft failure. In our study, graft failure in CT group was mostly because of haematoma, seroma and shear of the graft. NPWT/ROCF promotes wound healing by delayed primary or secondary intention through creating a moist wound environment, removing infectious material, improving microcirculation, providing tight adhesion, preparing the wound bed for closure, reducing oedema and promoting formation and perfusion of granulation tissue 12, 13, 14, 15, 16. NPWT/ROCF is indicated for use in all care settings and for a variety of wound types including STSGs 11, 22. At most institutions, NPWT/ROCF initial supply costs may seem higher than CT 28, 29. However, our results showed that NPWT/ROCF reduced the need for repeated grafting, which eliminates repeated surgical expenses, reduces the extra hospital days incurred by graft failure and potentially offsets the total cost of NPWT/ROCF.
This study has some limitations. As a retrospective study, group assignment (NPWT/ROCF versus CT) was not controlled, randomised or blinded. However, our data suggest that NPWT/ROCF over an STSG site may improve overall graft survival, as measured by fewer episodes of repeated grafting, maximum percentage of graft taken and complications.
CONCLUSION
We retrospectively reviewed a larger number of patients than have been previously described, and our results continue to suggest that V.A.C.® Therapy is an excellent method for securing split‐thickness skin grafts to the bed and for achieving stability of skin grafts. We believe further studies of NPWT/ROCF in a prospective, randomised fashion are warranted to better quantify outcome measures.
REFERENCES
- 1. Donato MC, Novicki DC, Blume PA. Skin grafting: historic and practical approaches. Clin Podiatr Med Surg 2000;17:561–91. [PubMed] [Google Scholar]
- 2. Blume PA, Key JJ. Skin grafts. In: Dockery GL, Crawford EC, editors. Lower Extremity Soft Tissue & Cutaneous Plastic Surgery. Philadelphia: Saunders,2004:157–71. [Google Scholar]
- 3. Rothstein AC. Skin grafting techniques. J Am Podiatr Med Assoc 1983;73:70. [DOI] [PubMed] [Google Scholar]
- 4. Hanasono MM, Skoracki RJ. Securing skin grafts to microvascular free flaps using the vacuum‐assisted closure (VAC) device. Ann Plast Surg 2007;58:573–6. [DOI] [PubMed] [Google Scholar]
- 5. Scherer LA, Shiver S, Chang M, Meredith W, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg 2002;137:930–3. [DOI] [PubMed] [Google Scholar]
- 6. Polk HC. Adherence of thin skin grafts. Surg Forum 1966;17:487–9. [PubMed] [Google Scholar]
- 7. Emerick KS, Deschler DG. Incidence of donor site skin graft loss requiring surgical intervention with the radial forearm free flap. Head Neck 2007;29:573–6. [DOI] [PubMed] [Google Scholar]
- 8. Blackburn JH, Boemi L, Hall WW. Negative pressure dressings as a bolster for skin grafts. Ann Plast Surg 1998;40:453–7. [DOI] [PubMed] [Google Scholar]
- 9. Carson SN, Overall K, Lee‐Jahshan S, Travis E. Vacuum‐assisted closure used for healing chronic wounds and skin grafts in the lower extremities. Ostomy Wound Manage 2004;50:52–8. [PubMed] [Google Scholar]
- 10. Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004;114:917–22. [DOI] [PubMed] [Google Scholar]
- 11. Stone P, Prigozen J, Hofeldt M, Hass S, DeLuca J, Flaherty S. Bolster versus negative pressure wound therapy for securing split‐thickness skin grafts in trauma patients. Wounds 2004;16:219–23. [Google Scholar]
- 12. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. [DOI] [PubMed] [Google Scholar]
- 13. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 14. Morykwas MJ, Argenta LC, Shelton‐Brown E, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 15. Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum‐assisted closure (VAC) device: a review. Am J Clin Dermatol 2005;6:185–94. [DOI] [PubMed] [Google Scholar]
- 16. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 17. McNulty AK, Schmidt M, Feeley T, Villanueva P, Kieswetter K. Effects of negative pressure wound therapy on cellular energetic in fibroblasts grown in a provisional wound (fibrin) matrix. Wound Repair Regen 2009;17:192–9. [DOI] [PubMed] [Google Scholar]
- 18. Kostiuchenok BM, Karlov VA, Gerasimov MV, Samykina TD. Vacuum treatment of suppurative wounds. Sov Med 1984;2:108–10. [PubMed] [Google Scholar]
- 19. Kostiuchenok BM, Kolker II, Karlov VA, Ignatenko LI, Muzykant TD. Vacuum treatment in the surgical management of suppurative wounds. Vestn Khir Im I I Grek 1986;137:18–21. [PubMed] [Google Scholar]
- 20. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–37. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 22. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum‐assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631–6. [DOI] [PubMed] [Google Scholar]
- 23. Braakenburg A, Obdeijn MC, Feitz R, Van Rooij IA, Van Griethuysen AJ. Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum‐assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006;118: 390–7. [DOI] [PubMed] [Google Scholar]
- 24. Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative‐pressure wound dressings for diabetic foot wounds. Ann Vasc Surg 2003;17:645–9. [DOI] [PubMed] [Google Scholar]
- 25. Llanos S, Danilla S, Barraza C, Armijo E, Pineros JL, Quintas M, Searle S, Calderon W. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double‐masked, controlled trial. Ann Surg 2006;244:700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moues CM, Van Den Bemd GJ, Heule F, Hovius SE. Comparing conventional gauze therapy to vacuum‐assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg 2007;60:672–81. [DOI] [PubMed] [Google Scholar]
- 27. Joseph EHC, Bergman S, Roaf E, Swann NF, Anatasi GW. Prospective randomized trial of vacuum‐assisted closure versus standard therapy of chronic non‐healing wounds. Wounds 2000;12: 60–7. [Google Scholar]
- 28. Philbeck TE Jr, Whittington KT, Millsap MH, Briones RB, Wight DG, Schroeder WJ. The clinical and cost effectiveness of externally applied negative pressure wound therapy in the treatment of wounds in home healthcare Medicare patients. Ostomy Wound Manage 1999;45: 41–50. [PubMed] [Google Scholar]
- 29. Flack S, Apelqvist J, Keith M, Trueman P, Williams D. An economic evaluation of VAC Therapy compared with wound dressings in the treatment of diabetic foot ulcers. J Wound Care 2008;17:71–8. [DOI] [PubMed] [Google Scholar]


